Abstract
BACKGROUND: While experimental data suggest that selective intracoronary hypothermia decreases infarct size, studies in patients with ST-elevation myocardial infarction (STEMI) are lacking.
AIMS: We investigated the efficacy of selective intracoronary hypothermia during primary percutaneous coronary intervention (PCI) to decrease infarct size in patients with STEMI.
METHODS: In this multicentre randomised controlled trial, 200 patients with large anterior wall STEMI were randomised 1:1 to selective intracoronary hypothermia during primary PCI or primary PCI alone. Using an over-the-wire balloon catheter for infusion of cold saline and a pressure-temperature wire to monitor the intracoronary temperature, the anterior myocardium distal to the occlusion was selectively cooled to 30-33°C for 7-10 minutes before reperfusion (occlusion phase), immediately followed by 10 minutes of cooling after reperfusion (reperfusion phase). The primary endpoint was infarct size as a percentage of left ventricular mass on cardiovascular magnetic resonance imaging after 3 months.
RESULTS: Selective intracoronary hypothermia was performed in 94/100 patients randomised to cooling. Distal coronary temperature decreased by 6°C within 43 seconds (interquartile range [IQR] 18-113). The median duration of the occlusion phase and reperfusion phase were 8.2 minutes (IQR 7.2-9.0) and 9.1 minutes (IQR 8.2-10.0), respectively. The infarct size at 3 months was 23.1±12.5% in the selective intracoronary hypothermia group and 21.6±12.2% in the primary PCI alone group (p=0.43). The left ventricular ejection fraction at 3 months in each group were 49.1±10.2% and 50.1±10.4%, respectively (p=0.53).
CONCLUSIONS: Selective intracoronary hypothermia during primary PCI in patients with anterior wall STEMI was feasible and safe but did not decrease infarct size compared with standard primary PCI. (ClinicalTrials.gov: NCT03447834)
ST-elevation myocardial infarction (STEMI) remains a major health problem and may lead to death or heart failure1. Early restoration of blood flow limits infarct size and improves long-term outcomes12. The treatment of choice in STEMI patients is early reperfusion and revascularisation by primary percutaneous coronary intervention (PCI)3. Paradoxically, reperfusion may also cause myocardial injury and increase infarct size. This is termed myocardial reperfusion injury4. Currently, no therapy exists to reduce such injury in humans456.
There is general agreement that reperfusion injury occurs during the first minutes of reperfusion, and preventive therapy before reperfusion may be cardioprotective4.
In animal models of myocardial infarction, hypothermia of 30-33°C reduces reperfusion injury and infarct size78. Importantly, this effect was only noted when hypothermia was established before reperfusion9101112. In contrast, studies in humans applying systemic cooling have not been able to confirm this protective effect13.
To overcome the intrinsic limitations of systemic cooling, we developed and tested a novel method to provide intracoronary hypothermia selectively to the infarct area just prior to reperfusion1415. This method was feasible and safe in a human pilot study16. Compared to systemic cooling, the target temperature of the threatened myocardium is achieved more rapidly by intracoronary cooling, within minutes and without noticeable side-effects17. In 2 of 4 patients with inferior STEMI and an occluded right coronary artery, symptomatic atrioventricular conduction disturbances were observed, one of which necessitated treatment with a temporary pacemaker. In 6 patients with large anterior STEMI, no complications were observed. Consequently, we conducted the randomised, controlled EUROpean Intracoronary Cooling Evaluation in patients with ST-elevation myocardial infarction trial (EURO-ICE) as a proof-of-principle study to investigate the ability of selective intracoronary hypothermia to decrease infarct size in patients with anterior wall STEMI18.
Methods
STUDY DESIGN
The trial protocol was approved by the institutional review boards of each participating site, and the study was conducted in accordance with the Declaration of Helsinki.
EURO-ICE was a prospective, multicentre randomised controlled trial to evaluate the effect of selective intracoronary hypothermia on infarct size as determined by cardiovascular magnetic resonance imaging (CMR) at 3 months. Patients with anterior wall STEMI and an occlusion of the proximal or mid-left anterior descending artery (LAD) with Thrombolysis in Myocardial Infarction (TIMI) grade 0 or 1 flow were randomised 1:1 to selective intracoronary hypothermia plus primary PCI versus standard primary PCI (control group), respectively18. The trial was conducted at 8 sites in Europe (Supplementary Table 1).
A data safety monitoring board (DSMB) had access to all data. Interim analyses, for which the investigators were blinded, were performed by the DSMB after the inclusion of 40 and 100 patients, respectively. CMR studies were analysed by an independent core laboratory, blinded to treatment (University of Glasgow Imaging Core Laboratory, United Kingdom). During the study, the authors had no access to outcome data, including CMR results. After the last CMR had been analysed by the core laboratory, the authors had unrestricted access to all data.
STUDY POPULATION
Patients were eligible if they were admitted for anterior wall STEMI with a summed ST-segment deviation of ≥5 mm on the qualifying electrocardiogram and presented within 6 hours after onset of symptoms. Patients were required to be conscious and capable of providing informed consent.
Major exclusion criteria were age <18 or >80 years, cardiogenic shock, previous anterior wall myocardial infarction, previous bypass surgery, severe concomitant disease with a life expectancy of less than 1 year, known contraindications to CMR, or inability to understand and give informed consent. The flowchart is shown in Supplementary Figure 1, and Supplementary Table 2 provides a list of all inclusion and exclusion criteria.
TRIAL PROCEDURES
Primary PCI was performed according to usual care. If the initial coronary angiogram demonstrated an occlusion in the proximal or mid-LAD with TIMI grade 0 or 1 flow, then the patient was eligible for the study. After a short verbal explanation, informed consent was obtained, and randomisation was performed. In case of randomisation to standard primary PCI, predilatation and stenting were performed as usual, and the procedure was finished according to regular routine.
In case of randomisation to selective intracoronary hypothermia, a regular guidewire was advanced beyond the culprit lesion, immediately followed by an over-the-wire balloon (OTWB) that was inflated to 4 atm at the location of the occlusion to prevent reperfusion. Next, a 0.014” coronary pressure wire (PW) capable of also measuring temperature (PressureWire [Abbott]) was equalised for pressure at the tip of the guiding catheter while simultaneously zeroing the blood temperature. Thereafter, it was positioned next to the regular guidewire in the balloon-occluded distal coronary artery (Central illustration). If necessary, the balloon was deflated for a few seconds to pass the PW into the distal LAD, after which the balloon was quickly reinflated to re-occlude the coronary artery.
Thereafter, the regular guidewire in the OTWB was removed, and the central lumen of the OTWB was connected to two parallel contrast infusion pumps (Supplementary Appendix 1), one filled with saline at room temperature and the other filled with saline at 4°C (Central illustration). Next, the intracoronary hypothermia protocol was performed as described below. Tympanic temperature was measured to monitor systemic temperature.
As an alternative to the use of a regular guidewire and OTWB, followed by introduction of the PW, it was also permitted to start the procedure with just a PW, cross the occlusion, and advance a specifically designed 2.7 Fr monorail balloon-infusion catheter (CoolCell catheter [CCC] [Hexacath]) to the site of the occlusion and inflate the balloon to 4 atm. No regular wire was then necessary, thus simplifying the procedure (Supplementary Figure 2).
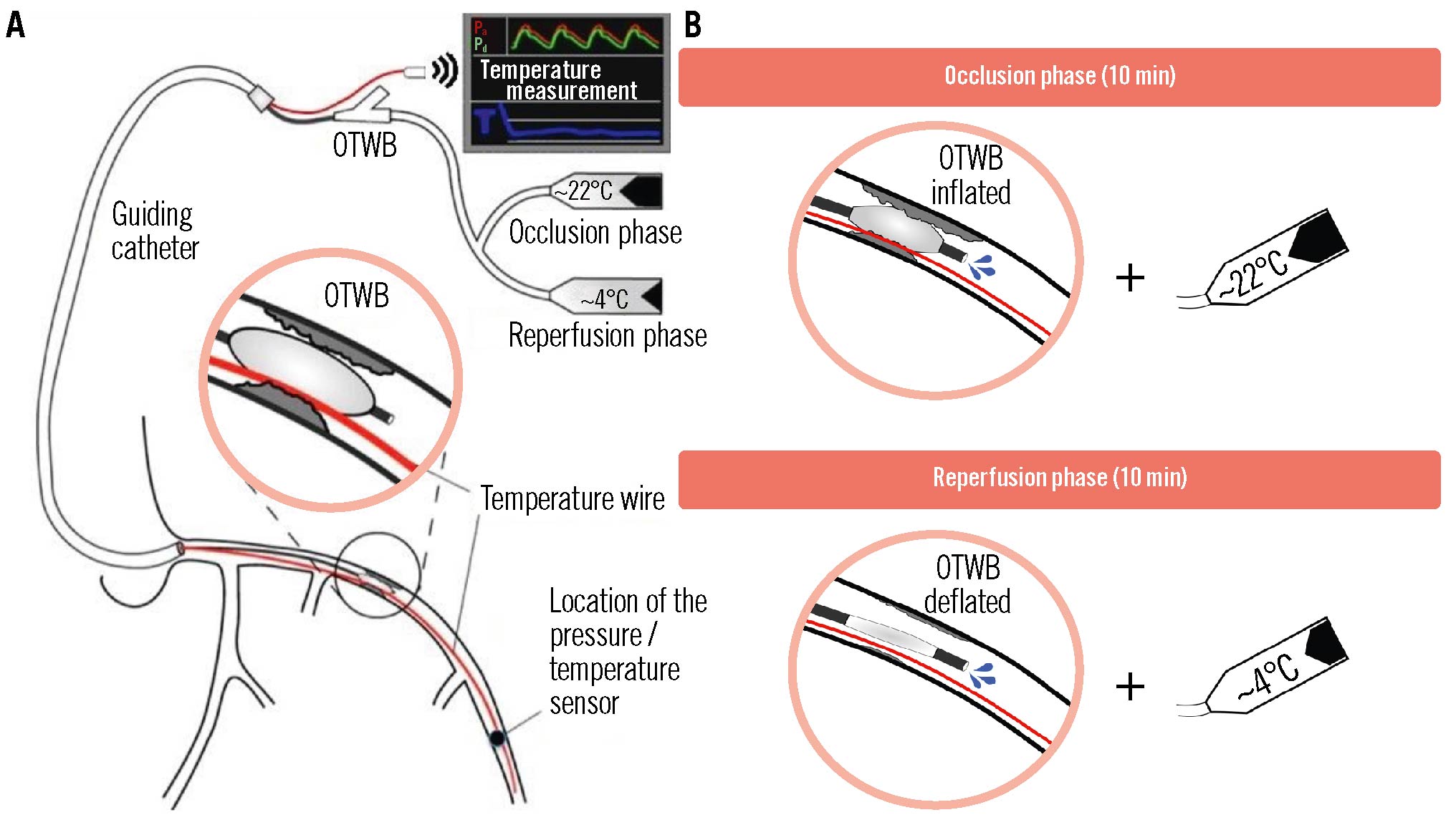
Central illustration. Selective intracoronary hypothermia during primary PCI in patients with anterior wall STEMI. A) A schematic overview of the instrumentation for selective intracoronary hypothermia is shown. The close-up shows the positioning of the balloon within the occlusion after removal of the regular guidewire in order to enable saline infusion through the central lumen of the over-the-wire balloon. The sensor-tipped pressure temperature wire is indicated in red. B) The occlusion phase (balloon inflated) and the reperfusion phase (balloon deflated) are shown with the associated saline temperatures. OTWB: over-the-wire balloon
INTRACORONARY HYPOTHERMIA PROTOCOL
Hypothermia was initiated for 7-10 minutes before the onset of reperfusion (occlusion phase, OTWB inflated) and continued for 10 minutes after reperfusion (reperfusion phase, balloon deflated). The first pump, filled with saline at room temperature, was connected to the central lumen of the OTWB, and infusion was started at a flow rate of 20-25 ml/min. If necessary, this flow rate was adjusted based upon the continuous measurement of the distal coronary temperature to maintain a temperature of approximately 6°C below body temperature, corresponding to a myocardial temperature in the infarcted area of roughly 4°C below body temperature during that occlusion phase (heat transport by conduction)19. After these 7-10 minutes, the balloon was deflated, and reperfusion was started. Just before deflating the balloon, the infusion was switched to saline at 4°C using the second infusion pump. Switching the temperature of the saline to 4°C during the reperfusion phase was necessary, because during the reperfusion phase, partial or complete reperfusion occurs, and the cooler saline mixes with warmer blood. In this way, an adequate reduction in the distal and myocardial temperatures can be maintained during the reperfusion phase (Figure 1, Supplementary Figure 3). It has been demonstrated in previous animal experiments that, during the reperfusion phase, coronary and myocardial temperatures are almost equal (heat transport by convection)15. The distal coronary pressure was measured continuously.
After the intracoronary infusions, the OTWB (or CCC) was removed, and PCI was finished using the PW as a guidewire. Medical treatment before and after the intervention followed contemporary guidelines.
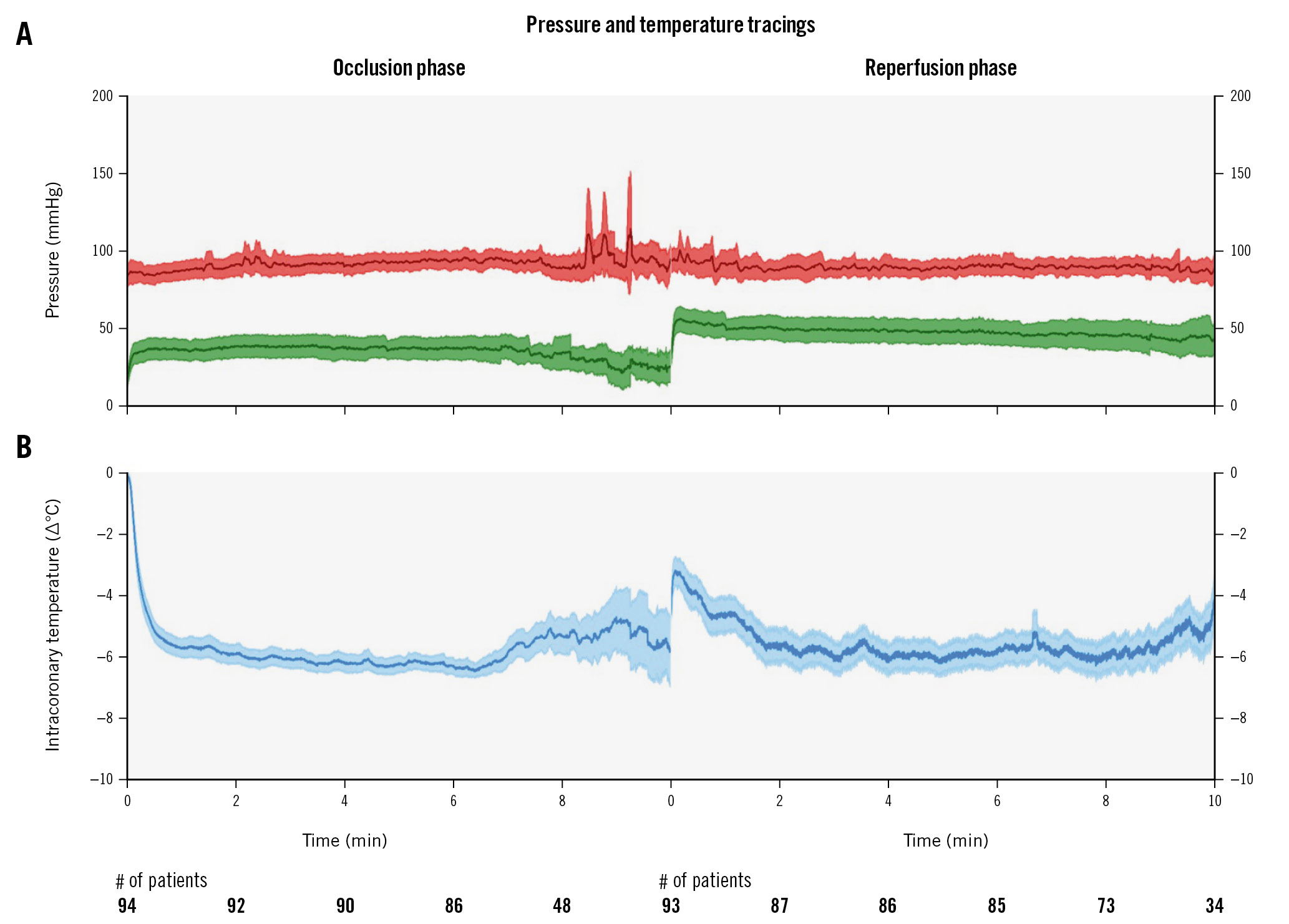
Figure 1. Composite tracing of all patients showing aortic pressure (red), distal coronary pressure (green) and intracoronary temperature (blue) during treatment with selective intracoronary hypothermia. Tracings are displayed as means with 95% confidence intervals (shading). Time in minutes is indicated on the horizontal axis with the number of patients participating at that timepoint written below. A) The procedure consisted of two parts: the occlusion phase with the inflated balloon and infusion of saline at room temperature, immediately followed by the reperfusion phase with the deflated balloon (allowing reperfusion) and infusion of saline at 4°C. B) Intracoronary temperature is expressed as °C relative to body temperature.
ENDPOINTS
The primary endpoint of the EURO-ICE trial was infarct size as a percentage of left ventricular mass (LVM) assessed at 3 months by CMR using late gadolinium enhancement (LGE) analysis. Key secondary endpoints were a composite of all-cause mortality or hospitalisation for heart failure at 3 months and at 1 year. Subgroup analyses were performed for the primary endpoint, and results were visualised as a forest plot for the prespecified characteristics. For a complete overview of the endpoints see Supplementary Table 3.
CARDIOVASCULAR MAGNETIC RESONANCE IMAGING
CMR at 1.5 Tesla was performed between 2 and 7 days and again at 3 months after the index STEMI. The protocol included T2* imaging (precontrast), T1 mapping (pre- and post-contrast), first pass myocardial perfusion imaging after administration of gadobutrol contrast media (Gadovist [Bayer]; 1.5 mmol/ml solution for injection), cine steady-state free precession imaging (SSFP), and finally, LGE imaging 10-15 minutes after contrast administration. LVM and function were calculated from the short-axis cine images. LGE imaging was used to calculate infarct size and to identify microvascular obstruction (MVO). Infarct size and MVO were expressed in grams and as a percentage of LVM. The T2* images were used to identify intramyocardial haemorrhage (IMH), and contrast-enhanced cine SSFP measured the area at risk. The Supplementary Appendix 2 provides a detailed description of the acquisition techniques, CMR parameters, and analyses.
SAMPLE SIZE AND STATISTICS
All endpoints were analysed in the intention-to-treat (ITT) population.
Since this study only included patients with anterior wall STEMI due to a proximal or mid-LAD occlusion, it was assumed that the mean infarct size in the control arm would roughly correspond to 25% of LVM. Assuming a normal distribution of infarct size with a mean of 25% in the control arm and a standard deviation of 15%, plus typical statistical assumptions (unpaired 2-tailed t-test, alpha of 0.05, and power 0.80), a sample size of 91 subjects per arm would be needed to detect an absolute reduction of 6.25% of LVM, i.e., a 25% relative reduction of infarct size. To account for patients lost-to-follow up, 200 patients were planned for the study.
The secondary endpoints of clinical outcomes at 3 months and at 1 year were compared by applying the chi-square or Fisher’s exact test to a 2x2 table of binary events per group. Similarly for the secondary endpoints involving imaging or blood samples, unpaired 2-tailed t-tests or Mann-Whitney U tests were used to compare values between the groups.
Predefined analyses included tests for heterogeneity of effect by lesion location (proximal vs mid-LAD occlusion), TIMI flow grade (0 vs 1), and assumed clinical characteristics (binary or using the cohort median as the threshold).
Finally, because the cooling procedure is not trivial in itself and because it was difficult to anticipate protocol deviations, per-treatment and per-protocol analyses for the primary endpoint were part of the statistical protocol.
Results
PATIENT CHARACTERISTICS
Patients were recruited between January 2019 and June 2022 in 8 European heart centres.
Baseline characteristics are presented in Table 1. The mean age (±standard deviation) of the patients was 62±11 years, and 86% were male.
Risk factors were equally distributed in both groups, and the summed ST-deviation on the qualifying electrocardiogram was 16 mm (interquartile range [IQR] 10-23) in the selective intracoronary hypothermia group and 13 mm (IQR 9-19) in the control group (p=0.07).
Angiography revealed the culprit occlusion in the proximal LAD in 51% of the patients and in the mid-LAD in 49% in the selective intracoronary hypothermia group, and in 49% and 51% in the control group, respectively (p=0.78).
The door-to-balloon time was 15 minutes longer in the hypothermia group compared with the control group: 37 (IQR 33-44) versus 22 minutes (IQR 18-26), respectively (p<0.001). Taking into account that the median duration of the occlusion phase was 8 minutes (Table 2), this indicates that an additional 7 minutes were needed for the specific instrumentation required for the cooling procedure.
Table 1. Baseline characteristics in both groups.
| Selective intracoronary hypothermia during primary PCI (N=100) | Standard primary PCI (N=100) | p-value | |
|---|---|---|---|
| Age, years | 61.7±9.9 | 61.6±11.2 | 0.92 |
| Sex | 0.55 | ||
| Male | 87 | 84 | |
| Female | 13 | 16 | |
| Medical history | |||
| Hypertension | 33 | 33 | 1.00 |
| Current smoker | 34 | 37 | 0.66 |
| Diabetes mellitus | 14 | 10 | 0.38 |
| Dyslipidaemia | 38 | 34 | 0.56 |
| Family history of CVD | 33 | 42 | 0.19 |
| Prior myocardial infarction | 6 | 1 | 0.05 |
| Prior PCI | 6 | 4 | 0.52 |
| Body temperature before procedure, °C | 36.3±0.8 | 36.3±0.8 | 0.96 |
| Coronary angiography | |||
| Left main disease | 1 | 0 | 0.32 |
| Multivessel disease | 24 | 24 | >0.99 |
| Anterior wall STEMI | |||
| ∑ ST deviation, mm | 16 (10-23) | 13 (9-19) | 0.07 |
| Culprit occlusion | 0.78 | ||
| Proximal LAD | 51 | 49 | |
| Mid-LAD | 49 | 51 | |
| Pre-PCI TIMI grade flow | 0.32 | ||
| 0 | 83 | 88 | |
| 1 | 17 | 12 | |
| Symptom onset-to-balloon time*, min | 149(117-213) | 156 (120-206) | 0.81 |
| Door-to-balloon time*, min | 37 (33-44) | 22 (18-26) | <0.001 |
| Data are given as mean±SD, n, or median (IQR). *In the selective intracoronary hypothermia during primary PCI group, symptom onset-to-balloon time and door-to-balloon time include the duration of the occlusion phase. CVD: cardiovascular disease; IQR: interquartile range; LAD: left anterior descending artery; PCI: percutaneous coronary intervention; SD: standard deviation; STEMI: ST-elevation myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction | |||
Table 2. Procedural data related to selective intracoronary hypothermia.
| Selective intracoronary hypothermia during primary PCI (N=94) | |
|---|---|
| Hypothermia – occlusion phase | |
| Time to target temperature, sec | 43 (18-113) |
| Duration of occlusion phase, min | 8.2 (7.2-9.0) |
| ∆ body temperature§, °C | −0.1 (−0.5 to 0.0) |
| Rate of infusion, ml/min | 20 (20-25) |
| Infused volume of saline, ml | 162 (140-200) |
| Atrial fibrillation | 0/94 |
| Sustained VT or VF# | 2/94 |
| Hypothermia – reperfusion phase | |
| Duration of reperfusion phase, min | 9.1 (8.2-10.0) |
| ∆ body temperature§, °C | −0.3 (−0.7 to 0.1) |
| Rate of infusion, ml/min | 20 (15-20) |
| Infused volume of saline, ml | 144 (123-200) |
| Atrial fibrillation | 0/94 |
| Sustained VT or VF# | 3/94 |
| Data are given as median (IQR) or n/N. §The difference in body temperature before the start of the procedure. #Necessitating defibrillation. PCI: percutaneous coronary intervention; VF: ventricular fibrillation; VT: ventricular tachycardia | |
PROCEDURAL RESULTS FOR PRIMARY PCI
Successful stenting was performed in 95 patients in the selective intracoronary hypothermia group and 95 patients in the control group. Patients were treated with current-generation drug-eluting stents. In the hypothermia arm, stenting was performed after completion of the hypothermia protocol. At the discretion of the interventional cardiologist, additional predilatation or direct stenting were both allowed.
No patients died during the procedure. Ventricular fibrillation occurred in 9 patients in the selective intracoronary hypothermia group versus 7 patients in the control group (Supplementary Figure 4) (p=0.60). Atrial fibrillation occurred in 0 versus 3 patients, respectively (p=0.08). Haemodynamic deterioration during the procedure occurred in 3 versus 2 patients, respectively (p=0.65). Two cases of acute stent thrombosis were encountered in the selective intracoronary hypothermia group versus 1 case in the control group (p=0.56).
PROCEDURAL RESULTS FOR SELECTIVE INTRACORONARY HYPOTHERMIA
The target temperature was achieved within 43 seconds (IQR 18-113) after the start of infusion. The duration of the occlusion phase was 8.2 minutes (IQR 7.2-9.0). The mean distal intracoronary temperature and distal coronary pressure for all patients are shown in Figure 1. The systemic temperature changed during the occlusion phase by −0.1â°C (IQR −0.5 to 0.0). The total infused volume of saline at room temperature was 162 ml (IQR 140-200) at a rate of 20 ml/min (IQR 20-25). No notable changes were observed in heart rate or aortic pressure.
The duration of the reperfusion phase was 9.1 minutes (IQR 8.2-10.0). The mean intracoronary temperature and distal coronary pressure for all patients during this phase are also shown in Figure 1. The systemic temperature changed by −0.3â°C (IQR −0.7 to 0.1). The total volume of cold saline used in the reperfusion phase was 144 ml (IQR 123-200) at a rate of 20 ml/min (IQR 15-20). Again, no notable changes were observed in heart rate or aortic pressure (Figure 1).
All details of cooling are presented in Table 2. An example of an individual patient’s recording during a complete cooling procedure is presented in Supplementary Figure 3.
PRIMARY ENDPOINT AND CMR RESULTS
Infarct size as a percentage of LVM at 3 months was available in 89 patients randomised to selective intracoronary hypothermia and in 97 patients randomised to the control group. Reasons for not performing CMR at 3 months are mentioned in Supplementary Figure 1.
Infarct size was 23.1±12.5% of LVM in the selective intracoronary hypothermia group and 21.6±12.2% in the control group (p=0.43). Absolute infarct size was 26.1±17.8 g in the selective intracoronary hypothermia group and 24.5±15.7 g in the control group (p=0.52). Left ventricular ejection fraction (LVEF) at 3 months was 49.1±10.2% in the selective intracoronary hypothermia group and 50.1±10.4% in the control group (p=0.53).
The myocardial salvage index was 0.54±0.24 in the selective intracoronary hypothermia group and 0.55±0.25 in the control group (p=0.82). All CMR results are presented in Table 3 and Supplementary Table 4.
The predefined analysis for heterogeneity showed a larger infarct size for proximal versus mid-LAD in both groups, with no difference between the selective intracoronary hypothermia and control groups (Supplementary Table 5). This was the same for TIMI grade 0 versus 1 flow. A forest plot of all predefined subanalyses of the primary endpoint is shown in Supplementary Figure 5.
The prespecified per-treatment analysis and per-protocol analysis did not significantly change the findings of the study (Supplementary Table 6, Supplementary Table 7).
Table 3. Primary endpoint and key secondary endpoints at the index admission and at 3-month follow-up.
| Selective intracoronary hypothermia during primary PCI (N=100) | Standard primary PCI (N=100) | p-value | |
|---|---|---|---|
| Primary endpoint | |||
| Infarct size at 3 months, % of LVM | 23.1±12.5 | 21.6±12.2 | 0.43 |
| Secondary laboratory endpoints during index admission | |||
| Maximum troponin level, ng/l | 5,749 (3,019-9,382) | 5,887 (2,365-8,979) | 0.78 |
| Maximum CK level, U/l | 2,460 (1,380-3,674) | 1,926 (712-3,228) | 0.06 |
| Maximum CK-MB level, U/l | 260 (185-400) | 239 (99-322) | 0.043 |
| Secondary clinical endpoints at 3 months | |||
| Composite of all-cause mortality or hospitalisation for heart failure | 2 | 1 | 0.56 |
| All-cause mortality | 1 | 0 | |
| Hospitalisation for heart failure | 1 | 1 | |
| Secondary laboratory endpoints at 3 months | |||
| NT-proBNP level, pg/ml | 355 (144-667) | 264 (114-527) | 0.25 |
| Secondary CMR endpoints | |||
| Infarct size at 3 months, g | 26.1±17.8 | 24.5±15.7 | 0.52 |
| Ejection fraction, % | 49.1±10.2 | 50.1±10.4 | 0.53 |
| Myocardial salvage index | 0.54±0.24 | 0.55±0.25 | 0.82 |
| MVO, % of LVM | 2.81±4.46 | 2.25±5.34 | 0.44 |
| IMH present | 49 (55) | 39 (42) | 0.08 |
| Secondary clinical endpoints at 1 year | |||
| Composite of all-cause mortality or hospitalisation for heart failure | 3 | 2 | 0.65 |
| All-cause mortality | 2 | 0 | |
| Hospitalisation for heart failure | 1 | 2 | |
| Implantation of ICD | 1 | 1 | |
| Secondary laboratory endpoints at 1 year | |||
| NT-proBNP level, pg/mL | 192 (92-432) | 183 (48-374) | 0.56 |
| Secondary echocardiography endpoint at 1 year | |||
| Ejection fraction, % | 52.1±10.5 | 50.9±9.6 | 0.44 |
| Data are given as mean±SD, median (IQR), n, or n (%). CK: creatine kinase; CK-MB: creatine kinase MB isoenzyme; CMR: cardiovascular magnetic resonance imaging; g: grams; ICD: implantable cardioverter-defibrillator; IMH: intramyocardial haemorrhage; IQR: interquartile range; LVM: left ventricular mass; MVO: microvascular obstruction; NT-proBNP: N-terminal pro-brain natriuretic peptide; PCI: percutaneous coronary intervention; SD: standard deviation | |||
SECONDARY ENDPOINTS AND CLINICAL FOLLOW-UP
All-cause mortality or hospitalisation for heart failure at 1 year occurred in 3 patients in the selective intracoronary hypothermia group and in 2 patients in the control group (p=0.65). One patient died in the selective intracoronary hypothermia group 6 weeks after the index admission because of intracranial haemorrhage and 1 patient died after 11 months because of respiratory failure due to COVID-19. No patients in the control group died.
Subacute stent thrombosis occurred in 1 patient in the selective intracoronary hypothermia group after 6 days; there were no cases of this in the control group.
During the index admission, no differences were present in the maximum creatine kinase (CK) level or maximum troponin level (Table 3). The maximum CK-MB isoenzyme (CK-MB) was 260 U/l (IQR 185-400) in the selective intracoronary hypothermia group and 239 U/l (IQR 99-322) in the control group (p=0.043). N-terminal pro-brain natriuretic peptide (NT-proBNP) at 3 months was 355 (IQR 144-667) pg/ml in the selective intracoronary hypothermia group and 264 (IQR 114-527) pg/ml in the control group (p=0.25). At 1 year, both NT-proBNP and LVEF (determined by echocardiography) were similar in both groups.
Discussion
The EURO-ICE trial demonstrated that selective intracoronary hypothermia during primary PCI in patients with large anterior wall STEMI was feasible and safe but did not reduce infarct size.
EURO-ICE is the largest hypothermia trial in patients with STEMI. As opposed to previous trials that investigated systemic cooling methods, we studied a technique of selective intracoronary cooling. This was performed using standard PCI equipment and consisted of 7-10 minutes of cooling of the threatened myocardium before the occluded coronary artery was opened, followed by an additional 10 minutes of cooling after reperfusion had been established.
The prevention of reperfusion injury in order to decrease myocardial infarct size remains a challenge. Previous attempts hitherto in humans using systemic hypothermia have been disappointing202122232425.
Although our technique of selective intracoronary hypothermia has several advantages compared to the systemic cooling methods used in previous trials, it remains unclear why selective intracoronary hypothermia was not effective to reduce infarct size in EURO-ICE. While the protocol was based on successful experimental studies, it cannot be excluded that the time intervals of cooling, depth of cooling, or other methodological issues related to humans affected the results. Moreover, it should be noted that the clinical outcomes, in patients randomised either to selective intracoronary hypothermia or to standard primary PCI, were better than expected. Infarct size, mortality and hospitalisation for heart failure were all low compared to historical data for patients with large anterior wall STEMI26.
Of particular interest is the relation between selective intracoronary hypothermia and rhythm disturbances. Using systemic hypothermia, atrial fibrillation occurs in approximately 40% of patients24, whereas atrial fibrillation did not occur in any of the patients who underwent cooling in our study. This may also have contributed to the observed difference in the incidence of cardiogenic shock between the EURO-ICE trial (incidence of cardiogenic shock in the cooling arm: 1%) and previously published systemic cooling trials (incidence of cardiogenic shock in the cooling arm: 10.3%)25.
Importantly, there was no difference in the incidence of ventricular fibrillation between the selective intracoronary hypothermia group and the control group (Supplementary Figure 4). The door-to-balloon time was longer in the selective intracoronary hypothermia group compared to the control group, but this did not result in a larger infarct size.
In the present study, no shivering was observed in the cooled patients even though no antishivering medications were used. In previous published (systemic) hypothermia trials, however, 20% of cooled patients had uncontrolled shivering despite pretreatment with antishivering medication (oral buspirone and intravenous pethidine)25.
Limitations
Our study has several limitations. First, the population was highly selective, and only patients with large anterior wall STEMI and a proximal or mid-LAD stenosis were included. However, there is no reason to suspect that the outcome would be any different in inferior wall STEMI. Second, our study was limited to patients with TIMI grade 0 or 1 flow. Patients with TIMI grade 2 or 3 flow were not included, because those patients had spontaneous reperfusion, and we believed that any advantage of cooling would not be pronounced in such cases. Third, although the cooling intervals were based on animal studies, it cannot be excluded that prolongation of the reperfusion phase may have been necessary and that, in fact, “delayed” reperfusion injury may have occurred in our study. Fourth, in 6 patients randomised to selective intracoronary hypothermia, the cooling protocol was not started at all. The main reason for this was an inability to place the PW into the distal LAD. This may have influenced the overall results. Fifth, LGE assessment for the primary endpoint could have been influenced by other causes for late enhancement, such as previous inferior infarction or myocarditis.
A major advantage of the technique used in this study is the possibility to have selective access to the infarct area before reperfusion occurs. A number of pharmacological therapies (such as cyclosporine, gap junction inhibitors and adenosine) have been investigated to prevent reperfusion injury during STEMI2627. In those studies, positive results from animal experiments could not be reproduced in humans. An important limitation in all of these studies was the fact that in humans it was difficult to deliver the respective drug to the myocardium at risk before reperfusion occurred, making it doubtful that the drug would have any timely effect. The technique used in the present study allows for the administration of drugs into the distal myocardium before reperfusion occurs.
Conclusions
The EURO-ICE trial demonstrates that selective intracoronary hypothermia during primary PCI – started shortly before and continued for 10 more minutes after reperfusion – in patients with large anterior wall ST-elevation myocardial infarction is feasible and safe but does not decrease infarct size compared to primary PCI alone.
Impact on daily practice
Myocardial reperfusion injury is a logical target to further decrease infarct size and improve clinical outcomes in patients with ST-elevation myocardial infarction. There is general agreement that reperfusion injury occurs during the first minutes of reperfusion, and as such, new therapies should be active in the myocardium at risk before reperfusion occurs. Selective intracoronary infusion, the technique used in the present study, allows for administration of drugs into the distal myocardium before reperfusion. New (pharmacological) therapies targeting reperfusion injury should consider using this technique to ensure adequate release in the myocardium at risk before reperfusion.
Acknowledgements
The authors wish to acknowledge the contributions of the research institutions, study investigators, research staff, and study participants.
Funding
The EURO-ICE trial was an investigator-initiated trial. The trial was financed by a research grant from Abbott. Their support remained limited to funding only, with no role in study design, data collection or analysis, or final manuscript publication.
Conflict of interest statement
N.H.J. Pijls reports personal fees from Abbott and OpSens; holding equity in Philips, ASML, HeartFlow, and GE HealthCare; and institutional research grants from Abbott and Hexacath. T. Engström reports personal fees from Abbott and Bayer, outside the submitted work. T.R. Keeble reports grants from Abbott, ZOLL Medical, and AstraZeneca, outside the submitted work. B. De Bruyne receives grant support from Abbott, Boston Scientific, Biotronik AG, and St. Jude Medical; and receives consulting fees from St. Jude Medical, OpSens, and Boston Scientific, outside of the submitted work; he is a shareholder for Siemens, GE HealthCare, Bayer, Philips, HeartFlow, Edwards Lifesciences, and Celyad. G. Karamasis reports personal fees from Abbott, outside the submitted work. K. Oldroyd reports employee fees from Biosensors International, outside the submitted work. C. Berry reports grants from Abbott, AstraZeneca, Boehringer Ingelheim, Coroventis, GSK, HeartFlow, Medyria, Neovasc, Novartis, Siemens Healthcare, and Menarini, outside the submitted work; and is supported by research funding from the British Heart Foundation (RE/18/6134217). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

