Abstract
BACKGROUND: Long-term follow-up is essential to evaluate the impact of polymer degradation in drug-eluting stents (DES).
AIMS: We aimed to compare durable-polymer DES (DP-DES) and biodegradable-polymer DES (BP-DES) during a 3-year follow-up to evaluate the entire period of polymer resolution (before, during, and after degradation).
METHODS: The HOST REDUCE POLYTECH RCT Trial was a randomised clinical trial enrolling patients with acute coronary syndrome (ACS) and comparing the efficacy and safety of DP-DES and BP-DES. The primary outcome was a patient-oriented composite outcome (POCO), and the key secondary outcome was a device-oriented composite outcome (DOCO).
RESULTS: A total of 3,413 ACS patients were randomised to either the DP-DES (1,713 patients) or BP-DES (1,700 patients) group. During the 3-year follow-up, the risk of the POCO was similar between the DP-DES and BP-DES groups (14.8% vs 15.4%, hazard ratio [HR] 0.96, 95% confidence interval [CI]: 0.80-1.14; p=0.613). However, the risk of the DOCO was lower in the DP-DES group (6.0% vs 8.0%, HR 0.73, 95% CI: 0.57-0.95; p=0.020). In a landmark analysis, the lower risk of the DOCO for the DP-DES group was evident during the transition from the early to the late period after percutaneous coronary intervention (PCI) (from 8 to 16 months post-PCI; 1.8% vs 3.3%, HR 0.54, 95% CI: 0.34-0.84; p=0.007), which was mainly driven by a risk reduction of target lesion revascularisation.
CONCLUSIONS: In ACS patients, DP-DES showed similar results to BP-DES regarding the POCO up to 3 years. For the DOCO, DP-DES were superior to BP-DES; this was due to the higher event rate during the period of polymer degradation.
Polymers in early-generation drug-eluting stents (DES) had issues of delayed healing, local hypersensitivity, inflammation, and neoatherosclerosis1. Therefore, recent development has focused on more biocompatible durable polymers (DP) and biodegradable polymers (BP). BP-DES seem to be theoretically better than DP-DES. However, this has not been confirmed in large-scale randomised trials, which have mostly reported comparable clinical outcomes between DP-DES and BP-DES23456789. Most of these studies were performed in all-comers, including a high proportion of stable angina patients; comparison of polymer technologies in dedicated acute coronary syndrome (ACS) patients is scarce. The HOST REDUCE POLYTECH RCT Trial (Harmonizing Optimal Strategy for Treatment of Coronary Artery Diseases Trial - Comparison of REDUCTION of PrasugrEl Dose & POLYmer TECHnology in ACS Patients) including a DES arm (HOST REDUCE ACS) and an antiplatelet arm (HOST REDUCE ACS) demonstrated the non-inferiority of DP-DES to BP-DES with regard to patient-oriented composite outcomes (POCO), but DP-DES showed a lower risk of device-oriented composite outcomes (DOCO) compared to BP-DES at 1 year10. However, regarding the mechanism of stent stabilisation, a 1-year follow-up period is too short to fully evaluate the impact of polymer degradation on the vascular tissue response and subsequent clinical events.
The performance of DES is determined by three essential components: the antiproliferative drug, the metallic stent strut and the polymer technology. Among these components, short-term results within the first year after percutaneous coronary intervention (PCI) may be majorly influenced by the drug and stent strut. Considering that the duration of polymer degradation in BP-DES varies from 4 to 15 months after PCI depending on the polymer11, long-term follow-up is required to evaluate the effect of different polymer technologies between DP-DES and BP-DES. Therefore, we initially planned the 3-year follow-up of the HOST-POLYTECH-ACS arm to compare two different polymer technologies in ACS patients.
Methods
STUDY DESIGN
This analysis evaluates long-term follow-up data of the DES arm of the HOST REDUCE POLYTECH RCT Trial (ClinicalTrials.gov: NCT02193971). The trial design and primary endpoint rate at 1 year have been published previously101213. Briefly, the trial was an investigator-initiated, randomised, parallel-group, open-label, adjudicator-blinded, multicentre trial performed at 35 centres in the Republic of Korea. The trial had a 2 by 2 factorial design evaluating two independent hypotheses and had two arms: the antiplatelet arm and DES arm. The antiplatelet arm, HOST REDUCE ACS, investigated the non-inferiority of prasugrel dose de-escalation therapy 1 month after index PCI compared to maintaining a conventional dose of prasugrel for 1 year in ACS patients13. The DES arm, HOST REDUCE ACS, explored the non-inferiority of DP-DES to BP-DES regarding patient-oriented outcomes in ACS patients at 1 year10. However, the performance of DES within the first year after PCI may be more influenced by the drug and stent strut rather than by the polymer technology. Therefore, to evaluate the clinical impact of two different polymer technologies, we planned a prespecified 3-year follow-up to compare the efficacy and safety of DP-DES and BP-DES.
STUDY POPULATION AND RANDOMISATION
Patients 19 years old or older diagnosed with ACS and having at least one culprit de novo coronary lesion eligible for stent implantation were considered for inclusion in this trial. The trial protocol, including the full inclusion/exclusion criteria and sample size calculation, is available in Supplementary Appendix 1. The study followed the provisions of the Declaration of Helsinki and was approved by the institutional ethics committee of each participating site. All patients provided written informed consent at enrolment. After diagnostic coronary angiography, eligible patients were randomly assigned to receive DP-DES (Promus Premier [Boston Scientific], XIENCE Alpine [Abbott], Resolute Onyx [Medtronic], DESyne or DESyneX2 [both Elixir Medical]) or BP-DES (BioMatrix, BioMatrix Flex [both Biosensors], Nobori, Ultimaster [both Terumo], SYNERGY [Boston Scientific], or Orsiro [BIOTRONIK]) in a 1:1 ratio (Figure 1). Randomisation was performed by a web-based application (Interactive Web Response System; software configuration: Apache 2, PHP 5, and MySQL 5, developed by the Medical Research Collaborating Center, Seoul National University Hospital), with information inputted by an independent clinical nurse or research nurse coordinator who was not involved with the rest of the trial.
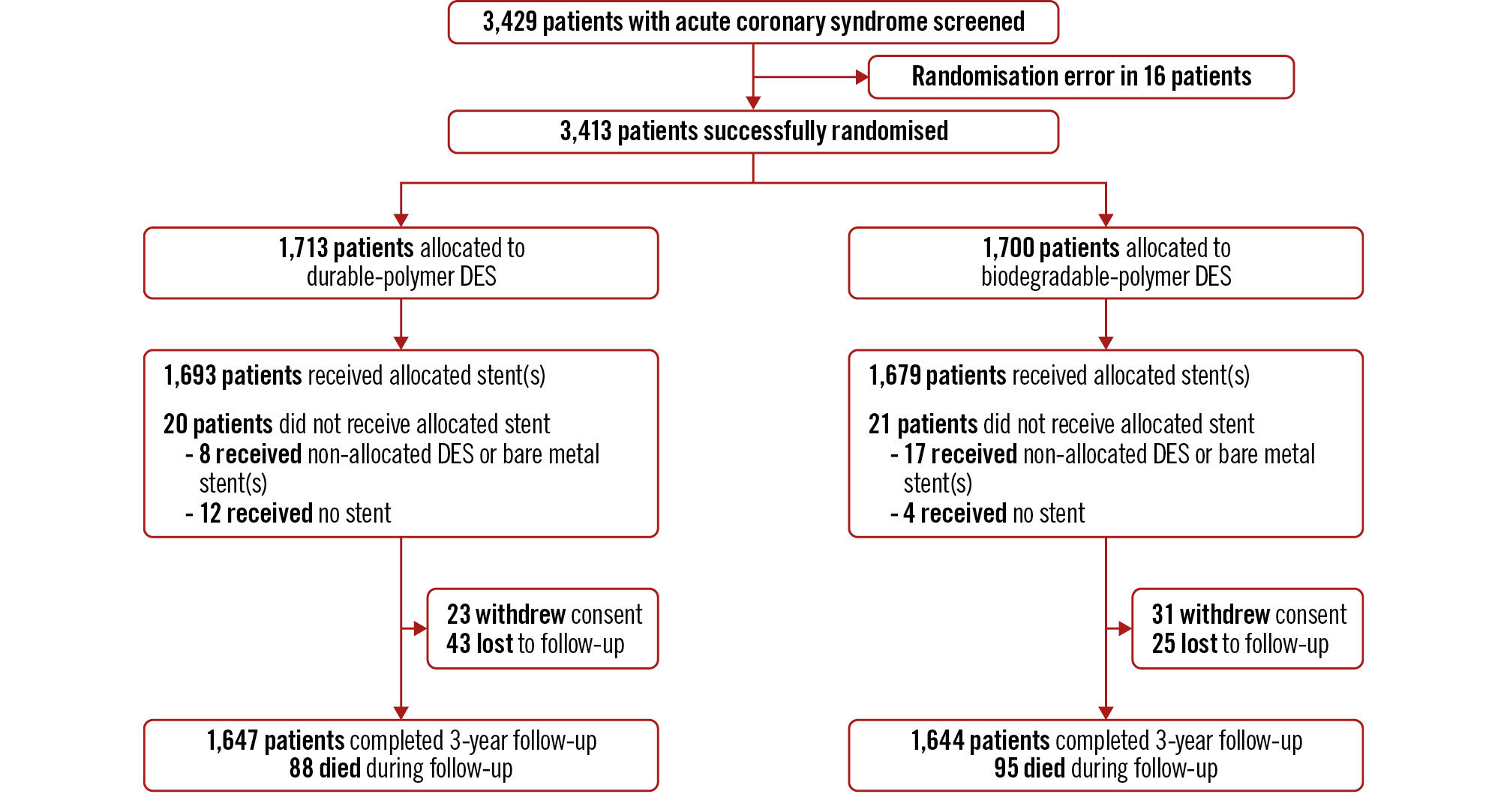
Figure 1. Study flowchart Patients who were diagnosed with ACS and who had at least 1 culprit coronary lesion in a native coronary artery with significant stenosis eligible for stent implantation were randomly assigned to the DP-DES group or the BP-DES group. Clinical follow-up was conducted up to 3 years after index PCI. ACS: acute coronary syndrome; BP: biodegradable-polymer; DES: drug-eluting stent; DP: durable-polymer; PCI: percutaneous coronary intervention
DEFINITIONS OF CLINICAL OUTCOMES
The primary outcome was the POCO at 3 years, a composite of all-cause death, non-fatal myocardial infarction (MI), and any repeat revascularisation. The key secondary outcome was the DOCO at 3 years, including cardiac death, target vessel MI, and target lesion revascularisation (TLR). The individual outcomes of the composite outcomes, stent thrombosis, target vessel revascularisation (TVR), non-TLR TVR, and non-TVR were also evaluated. All clinical outcomes were adjudicated by independent event adjudication committee members, who were blinded to the randomisation groups, throughout the 3-year follow-up period. The members of the independent event adjudication committee received medical records regarding adverse events without information on the randomisation groups.
STATISTICAL ANALYSIS
The sample size calculation was based on the working hypothesis of the original HOST REDUCE POLYTECH RCT Trial that DP-DES were non-inferior to BP-DES regarding the POCO at 1 year in patients with ACS10. A total of 3,384 patients were required to test the hypothesis of the original trial, and analyses were performed according to the intention-to-treat manner for this prespecified 3-year follow-up. The per-protocol analyses were also done as sensitivity analyses. To reflect the various stages of polymer degradation in BP-DES, we performed post hoc landmark analyses at 240 days and 480 days post-PCI11. The timepoints of the landmark analyses were determined by two factors: the time point of polymer degradation (120 to 360 days after PCI) and the temporal incidence of in-stent restenosis after PCI (the peak of restenosis of bare metal stents − into which the BP-DES transform − is 120 to 240 days after PCI). Adding these numbers together, we defined the “transition period” as the whole time period vulnerable to restenosis (when tissue responds to the microenvironment change induced by polymer degradation and bare metal exposure), which is 240 days to 480 days post-PCI. The time period up to 240 days after PCI (when the polymer degradation starts and is ongoing) was defined as the “early period” and that beyond 480 days post-PCI (when tissue stabilises) as the “late period”. Sensitivity analysis was performed on the event rate between stents within the BP-DES group: those exposing bare metal after polymer resolution (BioMatrix, BioMatrix Flex, Nobori, Ultimaster, SYNERGY) versus Orsiro, which does not expose bare metal even after polymer resolution due to the passive coating seal.
Categorical variables are reported as counts and percentages, and continuous variables are presented as means with standard deviations. Categorical variables were compared by the chi-squared test, while continuous variables were compared by the Student’s t-test. The cumulative incidence of each clinical outcome was presented with Kaplan-Meier estimates, and the group differences were compared by the log-rank test. The Cox proportional hazards assumptions were verified, and results were presented as a hazard ratio (HR) with its 95% confidence interval (CI). The consistency of the polymer effects among subgroups was assessed by a formal interaction test using the Cox proportional hazards regression model. All p-values were 2-sided, and p<0.05 was considered statistically significant. The statistical package R, version 4.0.3 (R Foundation for Statistical Computing) was used for statistical analysis.
Results
PATIENTS AND FOLLOW-UP
From September 2014 to December 2018, a total of 3,418 patients with ACS who had at least one culprit de novo coronary lesion requiring stent implantation were randomised to the DP-DES (1,713 patients) or the BP-DES (1,700 patients) group (Figure 1). Among them, 54 patients withdrew informed consent, and 68 patients were lost to follow-up at 3-year follow-up, leaving data available for 96.4% of the total population. The mean age was 63.1±11.1 years, and 45.0% of patients had diabetes mellitus (Table 1). The clinical diagnosis was unstable angina in 61.7% of patients, non-ST-segment elevation MI in 25.2%, and ST-segment elevation MI in 13.1%. Angiographic data showed that 54.4% of patients had multivessel disease, 30.5% underwent multilesion intervention, and the mean number of used stents was 1.7±1.0 (Table 1). More than 30% of patients underwent imaging-guided PCI. Baseline patient, lesion and procedural characteristics were well balanced between the two groups. Discharge medications and medications during follow-up are shown in Supplementary Table 1. There was no difference in the medications, including the antiplatelet pattern between the two groups.
Table 1. Baseline characteristics and lesion characteristics.
| DP-DES (N=1,713) | BP-DES (N=1,700) | p-value | |
|---|---|---|---|
| Demographics | |||
| Age, years | 63.0±11.1 | 63.1±11.1 | 0.717 |
| Male | 1,351 (78.9) | 1,337 (78.6) | 0.908 |
| Body mass index, kg/m2 | 24.9±3.1 | 25.0±3.2 | 0.647 |
| Hypertension | 1,092 (63.7) | 1,147 (67.5) | 0.023 |
| Diabetes | 789 (46.1) | 747 (43.9) | 0.227 |
| Dyslipidaemia | 1,280 (74.7) | 1,247 (73.4) | 0.382 |
| Chronic kidney disease | 79 (4.6) | 65 (3.8) | 0.289 |
| Current smoker | 515 (30.1) | 475 (27.9) | 0.331 |
| Prior myocardial infarction | 67 (3.9) | 70 (4.1) | 0.826 |
| Prior revascularisation | 220 (12.8) | 220 (12.9) | 0.973 |
| Prior stroke | 92 (5.4) | 110 (6.5) | 0.197 |
| Clinical presentation at index PCI | |||
| STEMI | 233 (13.6) | 214 (12.6) | 0.207 |
| NSTEMI | 448 (26.2) | 412 (24.2) | |
| Unstable angina | 1,031 (60.2) | 1,074 (63.2) | |
| Lesion characteristics | |||
| Number of diseased vessels | |||
| One vessel | 778/1,703 (45.7) | 769/1,689 (45.5) | 0.274 |
| Two vessels | 549/1,703 (32.2) | 512/1,689 (30.3) | |
| Three vessels | 376/1,703 (22.1) | 408/1,689 (24.2) | |
| Culprit lesion location | |||
| Left main coronary artery | 62/1,679 (3.7) | 58/1,669 (3.5) | 0.943 |
| Left anterior descending artery | 837/1,679 (49.9) | 845/1,669 (50.6) | |
| Left circumflex artery | 307/1,679 (18.3) | 308/1,669 (18.5) | |
| Right coronary artery | 473/1,679 (28.2) | 458/1,669 (27.4) | |
| Procedural complexity | |||
| Multilesion intervention | 512/1,687 (30.3) | 512/1,674 (30.6) | 0.912 |
| Heavy calcification | 317/2,353 (13.5) | 344/2,329 (14.8) | 0.217 |
| Bifurcation lesion | 422/2,351 (17.9) | 438/2,326 (18.8) | 0.460 |
| Thrombotic lesion | 204/2,353 (8.7) | 208/2,328 (8.9) | 0.789 |
| Type B2/C lesion | 1,165/2,351 (49.6) | 1,215/2,328 (52.2) | 0.076 |
| ISR lesion | 56/2,353 (2.4) | 42/2,328 (1.8) | 0.203 |
| IVUS use | 706/2,360 (29.9) | 752/2,339 (32.2) | 0.104 |
| Number of treated lesions per person | 1 [1, 2] | 1 [1, 2] | 0.958 |
| Number of stents per person | 1 [1, 2] | 1 [1, 2] | 0.475 |
| Data are presented as mean±standard devation, n (%), n/N (%) or median [interquartile range]. BP: biodegradable-polymer; DES: drug-eluting stent; DP: durable-polymer; ISR: in-stent restenosis; IVUS: intravascular ultrasound; NSTEMI: non-ST-segment elevation myocardial infarction; PCI: percutaneous coronary intervention; STEMI: ST-segment elevation myocardial infarction | |||
CLINICAL OUTCOMES
During 3 years of follow-up, the POCO occurred in 246 patients (Kaplan-Meier estimate: 14.8%) in the DP-DES group and in 254 patients (Kaplan-Meier estimate: 15.4%) in the BP-DES group (HR 0.96, 95% CI: 0.80-1.14; p=0.613) (Central illustration, Table 2). The DOCO occurred in 98 patients (Kaplan-Meier estimate: 6.0%) in the DP-DES group and 131 patients (Kaplan-Meier estimate: 8.0%) in the BP-DES group (Central illustration). The risk of the DOCO was lower in the DP-DES group than in the BP-DES group (HR 0.73, 95% CI: 0.57-0.95; p=0.021), which was mostly driven by a lower risk of TLR (HR 0.61, 95% CI: 0.42-0.88; p=0.008). Other secondary endpoints are shown in Table 2 and Supplementary Figure 1. The incidence of both non-TLR and non-TVR were similar between the two groups.
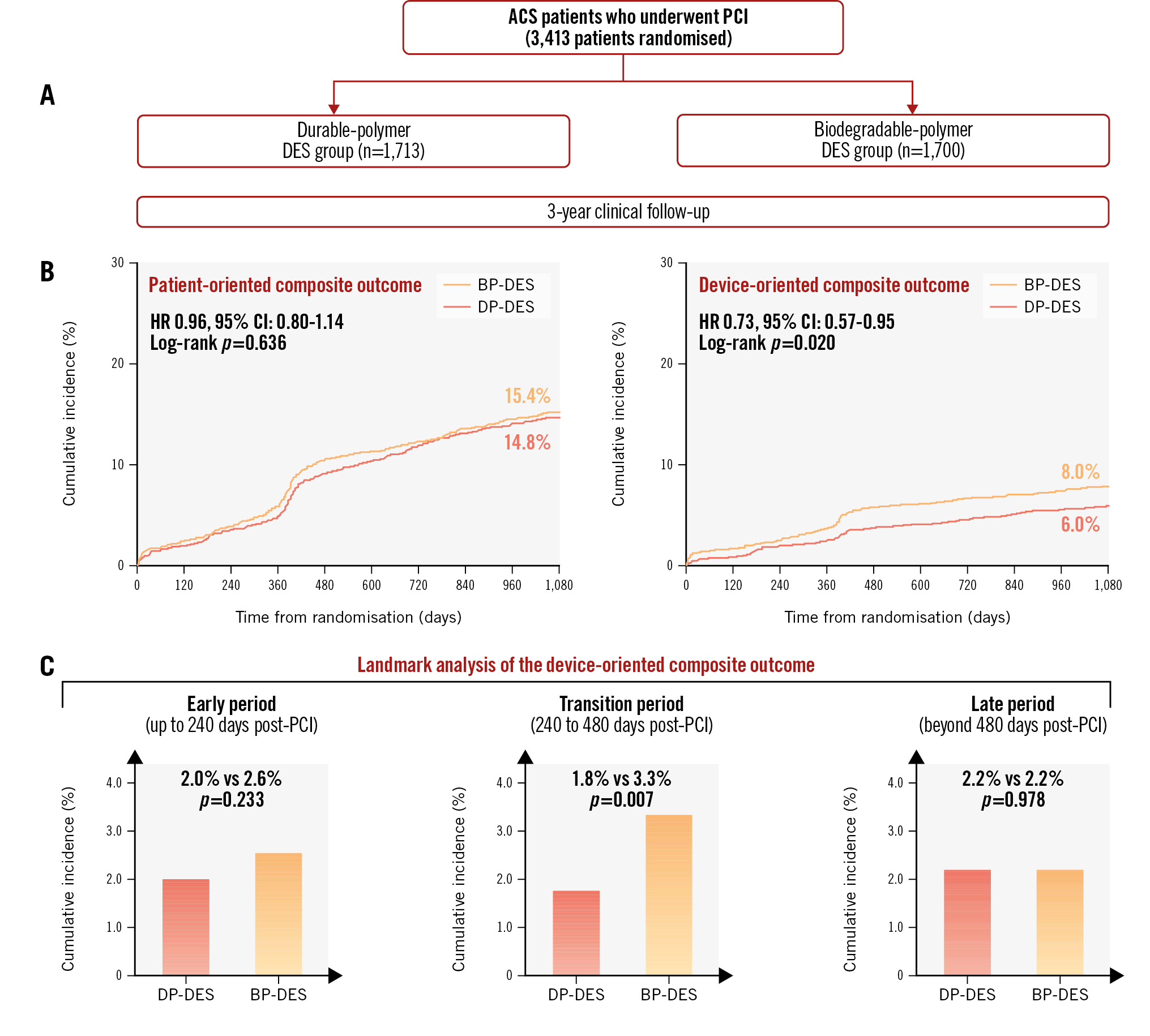
Central illustration. Three-year follow-up of polymer technology in ACS. A) A total of 313 patients, who were diagnosed with ACS and who had at least 1 culprit coronary lesion eligible for stent implantation, were randomly assigned to the DP-DES group or the BP-DES group. B) POCO and DOCO were evaluated during the 3-year clinical follow-up period. Risk of the POCO was similar between the two groups, while risk of the DOCO was higher in the BP-DES group. C) Landmark analyses of the DOCO at 240 days and 480 days post-PCI showed that this difference was mostly derived from events during the transition period. ACS: acute coronary syndrome; BP-DES: biodegradable-polymer drug-eluting stent; CI: confidence interval; DOCO: device-oriented composite outcome; DP-DES: durable-polymer drug-eluting stent; HR: hazard ratio; PCI: percutaneous coronary intervention; POCO: patient-oriented composite outcome
Table 2. Clinical outcomes at 3 years in the intention-to-treat population.
| DP-DES (N=1,713) | BP-DES (N=1,700) | Hazard ratio (95% CI) | p-value | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Patient-oriented composite outcome* | 246 (14.8) | 254 (15.4) | 0.96 (0.80-1.14) | 0.613 |
| Key secondary endpoint | ||||
| Device-oriented composite outcome† | 98 (6.0) | 131 (8.0) | 0.73 (0.57-0.95) | 0.021 |
| Other secondary endpoints | ||||
| All-cause death | 88 (5.3) | 95 (5.7) | 0.92 (0.69-1.23) | 0.562 |
| Cardiac death | 49 (3.0) | 59 (3.6) | 0.82 (0.56-1.20) | 0.312 |
| Any MI | 25 (1.6) | 24 (1.5) | 1.03 (0.59-1.81) | 0.911 |
| Target vessel myocardial infarction | 12 (0.7) | 13 (0.8) | 0.91 (0.42-2.00) | 0.821 |
| Stent thrombosis | ||||
| Definite/probable | 9 (0.6) | 8 (0.5) | 1.11 (0.43-2.89) | 0.824 |
| Definite/probable/possible | 9 (0.6) | 9 (0.6) | 0.99 (0.39-2.49) | 0.983 |
| Acute (<24h) | 1 (0.1) | 2 (0.1) | ||
| Subacute (1 day-1 month) | 1 (0.1) | 2 (0.1) | ||
| Late (1 month-1 year) | 7 (0.4) | 5 (0.3) | ||
| Any repeat revascularisation | 162 (10.0) | 168 (10.5) | 0.95 (0.77-1.18) | 0.644 |
| Target vessel revascularisation | 71 (4.4) | 106 (6.6) | 0.66 (0.49-0.89) | 0.006 |
| Target lesion revascularisation | 46 (2.9) | 74 (4.6) | 0.61 (0.42-0.88) | 0.008 |
| Non-target lesion revascularisation | 25 (1.6) | 32 (2.0) | 0.77 (0.46-1.31) | 0.337 |
| Non-target vessel revascularisation | 102 (6.3) | 82 (5.1) | 1.24 (0.93-1.66) | 0.150 |
| Data are presented as n (%). *Patient-oriented composite outcome: a composite of all-cause death, non-fatal myocardial infarction, and any repeat revascularisation. †Device-oriented composite outcome: a composite of cardiac death, target vessel myocardial infarction, and target lesion revascularisation. BP: biodegradable-polymer; CI: confidence interval; DES: drug-eluting stent; DP: durable-polymer; MI: myocardial infarction | ||||
PER-PROTOCOL ANALYSES AND SUBGROUP ANALYSES
The per-protocol analyses demonstrated similar results to the intention-to-treat analyses for the primary outcome (Kaplan-Meier estimates: 14.7% vs 15.2% in the DP-DES and BP-DES groups, HR 0.96, 95% CI: 0.81-1.15; p=0.690) and for the key secondary outcome (Kaplan-Meier estimates: 6.0% vs 7.9% in the DP-DES and BP-DES groups, HR 0.75, 95% CI: 0.57-0.97; p=0.030) (Supplementary Figure 2, Supplementary Table 2). Also, no significant interaction was observed between the antiplatelet arm and DES arm, with regard to the primary and key secondary outcomes (Supplementary Figure 3). In subgroup analyses, the impact of polymer types on the primary and key secondary outcomes was consistent across various subgroups without significant interactions (Supplementary Table 3, Supplementary Table 4). In a sensitivity analysis excluding patients receiving stents with thick stent struts (stent strut thickness >100 μm), such as the BioMatrix, BioMatrix Flex and Nobori DES, the clinical outcomes were consistent across the total study population as shown in Supplementary Figure 4.
LANDMARK ANALYSIS
Landmark analyses were performed at 240 and 480 days after index PCI (Central illustration). Regarding the POCO, there was no difference in outcomes between the DP-DES versus BP-DES groups during the early period (Kaplan-Meier estimate: 3.7% vs 4.0%, HR 0.91, 95% CI: 0.65-1.29; p=0.612), transition period (Kaplan-Meier estimate: 5.8% vs 6.9%, HR 0.82, 95% CI: 0.62-1.09; p=0.167), or late period post-PCI (Kaplan-Meier estimate: 6.1% vs 5.2%, HR 1.18, 95% CI: 0.87-1.60; p=0.278). Regarding the DOCO, there were no differences in outcomes between stent groups during the early period (Kaplan-Meier estimate: 2.0% vs 2.6%, HR 0.76, 95% CI: 0.49-1.19; p=0.233) or late period post-PCI (Kaplan-Meier estimate: 2.2% vs 2.2%, HR 1.01, 95% CI: 0.63-1.61; p=0.978). However, during the transition period, the risk of the DOCO was significantly lower in the DP-DES group (Kaplan-Meier estimate: 1.8% vs 3.3%, HR 0.54, 95% CI: 0.34-0.84; p=0.007). We also performed a landmark analysis 1 year post-PCI (Supplementary Figure 5). Both stent arms showed similar performance regarding the POCO and DOCO, before and after 1 year post-PCI.
Discussion
In this 3-year follow-up of the HOST POLYTECH ACS arm of the HOST REDUCE POLYTECH RCT Trial, we were able to observe differences between the clinical outcomes of patients who received DP-DES and BP-DES. The current study is the largest single study with a dedicated ACS population and meets the requirement to assess different polymer technologies, i.e., the long follow-up duration covering before, during, and after polymer degradation. In terms of patient-oriented outcomes, both DP-DES and BP-DES demonstrated excellent outcomes and showed similar performance. However, the rate of device-oriented outcomes was significantly lower in the DP-DES group than the BP-DES group. In a post hoc landmark analysis, we found that this was mainly driven by the reduction of adverse events between 8 and 16 months post-PCI, the transition period when polymer degradation and subsequent bare metal exposure of BP-DES probably induce inflammation in the micromilieu and then aggravate neointimal growth or thrombosis in the stented segment (Central illustration). These findings were consistent in a post hoc analysis which excluded thick-strut stents from the BP-DES group. The present analysis extends our understanding on the dynamic change in performance between BP-DES and DP-DES depending on the time period after implantation.
COMPARISON OF THE POLYMERS ON DP-DES AND BP-DES
The first-generation DES were effective in reducing restenosis compared to bare metal stents. However, their polymers were deemed to play an important role in chronic inflammatory reactions, leading to late events14. Naturally, the objective of further DES development was to make polymers biocompatible with less inflammation or to have polymers degrade within a few months, after they have served their role of drug elution. Numerous clinical trials have tested whether BP-DES have clear benefits compared with DP-DES, but the majority have failed, since outcomes were mostly comparable between the two types of DES23456789. A recent large meta-analysis comparing BP-DES to second-generation DP-DES found no significant differences in the rates of TVR, stent thrombosis, MI, or cardiac death between the two platforms, suggesting similar safety and efficacy15. However, the follow-up duration of most of the studies included in the meta-analysis was 12 months, and therefore, the impact of polymer degradation on clinical outcomes could not be fully evaluated. Moreover, most of the available literature on comparison of polymer technologies reported clinical outcomes based on all-comers. When we consider the pathophysiology of ACS, which mainly occurs by plaque rupture or erosion followed by platelet-rich thrombosis and inflammation16, the clinical performance may be more profoundly affected by differences in the polymer technology in ACS patients.
TIME-SPECIFIC LANDMARK ANALYSIS
In our study, we performed a post hoc time-specific landmark analysis, revealing that DP-DES had a lower risk of the DOCO compared to BP-DES in patients with ACS. This was mainly due to the difference in DOCO rates between 240 to 480 days after implantation (transition period). Among a total of 131 DOCO events in the BP-DES group, about 40% (53 events) occurred during the transition period. The difference between the two groups disappeared beyond 480 days post-PCI (late period). The lower DOCO rate in the DP-DES group, compared to in the BP-DES group, during the transition period can be explained by certain mechanisms. First, the inflammatory process induced by polymer degradation may lead to endothelial dysfunction, delayed endothelialisation, neointimal hyperplasia and late thrombosis17. This process may be exaggerated and have a larger impact on clinical outcomes in patients with ACS. Considering the different polymer degradation time of each BP-DES (3-4 months for Ultimaster and SYNERGY, 9-12 months for BioMatrix/Nobori, and 12-15 months for Orsiro), the transition period is the time frame when polymers degrade and then probably induce an acidic and inflammatory local milieu with bare metal exposure at the stented segment, leading to neointimal tissue growth and thrombosis. This finding of a lower risk of the DOCO with DP-DES may have been magnified by the bump in the event curves that was observed around 1 year post-PCI. This may reflect the tendency to perform angiographic surveillance at this time. However, this may have a minor influence on the study results, because these events were clinically driven revascularisation events, mostly at the target lesion site, which were adjudicated by independent event adjudication committee members, who were blinded to the randomisation groups.
Second, the durable polymers used in new-generation DES have superior biocompatibility compared to bare metal stents. The polymers of newer-generation DES have been shown to reduce thrombogenicity and vascular injury, accelerate endothelialisation, and potentially improve clinical outcomes18. Moreover, the extent of fibrin and inflammatory response is significantly less in the second-generation DES, inducing greater neointimal coverage and re-endothelialisation over struts1920. A superior albumin retention rate and thromboresistance of durable polymers may also lead to lower late restenosis in DP-DES21.
Our finding of higher DOCO rates in BP-DES patients during the transition period after PCI is reflected in higher DOCO rates in BP-DES patients after 1 year post-PCI in other studies. The BIO-RESORT study showed a late catch-up of TLR rates for the SYNERGY BP-DES compared to the Onyx DP-DES5. In the SORT-OUT VI trial, 1-year results reported that the TLR rate was lower in the BP-DES (BioMatrix Flex) arm; however, the 3-year results showed the TLR rate was lower in the DP-DES (Resolute Integrity [Medtronic]) arm owing to events that occurred in the BP-DES arm after the first year of follow-up4. In the Japanese NEXT Trial, the safety and efficacy of BP-DES (Nobori) were non-inferior to those of DP-DES (XIENCE [Abbott]/Promus [Boston Scientific]) at 3 years6. But, similarly, the Japanese CENTURY II Trial showed that there was a late catch-up of TLR rates in the BP-DES (Ultimaster) group compared to the DP-DES (XIENCE) group at 5-year follow-up22.
Limitations
The results of the current study should be interpreted in view of several limitations. First, the randomisation of the current arm of the study was only for polymer technology, which resulted in the inclusion of various stents in each group. Clinical outcomes may be influenced by not only the polymer technology but also from numerous stent-related factors, such as stent design, strut thickness, release kinetics and the type of antiproliferative drug applied. These stent-related factors may influence the clinical events in the early period after PCI, while differences in polymers may influence the events in the transition period, when polymers are degraded and microenvironmental changes, inflammation and then vascular tissue response can occur. In our current analysis, the increase in TLR rates in BP-DES patients, compared to DP-DES patients, was significant during the transition period but not during the early phase or late phase post-PCI, suggesting that this difference in TLR rates may be derived from differences in the polymers. Previous studies have failed to evaluate the potential impact of polymer degradation on clinical events depending on the period of polymer degradation after stent implantation. Moreover, we performed a sensitivity analysis by excluding BP-DES with thick stent struts, which revealed consistent results. The strut thickness of the different BP-DES and DP-DES platforms in our study were as follows: for the BP-DES: Orsiro 60 μm, SYNERGY 74 μm, Ultimaster 80 μm, BioMatrix 120 μm, BioMatrix Flex 120 μm, and Nobori 120 μm; and for the DP-DES: Onyx 81 μm, XIENCE Alpine 81 μm and Promus Premier 81 μm. Since the reduction of strut thickness has been associated with a reduced risk of restenosis23, we performed another sensitivity analysis after excluding stents with a strut thickness of 100 μm or more. Nevertheless, the superiority of DP-DES was still persistent, showing that the higher TLR rate of BP-DES patients during the transition period after PCI was not attributed to the strut thickness or stent design. However, there is still a possibility that the results might have been driven by a particular DP-DES or BP-DES rather than by the polymer or strut thickness.
Second, the timepoints of the landmark analyses at 240 and 480 days were not included in the prespecified analysis plan, therefore the results should be interpreted as hypothesis-generating analyses. Third, we observed a bump in the event curves during the transition period in both the DP-DES and BP-DES groups. Angiographic surveillance was not performed in this study, and the primary endpoint only included clinically driven revascularisation events. Moreover, the prescription patterns of all medications, including antiplatelet agents, were similar between the two groups. Fourth, although we reported a significant difference in the DOCO incidence, our study was not adequately powered to assess the individual components of the composite endpoint, let alone rare device-oriented clinical events, such as stent thrombosis. Additionally, our study could not demonstrate a difference in patient-oriented outcomes, and thus, the current findings should be considered hypothesis-generating. Fifth, we did not evaluate the quality of PCI post-procedure, such as with quantitative coronary angiography of the target lesion, assessment of post-PCI minimal stent area when intravascular ultrasound was utilised, or physiological assessment when fractional flow reserve was utilised. Sixth, this was a Korean-only study, therefore caution is needed in interpretation. Our findings may not be generalisable to other populations who may respond differently to the stent characteristics. Lastly, this was an open-label study, and the clinical events committee relied on information from medical charts and reports of telephone interviews, which may have been biased by the primary physician.
Conclusions
During 3-year follow-up of ACS patients undergoing PCI, DP-DES showed similar rates of patient-oriented outcomes to BP-DES, while DP-DES were superior to BP-DES in terms of device-oriented outcomes, due to a lower risk during the transition period after PCI. These results suggest that polymer degradation may transiently change the local milieu and induce inflammation, tissue growth, or thrombosis at the stented segment, leading to adverse events.
Impact on daily practice
Data from long-term follow-up of the HOST REDUCE POLYTECH RCT Trial present a comparison of biodegradable-polymer and durable-polymer drug-eluting stents (DP-DES) for the entire period of polymer degradation. During the total 3-year follow-up period, both stent arms showed similar patient-oriented composite outcome rates. However, the device-oriented composite outcome rate was lower in the DP-DES arm, which was majorly driven by target lesion revascularisation events that occurred when the polymer degradation was ongoing, defined as 8 to 16 months after percutaneous coronary intervention. This can be explained by the fact that polymer degradation may induce inflammation, leading to tissue growth or thrombosis at the stented segment. Medical treatment that can control inflammation during the polymer degradation period may improve clinical outcomes, and next-generation stents may improve performance by stabilising the polymer degradation mechanism.
Study investigators
Hyo-Soo Kim, MD; Seoul National University Hospital, Seoul, Republic of Korea; Jay Young Rhew, MD; Presbyterian Medical Center, Jeonju, Republic of Korea; Kook Jin Chun, MD; Pusan National University Yangsan Hospital, Yangsan, Republic of Korea; Young-Hyo Lim, MD; Hanyang University Seoul Hospital, Seoul, Republic of Korea; Jung Min Bong, MD; Hanlim General Hospital, Incheon, Republic of Korea; Jang-Whan Bae, MD; Chungbuk National University, Cheongju, Republic of Korea; Bong Ki Lee, MD; Kangwon National University, Chuncheon, Republic of Korea; Seok-Yeon Kim, MD; Seoul Medical Center, Seoul, Republic of Korea; Keun-Ho Park, MD; Chosun Medical Center, Gwangju, Republic of Korea; Seung-Woon Rha, MD; Korea University Guro Hospital, Seoul, Republic of Korea; Won-Yong Shin, MD; Soonchunhyang University Cheonan Hospital, Cheonan, Republic of Korea; Hong-Seok Lim; Ajou University Medical Center, Suwon, Republic of Korea; Kyungil Park, MD; Dong-A University Hospital, Busan, Republic of Korea; Yun-Kyeong Cho, MD; Keimyung University Dongsan Medical Center, Daegu, Republic of Korea; Soon Jun Hong, MD; Korea University Anam Hospital, Seoul, Republic of Korea; Sanghyun Kim, MD; Seoul Boramae Hospital, Seoul, Republic of Korea; Sang-Ho Jo, MD; Hallym University Sacred Heart Hospital, Anyang, Republic of Korea; Yong Hoon Kim, MD; Kangwon National University, Chuncheon, Republic of Korea; Won Kim, MD; Kyung Hee University Medical Center, Seoul, Republic of Korea; Sung Yun Lee, MD; Ilsan Paik Hospital, Goyang, Republic of Korea; Seok Kyu Oh, MD; Wonkwang University Hospital, Iksan, Republic of Korea; Ung Kim, MD; Yeungnam University Hospital, Daegu, Republic of Korea; Dong-Bin Kim, MD; Bucheon St. Mary’s Hospital, Bucheon, Republic of Korea; In-Ho Chae, MD; Seoul National University Bundang Hospital, Seongnam, Republic of Korea; Keon-Woong Moon, St. Vincent’s Hospital, Suwon, Republic of Korea; Hyun Woong Park, MD; Gyeongsang National University Hospital, Jinju, Republic of Korea; Eun Seok Shin, MD; Ulsan University Hospital, Ulsan, Republic of Korea; Dong Woon Jeon, MD; National Health Insurance Service Ilsan Hospital, Goyang, Republic of Korea; Kyu-Rok Han, MD; Kangdong Sacred Heart Hospital, Seoul, Republic of Korea; Si Wan Choi, MD; Chungnam National University Hospital, Daejeon, Republic of Korea; Jae Kean Ryu, MD; Daegu Catholic University Medical Center, Daegu, Republic of Korea; Myung Ho Jeong, MD; Chonnam University Hospital, Gwangju, Republic of Korea; Kwang Soo Cha, MD; Pusan National University Hospital, Pusan, Republic of Korea; Namho Lee, MD; Kangnam Sacred Heart Hospital, Seoul, Republic of Korea. Clinical Event Adjudication Members: Do-Yoon Kang MD; Asan Medical Center, Seoul, Republic of Korea; Young Bin Song, MD; Samsung Medical Center, Seoul, Republic of Korea
Funding
This study was supported by Daiichi Sankyo, Boston Scientific, Terumo, Biotronik, Qualitech Korea, and Dio.
Conflict of interest statement
H. Kim has received research grants or speaker fees from Daiichi Sankyo, Boston Scientific, Terumo, Biotronik, Dio, Medtronic, Abbott, Edwards Lifesciences, Amgen, and Boehringer Ingelheim. K.W. Park reports fees from Daiichi Sankyo, AstraZeneca, Sanofi, Bristol-Myers Squibb, Bayer, and Pfizer, outside of the submitted work. The other authors and investigators have no conflicts of interest to declare in relation to this paper.
Supplementary data
To read the full content of this article, please download the PDF.

