During the 4-year follow-up of a patient with a Tendyne valve (Abbott) implanted in 2019, the transthoracic echocardiogram (TTE) showed valve dysfunction with severe mitral regurgitation (MR). Transoesophageal echocardiography (TOE) displayed multiple jets at the level of the outer frame, normal leaflet excursion, and no endocarditic foci. On a cardiac computed tomography (CT) scan, the Tendyne valve showed no structural anomalies and appeared normopositioned (Supplementary Figure 1, Supplementary Figure 2, Moving image 1).
Surgical intervention was excluded because of an extremely high operative risk, but even the transcatheter approach was challenging. A two-step strategy, involving the implantation of a covered stent followed by a SAPIEN 3 (S3 [Edwards Lifesciences]), would have led to a prolonged period of massive MR and haemodynamic instability. The implant of a standard S3 alone would have maintained a more stable positioning, but its upper free-flow segment would not permit leak closure. To overcome this issue, we decided to perform a direct valve-in-valve (ViV) procedure with a customised S3 (Figure 1), sewing a polytetrafluoroethylene (PTFE) patch to cover the free-flow segment of the valve stent1. ViV implantation in the Tendyne valve remained challenging2, since the S3 valve needed to be crimped directly over the balloon, and to be sure that the nose cone entered the flower-shaped frame of the Tendyne, a double-wire strategy was necessary, with selective crossing of the same petal and ballooning prior to implantation. Furthermore, the Tendyne structure exposed the ViV procedure to the risk of S3 atrialisation during the final deployment. To test our potential solution, an in silico implant was performed using a three-dimensionally (3D) printed heart model based on the latest available CT scan acquisition (Supplementary Figure 3-Supplementary Figure 4-Supplementary Figure 5).
Under TOE guidance we performed a posterior-inferior transseptal puncture: the valve was crossed in the correct petal portion using an Agilis NxT catheter (Abbott) and a guidewire (Terumo). A Safari XS wire (Boston Scientific) was secured into the left ventricle (LV) and used to advance a 14 Fr Armada balloon (Abbott) for septostomy. A second wire was advanced through the septum into the left atrium (LA), and using an Agilis catheter, the valve was recrossed in the same petal to position the second Safari wire in the LV. Meanwhile, a customised 29 mm S3 valve was prepared as planned and then crimped directly onto the balloon of a standard Edwards Commander delivery system (Edwards Lifesciences). The system was advanced through the septum to the left atrium over the second Safari wire, using the “Buddy Balloon Technique” (BBT) with a 14 Fr Armada balloon that had previously been positioned. The BBT was also used to facilitate valve crossing and implantation of the S3. The modified S3 crossed the Tendyne without any impingement; the first Safari wire was removed. Under fluoroscopic and echo guidance, using rapid ventricular pacing, the S3 was implanted as deeply as possible (Supplementary Figure 6). However, as expected, the interaction with the Tendyne ventricular nitinol struts produced an “atrialisation” movement of the S3.
We optimised the implant with postdilatation, yet on the control TOE, the regurgitant jets, despite being reduced, appeared to still be significant. A second customised S3 prosthesis that had been crimped directly and even more distally onto the balloon was implanted; a slight atrialisation movement was present, but on the control TOE, the residual regurgitation was mild (Supplementary Figure 7).
Prior to discharge, the TTE showed a mitral transvalvular mean gradient of 3 mmHg, and at 6 months, the TTE revealed mild regurgitation (Supplementary Figure 8, Moving image 2-Moving image 3-Moving image 4).
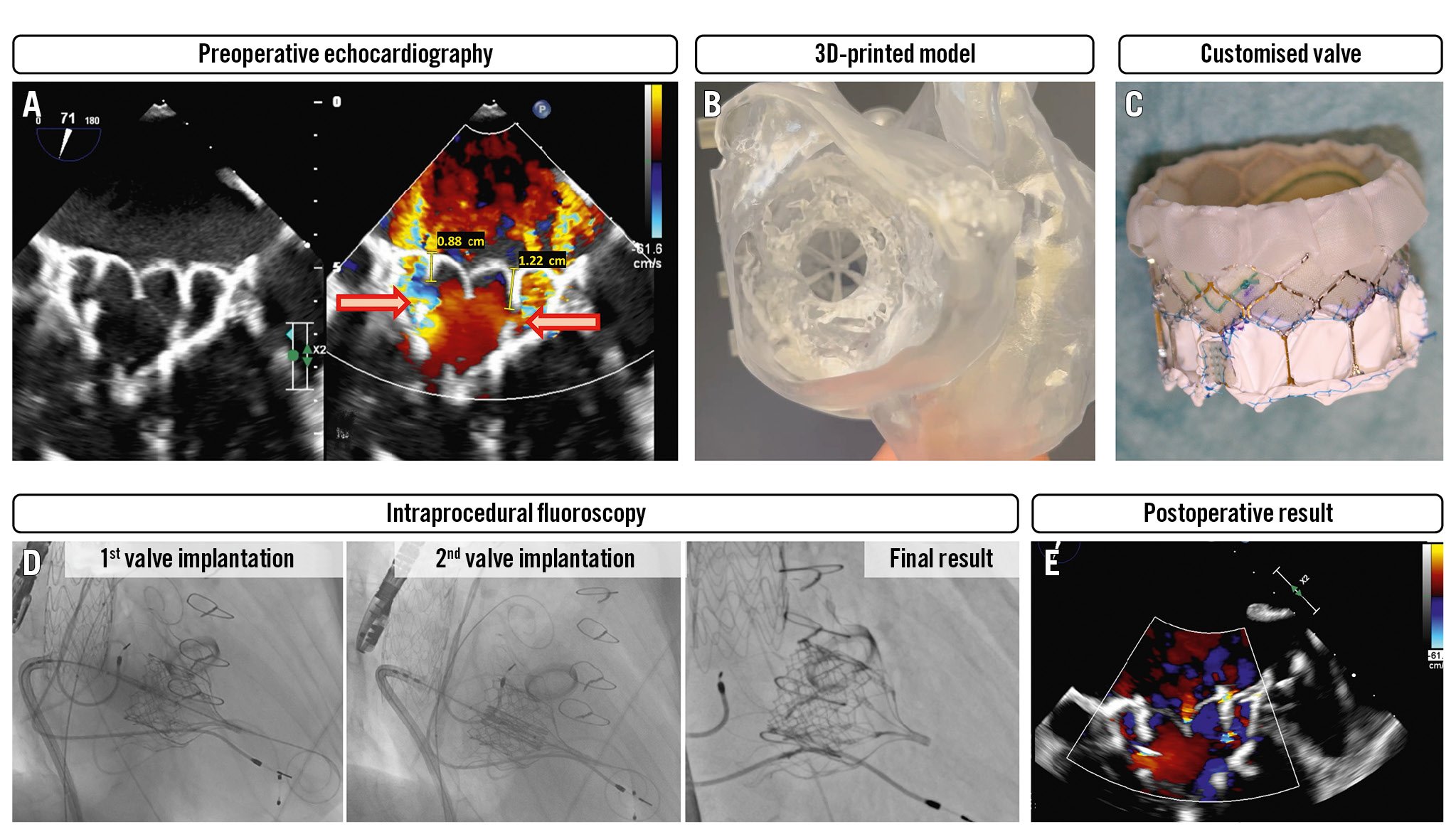
Figure 1. S-Dyne procedure. A) Transoesophageal echocardiography (TOE) displaying severe regurgitation at the level of the outer frame with multiple jets, normal leaflet excursion, and no endocarditic foci. B) A 3D printed heart model based on the patient’s CT scan. C) The customised SAPIEN (Edwards Lifesciences) valve with a PTFE sutured neoskirt. D) The valve-in-valve procedure. E) Postoperative TOE findings with mild residual mitral regurgitation. 3D: three-dimensionally; CT: computed tomography; PTFE: polytetrafluoroethylene
Conflict of interest statement
P. Denti has received speaker honoraria from Abbott and Edwards Lifesciences; and is a consultant for Pi-Cardia, InnovHeart, and Approximate Labs. F. Maisano reports grants and personal fees from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, Terumo, Perifect, Xeltis, Transseptal Solutions, Cardiovalve, Magenta, 4Tech, Coregard, SwissVortex, and Occlufit. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Tendyne bioprosthesis normopositioned and functioning well when initially deployed.
Moving image 2. TOE showing severe regurgitation at the level of the outer frame with multiple jets, normal leaflet excursion, and no endocarditic foci.
Moving image 3. TOE direct comparison between pre- and postprocedural valve function.
Moving image 4. Recorded procedure.

