Abstract
This clinical consensus statement of the European Association of Percutaneous Cardiovascular Interventions was developed in association with the European Society of Cardiology Working Group on Cardiovascular Surgery. It aims to define procedural and contemporary technical requirements that may improve the efficacy and safety of percutaneous coronary intervention (PCI), both in the acute phase and at long-term follow-up, in a high-risk cohort of patients on optimal medical therapy when clinical and anatomical high-risk criteria are present that entail unacceptable surgical risks, precluding the feasibility of coronary artery bypass grafting (CABG). This document pertains to patients with surgical contraindication according to the Heart Team, in whom medical therapy has failed (e.g., residual symptoms), and for whom the Heart Team estimates that revascularisation may have a prognostic benefit (e.g., left main, last remaining vessel, multivessel disease with large areas of ischaemia); however, there is a lack of data regarding the size of this patient population. This document aims to guide interventional cardiologists on how to proceed with PCI in such high-risk patients with reduced left ventricular ejection fraction after the decision of the Heart Team is made that CABG − which overall is the guideline-recommended option for revascularisation in these patients − is not an option and that PCI may be beneficial for the patient. Importantly, when a high-risk PCI is planned, a multidisciplinary decision by interventional cardiologists, cardiac surgeons, anaesthetists and non-invasive physicians with expertise in heart failure management and intensive care should be agreed upon after careful consideration of the possible undesirable consequences of PCI, including futility, similar to the approach for structural interventions.
The incidences of complex coronary artery disease (CAD) and heart failure (HF) are growing in view of ageing populations and an increasing comorbidity burden1. Approximately 1-2% of hospitalisations in Europe and the United States are due to HF, and the treatment of patients suffering from CAD and reduced left ventricular ejection fraction (LVEF) represents a major clinical and public health challenge23. The European Society of Cardiology (ESC) 2018 guidelines on myocardial revascularisation recommend a coronary artery bypass graft (CABG) as the primary revascularisation strategy for patients with CAD and reduced LVEF. This recommendation is based on studies in a patient population with low or intermediate surgical risk and chronic coronary syndrome. In clinical practice, there are an increasing number of patients with depressed LVEF and high-risk features of CAD, including stabilised acute coronary syndromes (ACS), for whom the Heart Team votes against CABG due to high, or even prohibitive, surgical risk. Data from real-world practice indicate that percutaneous coronary intervention (PCI) is preferred over CABG in approximately 50% of patients due to high surgical risk or excessive frailty features456. In addition, an unknown number of patients decide against CABG due to a personal decision-making process after careful informed consent. Currently, given the absence of randomised controlled trials (RCTs), recommendations − weighing the efficacy and safety of PCI and CABG in the setting of left ventricular (LV) dysfunction in high-risk patients − are based on registries and secondary analyses of RCTs performed in the setting of stable CAD4789. The results of these studies are prone to multiple confounders and selection bias, whereas reality leads to frequent performance of PCI in very high to prohibitive surgical risk patients, for whom medical management is believed not to be a viable option510. This clinical consensus statement of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) was developed in association with the European Society of Cardiology (ESC) Working Group on Cardiovascular Surgery; it aims to define procedural and contemporary technical requirements that may improve the efficacy and safety of PCI, both in the acute phase and at long-term follow-up, in this high-risk cohort of patients on optimal medical therapy (OMT) when clinical and anatomical high-risk criteria are present that entail unacceptable surgical risks, precluding the feasibility of CABG (Figure 1).
This document pertains to patients in whom CABG, which is the overall guideline-recommended option for revascularisation in patients with complex CAD and reduced LVEF, is not an option according to the Heart Team, in whom medical therapy has failed (e.g., residual symptoms), and for whom the Heart Team estimates that revascularisation may have a prognostic benefit (e.g., left main, last remaining vessel, multivessel disease with large areas of ischaemia).
A multidisciplinary decision-making process by interÂventional cardiologists, cardiac surgeons, anaesthetists and non-invasive physicians with expertise in HF management and intensive care will be discussed within the consensus statement to evaluate the pros and cons of high-risk PCI. This is of high importance, since data are limited or non-existing on (1) any patient benefit from revascularisation, (2) patient benefit from additional mechanical circulatory support (MCS) during revascularisation, and (3) post-PCI care of this high-risk cohort.
We provide a consensus for an interdisciplinary, case-by-case decision-making process for patients with significantly impaired LVEF and complex CAD with high-risk features for whom the Heart Team has voted to carry out a high-risk PCI procedure due to risk of surgical futility (surgical turndown patients) or when the patient has decided against CABG (Central illustration).
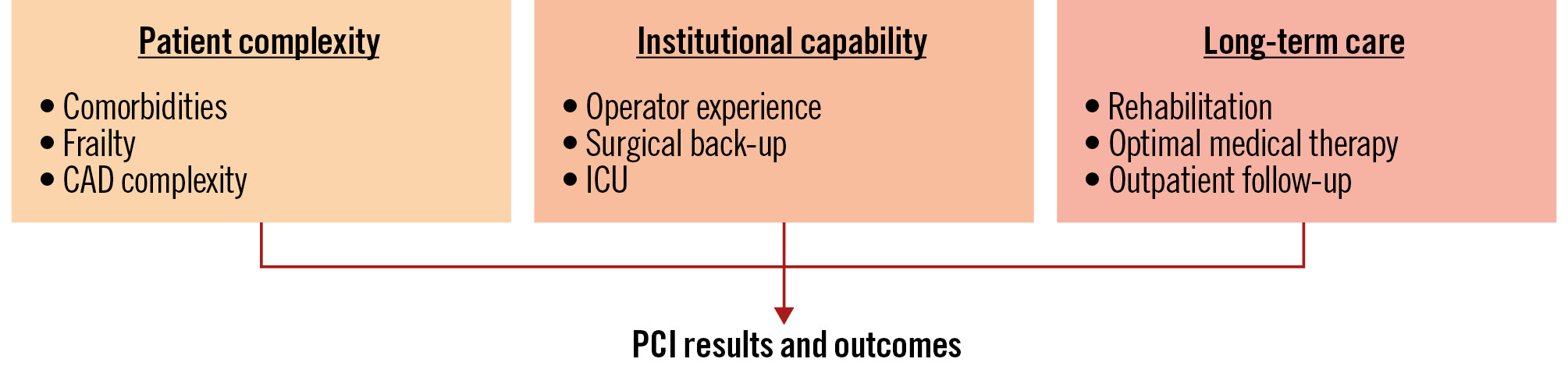
Figure 1. Patient and institutional factors influencing PCI outcomes. In addition to reduced ejection fraction, patient complexity − such as the presence of other significant comorbidities, remaining myocardial viability and overall frailty − critically influences both surgical risk and PCI risk. Once patients are classified as having high perioperative risk, coronary artery disease (CAD) complexity together with coexistent peripheral and/or valvular disease increase the interventional challenge. This must be matched by general institutional capabilities, including the availability of mechanical circulatory support and intensive care unit (ICU) expertise, and individual levels of competence in procedural skills, including knowledge, behaviour and attitudes for adequate surgical back-up and bailout situations. For long-term care, cardiovascular rehabilitation, adherence to optimal medical therapy and dedicated follow-up in a specialised outpatient setting are required. PCI: percutaneous coronary intervention
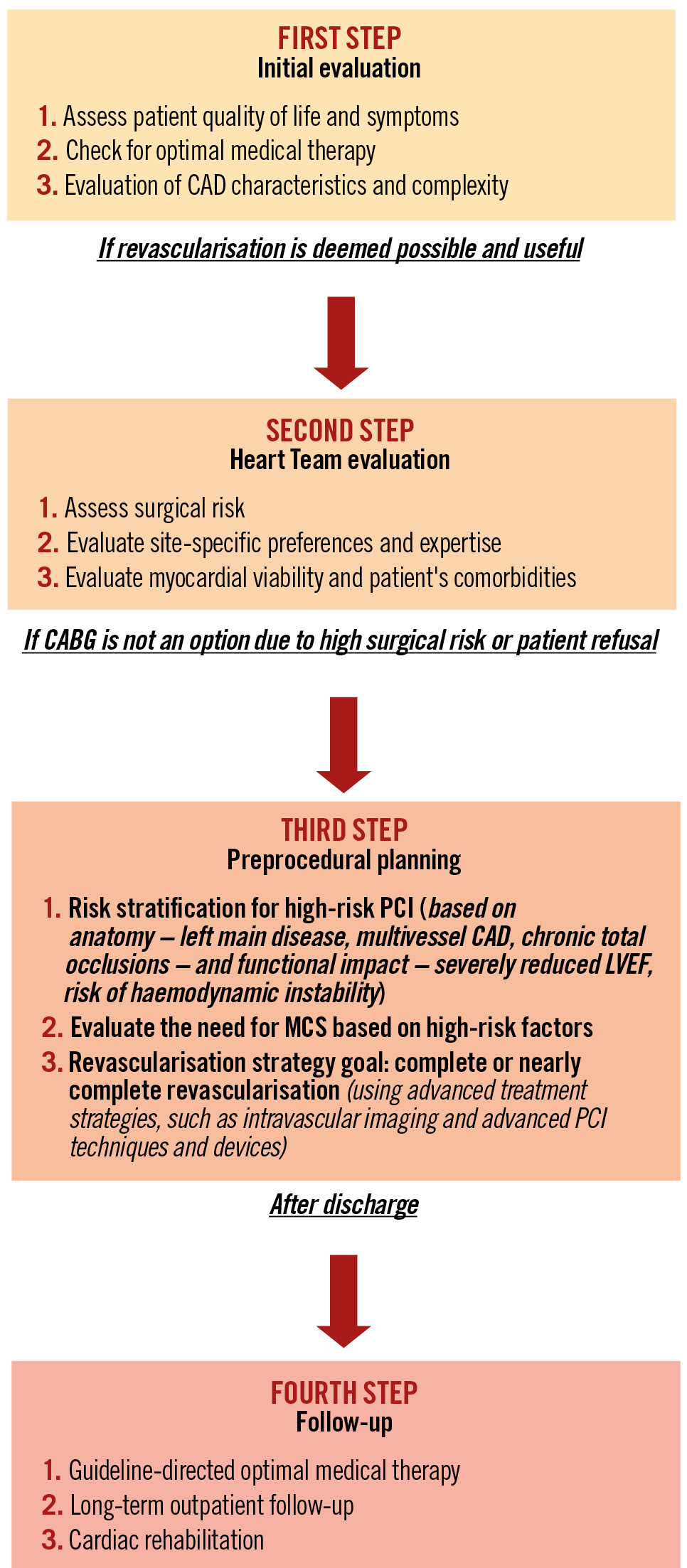
Central illustration. Revascularisation in patients with complex CAD and reduced LVEF. CABG: coronary artery bypass graft; CAD: coronary artery disease; LVEF: left ventricular ejection fraction; MCS: mechanical circulatory support; PCI: percutaneous coronary intervention
Revascularisation strategies in patients with severe LV dysfunction
Patients presenting with low LVEF and complex CAD potentially requiring revascularisation are discussed in the Heart Team with a comprehensive, multidisciplinary evaluation of risks and benefits including, but not limited to, anaesthesia, cross-clamping of the aorta, coronary artery disease pattern, concomitant valve disease or aortic dilatation, and degree of LV dysfunction411. Regarding the evidence for revascularisation from RCTs for this patient cohort, the extended observation, up to 10 years, of the Surgical Treatment for Ischemic Heart Failure (STICH) trial revealed that many patients with severely impaired LVEF can benefit from coronary revascularisation with CABG compared to OMT alone912, thereby driving the guideline recommendations for CABG in this patient cohort4.
Comparisons between PCI and CABG are primarily limited to analyses of RCTs enrolling patients suitable for both CABG and PCI, in which the average LV function was normal in the majority of patients7131415. The eligibility criteria of these available RCTs led to the exclusion of most patients with very complex clinical and anatomical conditions as well as patients who would not accept a surgical option. While this may have excluded patients with higher probability of benefits by CABG, it also excluded inoperable patients potentially amenable to PCI15.
Regarding the comparison between PCI and OMT, the recent REvascularisation for Ischemic VEntricular Dysfunction-BCIS2 (REVIVED-BCIS2) trial investigated whether PCI in combination with OMT reduces mortality and HF hospitalisation compared to OMT alone. Most of these patients did not have angina or severe HF symptoms (New York Heart Association Class I-II). As such, the population included in the REVIVED-BCIS2 trial may not be representative of patients with ischaemic symptoms and impaired LVEF requiring recurrent HF hospitalisations. A significant proportion of patients in the REVIVED-BCIS2 trial had intermediate-complexity coronary artery disease with only half of the patients having either 3-vessel or left main CAD. The trial was powered for 700 patients with an expected overall event rate of 43%. Over a median of 41 months, the trial did not provide evidence of benefit of PCI over OMT16. The overall event rate was lower than anticipated, and many HF events occurred in patients who died during the follow-up period16. To observe a clinically relevant improvement in LV function and HF events in otherwise asymptomatic patients, one would assume the presence of rather extensive CAD causing the compromised LV function. Table 1 provides an overview of current data.
Observational studies have demonstrated the feasibility of PCI in patients with severe LV dysfunction with acceptable rates of in-hospital and long-term mortality1718192021. In the absence of RCTs, registry data suggest that PCI using modern drug-eluting stents (DES) may be an acceptable alternative to CABG in patients with multivessel disease and impaired LVEF when complete revascularisation can be achieved22. However, observational data indicate a benefit of CABG over PCI in patients with impaired LVEF232425. As such, a comprehensive assessment of patients is advocated, and reduced LVEF needs to be considered along with other major comorbid conditions.
Table 1. Characteristics of STICH and REVIVED-BCIS2 trials.
| Parameter | STICH1213 | REVIVED-BCIS217 |
|---|---|---|
| Patients enrolled | 610 (CABG)/602 (OMT) | 347 (PCI)/353 (OMT) |
| Inclusion criteria | LVEF ≤35%, extensive CAD | LVEF ≤35%, extensive CAD, myocardial viability |
| Mean age, years | 60/59 | 70/69 |
| Mean LVEF, % | 27/28 | 27/27 |
| Mean BMI, kg/m2 | 27/27 | 28/29 |
| Diabetes | 39/40 | 39/43 |
| NYHA III-IV | 37/37 | 23/29 |
| Angina CCS III-IV | 5/4 | 2/2 |
| Previous MI | 76/78 | 50/56 |
| Previous PCI/CABG | 17/14 | 22/28 |
| Left main CAD | 3/2 | 14/13 |
| 3-vessel CAD | 62/59 | 38/42 |
| Primary endpoint | All-cause death | Death or HF |
| Result | CABG: 218 (36)OMT: 244 (41)HR 0.86 (95% CI: 0.72-1.04) | PCI: 129 (37.2)OMT: 134 (38.0)HR 0.99 (95% CI: 0.78-1.27) |
| Secondary endpoint | Death or HF | All-cause death |
| Result | CABG: 290 (48)OMT: 324 (54)HR 0.84 (95% CI: 0.71-0.98) | PCI: 110 (31.7)OMT: 115 (32.6)HR 0.98 (95% CI: 0.75-1.27) |
| Median follow-up, months | 56 (IQR 48-68) | 41 (IQR 28-60) |
| Conclusions | There was no significant difference between medical therapy alone and medical therapy plus CABG with respect to all-cause death. Lower rates of death from cardiovascular causes and of death from any cause or hospitalisation for cardiovascular causes were noted in those undergoing CABG plus OMT. | PCI in addition to OMT in patients with severe LV dysfunction & coronary artery disease with viable myocardium did not significantly improve overall mortality or rates of heart failure hospitalisation.PCI with OMT was not superior to OMT alone in improving LV systolic function, NYHA Functional Class, or quality of life. |
| Limitations | Younger cohort with a mean age 60 years; effect of CABG on all-cause mortality diminished with increasing age (p-interaction=0.062). | Older population not revascularised by CABG, introducing selection bias early on in patients with few symptoms of angina or HF. |
| Data are given as % or n (%), unless stated otherwise. Baseline characteristics and outcome parameters of the Surgical Treatment for Ischemic Heart Failure (STICH)12 and the Revascularisation for Ischaemic Ventricular Dysfunction-British Cardiovascular Intervention Society-2 (REVIVED-BCIS2)16 trials. BMI: body mass index; CABG: coronary artery bypass graft; CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; CI: confidence interval; HF: heart failure; HR: hazard ratio; IQR: interquartile range; LV: left ventricular; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; OMT: optimal medical therapy; PCI: percutaneous coronary intervention | ||
Consensus statement
OMT forms the foundation of all revascularisation strategies. In cases where patients meet the criteria observed in STICH patients, the Heart Team must reassess surgical strategies. For patients who have been declined surgical intervention and for those who refuse surgical options, PCI-based treatment strategies may present a viable alternative, despite the absence of randomised data.
Revascularisation based on the anatomical pattern of CAD
HF with reduced LVEF is associated with worse outcomes, both periprocedurally and long term, in patients treated with PCI26. However, robust prospective data with respect to the mode of revascularisation in patients with HF and reduced LVEF are still lacking162728.
Regarding coronary anatomy, a thoughtful evaluation between safety and efficacy is of paramount importance, especially in a high-risk population of patients with a higher propensity for complications. If intraprocedural vessel occlusion or flow-limiting dissection occurs in patients with an impaired LVEF, further severe haemodynamic deterioration can ensue, with the potential to rapidly lead to shock or arrest. Several angiographic scoring systems that validly estimate plaque burden and coronary anatomy complexity are available, but they do not account for the reduced LVEF29. The SYNTAX score II includes LVEF as a variable, but patients with significantly impaired LVEF were significantly underrepresented in the score development cohort, accounting for only 2%.
Therefore, rather than relying on these scores, the anatomical complexity of high-risk PCI can be more pragmatically described by patterns that are encountered in clinical practice. These include the last remaining vessel, chronic total occlusions (CTOs), left main disease, and diffuse 3-vessel CAD. Treatment of these high-risk patients should be limited to experienced operators with technical expertise and routine performance of such complex procedures. Similar cautioning applies to patients with severely depressed LVEF shortly after a myocardial infarction.
Multiple clinical studies and guidelines have addressed infrastructural and technical aspects of complex coronary interventions. This includes position statements on decision-making within the Heart Team for complex coronary anatomy30, strategies on bifurcation intervention including left main PCI31, the use of left ventricular assist devices in patients with an indication for complex PCI and reduced LVEF32, the use of intravascular imaging3334, lesion preparation including rotational atherectomy35, and strategies to deal with revascularisation failure36. In addition, a vast number of publications address treatment options in specific coronary anatomical challenges, e.g., ostial and long lesions, CTOs, calcified lesions, in-stent stenosis, bypass graft interventions and others, which are extensively reviewed and discussed in The PCR-EAPCI Textbook of Percutaneous Interventional Cardiovascular Medicine (www.pcronline.com/eurointervention/textbook/pcr-textbook/table-of-contents/).
Complex anatomy often necessitates intracoronary imaging guidance during PCI, but the additive information obtained by intravascular ultrasound and optical coherence tomography should be weighed against the increased ischaemic and procedural times and amount of contrast media. Nevertheless, in complex CAD, it reduces the risk of postprocedural cardiovascular events3738. When intravascular imaging is not feasible or the expected information is not relevant, stenting optimisation can be adequately assessed by stent enhancement using subtraction imaging algorithms39. Fractional flow reserve or instantaneous wave-free ratio, in principle, are other options to confirm acute PCI success40; however, these measurements are affected by intra-aortic balloon pump (IABP) use and are not clearly validated on LV support414243. It remains unclear whether either anatomical or physiological lesion selection is beneficial to guide PCI in patients with LV dysfunction or those on MCS devices.
Consensus statement
State-of-the-art functional and imaging studies, along with advanced revascularisation techniques, are essential for complex PCI procedures. Intracoronary imaging has demonstrated its ability to enhance outcomes and is emerging as a necessary standard in high-risk, complex PCI procedures. In high-risk PCI, its use should be weighed against the increased ischaemic and procedural times and amount of contrast media.
Completeness of myocardial revascularisation
The strong relationship between the extent of ischaemia and clinical endpoints suggests that complete revascularisation is preferable in order to improve outcomes and quality of life. A residual SYNTAX score indicative of incomplete revascularisation portends a higher risk of mortality and major adverse cardiac events (MACE), irrespective of whether an anatomical or score-based definition of incomplete revascularisation is employed44. The degree of risk correlates with the degree of incomplete revascularisation. In 2018, the ESC/European Association for Cardio-Thoracic Surgery (EACTS) guidelines on myocardial revascularisation stated that the ability to achieve complete revascularisation should be considered when choosing between revascularisation modalities4. This was re-emphasised in the 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes11. Nevertheless, since the decision on revascularisation in ischaemic cardiomyopathy is complex (anatomical and/or functional incomplete/complete/extensive revascularisation), decision-making cannot be based solely on guidelines.
A few non-randomised studies have evaluated completeness of revascularisation in high-risk PCI patients treated with MCS devices. These registries have shown that the extent of revascularisation in symptomatic patients with complex coronary anatomies, often unsuitable for surgical treatment, demonstrates improvement in LVEF (in segments correlating with severity of revascularisation scores) and outcomes18204546. The European best practice consortium suggests that decision-making on revascularisation with MCS-facilitated PCI should be based on the capability to achieve complete and extensive revascularisation47. However, at present, the choice between the two types of intervention and the completeness of treatment is left to the clinician following an in-depth evaluation of patient comorbidities. Individualised therapy may include staged complete revascularisation supported by LV unloading.
Consensus statement
When undertaking revascularisation via PCI in critically ill patients with severe LV dysfunction and no surgical options, the procedure should strive for nearly complete revascularisation whenever feasible. Moreover, in situations where MCS is utilised to facilitate high-risk PCI, the stabilised haemodynamic environment should be leveraged to achieve as comprehensive a revascularisation as possible. This approach may enable the undertaking of more complex interventions, particularly in the left main or in patients with compromised LV function following recent myocardial infarction. In instances where the potential target lesion involves a CTO, a specialised CTO operator should have assessed the patient’s angiogram beforehand and be readily available for that aspect of the procedure if warranted.
Extent of myocardial ischaemia and residual myocardial viability
The presence and extent of myocardial ischaemia has been strongly associated with poor patient prognosis and adverse events, especially in patients with HF and reduced LVEF. Several studies have demonstrated that significant myocardial ischaemia, as detected by perfusion imaging tests, is associated with unfavourable clinical outcomes at long-term follow-up484950. Furthermore, residual ischaemia after treatment has been similarly associated with long-term mortality51. Consistently, a more complete revascularisation by a surgical approach has been shown to improve survival in stable patients with ischaemic HF and reduced LVEF, compared with OMT alone9.
A controversial point is the evaluation of residual myocardial viability and, most importantly, its impact on clinical outcomes. Viability testing in revascularisation trials, including examples such as STICH or COURAGE, was not a determinant of outcomes and hence limits the clinical importance of myocardial ischaemia assessment in influencing the decision-making for revascularisation. Of note, myocardial viability was a part of the protocol design in REVIVED-BCIS2, in order to unequivocally confirm a diagnosis of ischaemic cardiomyopathy. Previous registries have demonstrated favourable associations between myocardial viability, coronary revascularisation, and clinical outcomes52535455. On the contrary, a post hoc analysis of the randomised STICH trial failed to find an impact of viability on all-cause mortality, regardless of revascularisation. Of note, the vast majority of the patients had evidence of myocardial viability on single-photon emission computed tomography/echo-dobutamine tests only but not on other validated modalities, such as cardiac magnetic resonance. Notwithstanding, myocardial viability was associated with a significant improvement in LVEF. Interestingly, a trend towards a significant interaction between the presence of myocardial viability and the treatment arm was shown for long-term cardiovascular mortality, suggesting a possible beneficial role of revascularisation of viable myocardium. Accordingly, current ESC/EACTS guidelines on myocardial revascularisation support the assessment of myocardial viability in patients with CAD and HF before revascularisation4. The recently published REVIVED-BCIS2 trial did not show improvement in LV function in patients with low LVEF and CAD treated with PCI on top of OMT despite viable myocardium16. No data were provided on the anatomical location and extent of CAD nor the rate of appropriate complete revascularisation, as suggested before56. Patients with functional incomplete revascularisation had a significantly higher rate of MACE compared with those with functional complete revascularisation. A combined anatomical and physiological scoring system after PCI demonstrated a higher discriminatory value for risk prediction57.
Currently, no subanalysis argues for a PCI strategy in patients with similar characteristics of the REVIVED-BCIS2 cohort. A strategy of initial OMT and postponing additional revascularisation therapies may help in the decision-making process.
The latest guidelines give a Class I recommendation for CABG in patients with an LVEF of 30% with multivessel disease and no diabetes4. Since severely reduced LV function remains a leading reason for surgical turndown, a first-line strategy of OMT seems appropriate, bringing the patient to a different risk class which may then argue for revascularisation.
The ongoing PROTECT IV Trial (ClinicalTrials.gov: NCT04763200; described in more detail below) will provide new evidence in this field with a prespecified analysis on the association between myocardial viability and post-revascularisation outcomes in high-risk PCI patients.
Consensus statement
Patients classified as having low to intermediate surgical risk with complex CAD are considered suitable candidates for CABG, irrespective of their LVEF, per current guideline recommendations. Conversely, patients with high to prohibitive risk with complex CAD derive benefits from OMT, regardless of their LVEF. However, there is a lack of randomised trial data providing guidance on revascularisation strategies for this patient cohort, particularly in cases of surgical turndown or high-risk patients declining CABG. For PCI, achieving functional complete revascularisation of all haemodynamically flow-limiting lesions, allowing for anatomically “incomplete” revascularisation of physiologically non-significant lesions and vessels, is the preferred approach, whereas anatomically complete revascularisation after CABG is the current standard of care58. It is anticipated that symptom improvement will occur in this group, although the debate on LVEF improvement persists, as demonstrated by REVIVED-BCIS2. In instances where LVEF improvement is observed, positive effects on outcomes may be expected. A clear correlation between myocardial ischaemia and coronary artery stenosis in large and prognostically relevant coronaries, preferably measured with fractional flow reserve, serves as a treatment indication.
Expected benefit balanced with procedural risk
High-risk PCI aims primarily to improve symptoms and quality of life in patients with advanced CAD and reduced LVEF, whether in chronic or acute coronary syndromes. The potential benefits in quality of life have to be balanced against procedural risks such as target vessel failure, vascular complications, bleeding, renal impairment due to higher contrast volume, and potential ischaemia-related haemodynamic instability during or after the procedure.
If the Heart Team decides to use MCS to prevent haemodynamic instability, the increased risks of vascular complications and bleeding associated with MCS have to be weighed against the potential benefit of more successful and/or more complete revascularisation. This balance is crucial when discussing the use of MCS in high-risk PCI patients. Even in cardiogenic shock, this issue remains unresolved: MCS use is linked to more complications and, in some analyses, higher mortality rates, though these patients were generally sicker59. The Heart Team needs to evaluate whether the potential revascularisation benefit justifies the use of large-bore access to stabilise the patient for procedural success. Lessons learned from MCS use in shock indicate that improved outcomes can be achieved despite bleeding and vascular complications60, highlighting the importance of thorough preprocedural planning. This includes selecting the appropriate MCS device and optimising vascular access, similar to structural interventions, where complications have been reduced over time.
The following section covers the available data and provides the consensus. For detailed guidance on planning and procedure to minimise risk and improve success, please refer to Supplementary Appendix 1-Supplementary Appendix 2-Supplementary Appendix 3.
MCS in high-risk PCI
The use of MCS devices is an option in high-risk PCI patients with severely depressed LV function but should not be assumed as the default treatment strategy. There are three categories of MCS: IABP, microaxial flow pumps, and venoarterial extracorporeal membrane oxygenation (VA-ECMO)6162. While traditionally used in patients with cardiogenic shock606364, their use in high-risk PCI has increased, particularly in elderly patients with multiple comorbidities6566. However, there are limited supportive outcome data, compared to OMT alone, and no clear evidence on the superiority of one device over another or their cost-effectiveness in high-risk PCI67686970.
Regarding RCTs in this field, two larger studies have been conducted but have provided limited supportive data:
1. Balloon Pump Assisted Coronary Intervention Study (BCIS-1) investigated elective IABP support in patients with LVEF <30% and severe CAD. The trial did not show a reduction in composite endpoints (death, acute myocardial infarction, cerebrovascular event, or revascularisation) at discharge, suggesting no benefit for routine IABP placement before PCI in severe LV dysfunction7172. However, an extended assessment indicated a 33% reduction in all-cause mortality with IABP support during high-risk PCI, though this trial was not designed to address mortality outcomes specifically73.
2. PROTECT II Trial evaluated the Impella 2.5 (Abiomed) in high-risk PCI patients with impaired LVEF ≤35% and severe CAD. The trial was halted early due to assumed futility, and the primary endpoint (composite of all-cause death, myocardial infarction, stroke or transient ischaemic attack, any repeat revascularisation by PCI or CABG, need for a cardiac or vascular operation, acute renal insufficiency, severe intraprocedural hypotension requiring therapy, cardiopulmonary resuscitation or ventricular tachycardia requiring cardioversion, aortic insufficiency and angiographic failure of PCI) was not significantly reduced19. Although exploratory analyses suggested symptom reduction and improved LVEF at 90 days, there was no significant reduction in the composite endpoint (death, myocardial infarction, stroke). Real-world data indicate a sicker patient cohort undergoing PCI with microaxial flow pump support compared to the trial population2174.
Current use and future research
Despite a lack of prospective data showing clinical improvement, the prophylactic use of MCS, especially microaxial flow pumps, has increased. The ongoing PROTECT IV Trial aims to determine if PCI with a microaxial flow pump is superior to PCI without it in reducing composite rates of adverse outcomes at 3-year follow-up in high-risk patients with complex CAD and reduced LVEF ≤40%.
In summary, the interventional community faces neutral or inconclusive trial results, yet MCS devices, particularly microaxial flow pumps, continue to be used broadly in complex PCI procedures75. While some experts question their cost-effectiveness, others favour their use due to anticipated haemodynamic benefits during complex PCI. The existing lack of definitive evidence underscores the need for further trials, although conducting such trials is challenging because of the perceived high risk in standard-care groups.
Consensus statement
The pathophysiological effects of MCS devices can bring safety to the procedure and reduce the consequences of complications during complex PCI. However, severe complications, particularly related to access site, may impact outcomes, and recent studies have not clearly established a morbidity or mortality benefit. Until the randomised PROTECT IV study is published, careful patient selection, personal preference, and active prevention and surveillance of vascular complications are essential for safe MCS device use.
Therefore, MCS should not be used as the default strategy for high-risk PCI in patients with severely depressed LVEF. The potential benefit of haemodynamic stability must be weighed against the risks of bleeding, vascular complications and increased contrast exposure. The selected MCS device should represent the least invasive yet haemodynamically adequate device. Retrospective analyses suggest that VA-ECMO is extremely invasive, while IABP may not provide sufficient haemodynamic stability in some cases, making microaxial pumps a suitable choice in most situations. MCS devices should be used for the shortest time necessary. For most patients, the duration is purely periprocedural, with explantation and access closure performed in the cath lab. However, in some cases, extended MCS use for several hours post-procedure will be required, necessitating intensive care unit capabilities familiar with managing MCS patients.
Postprocedural management
Antithrombotic therapy
Periprocedural intravenous anticoagulation and antiplatelet therapy should, in general, follow the current ESC/EACTS guidelines4. While PCI is possible in patients on oral anticoagulants in principle, in high-risk PCI, an interruption of these is commonly required based on the individual risk, use of large-bore access, and intensive intravenous anticoagulation during PCI76. Dual antiplatelet therapy is loaded before elective stenting4 and, after high-risk PCI, is prescribed for the usually recommended duration77. Patients with substantial individual ischaemic risk, particularly after extensive stent implantation, may benefit from more intense and/or prolonged antithrombotic strategies11.
Optimal medical therapy
Optimal medical therapy of CAD is important for symptom control and prevention of associated events11. This comprises antianginal medications, if indicated, and secondary prevention pharmacotherapy in accordance with clinical practice guidelines1178. Medication adherence is paramount to ensure maximal efficacy. HF therapy should be optimised according to guideline recommendations, ideally in consultation with an HF specialist79. The aim is to reduce mortality; improve clinical status, functional capacity and quality of life; and prevent hospitalisation79. Unless contraindicated or not tolerated, the use of inhibitors of the renin-angiotensin-aldosterone system, beta blockers, mineralocorticoid-receptor antagonists and sodium glucose transporter 2-inhibitors are indicated in all patients with HF and reduced LVEF, with additional pharmacological and/or implantable device therapies when indicated79. Since medical therapy is continuously developing, not all drugs considered to be part of optimal medical therapy today were used in the revascularisation and device trials mentioned before.
Follow-up and heart failure specialist consultation
OMT and lifestyle modification, with smoking cessation and dietary adaptations, are paramount for risk reduction and prevention of future cardiovascular events78. HF is the most common reason for hospital readmission after PCI4680. Cardiac rehabilitation is recommended by current ESC guidelines for all patients after PCI for acute infarction4. However, patients with reduced LVEF deemed ineligible for surgery who have undergone high-risk PCI are more likely to present with coexisting conditions, such as severe lung disease, liver dysfunction, chronic kidney disease, peripheral vascular disease, and cerebrovascular disease. In these circumstances, rehabilitation may need to be realistically adapted to meet individual needs5. Careful discharge planning is essential, with planned early review. Despite a successful procedure, impaired LVEF is likely to persist in this high-risk patient group and HF therapy should be adapted79. Long-term follow-up requires cardiac services with referral to specialist teams, such as HF services and cardiac device specialists.
Training and protocols
To optimise patient care, multidisciplinary and interprofessional institutional protocols for patients with reduced LVEF undergoing invasive cardiac procedures and/or requiring MCS support should be established, as has been described for cardiogenic shock in the acute setting818283. Similar to the 2020 EAPCI Core Curriculum for Percutaneous Cardiovascular Interventions, we suggest a defined minimum qualification for operators performing high-risk PCI with or without MCS support, which requires a higher level of competence in several techniques. Operators should be experienced in techniques such as radial access in general; femoral access with devices ≥10 Fr; rescue pericardiocentesis; right and left haemodynamic assessment; PCI in all cases of ACS including multivessel disease, bypass grafts, bifurcation lesions and left main disease; lesion preparation including rotational, laser and orbital atherectomy, as well as shockwave lithotripsy; invasive physiology and intracoronary imaging; strategies to manage revascularisation failure or complications; and, in particular, the use of percutaneous MCS devices84.
Gaps in knowledge
The proportion of high-risk procedures in elderly populations with complex disease, reduced LVEF causing HF, and comorbidities precluding surgical revascularisation is steadily growing. The prognostic impact of high-risk PCI in this setting of patients still warrants high-quality data. Generally, the evidence on MCS devices for elective procedures in patients with impaired LV function is controversial, with underpowered trials assessing a wide spectrum of patients19. While registries support the idea of MCS support in certain disease constellations204685, it still remains unknown whether interventional, surgical or medical treatment is the best modality. In case of circulatory support, it remains unclear whether the different types of MCS used in HF patients with high surgical risk are equivalent in terms of cardioprotection or do themselves impose negative effects. Last but not least, we do not know whether extensive revascularisation improves outcomes regarding mortality or quality of life in these patients. Based on this lack of prospective data, current ESC guidelines on myocardial revascularisation and heart failure do not provide any recommendations regarding the use of MCS when discussing revascularisation in heart failure patients479.
Conclusions
High-risk PCI in patients with reduced LVEF deemed unsuitable for surgical revascularisation is growing. Percutaneous options often entail complex revascularisation strategies requiring plaque debulking, multistent procedures, and circulatory support during extended revascularisation procedures. It is critical for an early Heart Team discussion to take place during risk stratification and management planning that extends beyond the PCI and includes optimising guideline-directed medical therapy and treating concomitant valvular heart disease. As such, establishing a more comprehensive treatment plan − assessing individual risk, identifying viable myocardium, offering complete revascularisation, determining the need for circulatory support and providing cardiac rehabilitation − is necessary for these patients (Table 2, Figure 2). Discussion by two experienced operators suggesting alternative treatment options might be helpful early in the patient assessment.
Randomised trials have often excluded these patients, making real-world registries important in capturing short-term outcomes. In the future, developing predictive risk scores will permit a more tailored and individualised patient-centred approach.
Table 2. Appropriate approach to the patient and consensus on the management plan.
| Logistics prior to high-risk PCI in patients with severely compromised LV function not suitable for surgery |
|---|
| Obtain proper patient consent after ensuring that the patient is fully informed about the substantial risk and probable benefit of the procedure |
| Offer a second opinion |
| Preload the patient with acetylsalicylic acid and a P2Y12 inhibitor prior to PCI |
| Assess kidney function and hydrate as much as possible |
| Assess access site with ultrasound |
| Assess the potential need for MCS and, if necessary, decide on timing (upfront or as bailout) and type of MCS (IABP, ECMO or microaxial flow pump) |
| Ensure availability of ICU bed in case of need for postprocedural circulatory support |
| Formally assess bleeding and ischaemic risks |
| Provide a stepwise plan for the intervention including bailout procedures |
| Logistics to be resolved prior to high-risk PCI in patients with severely compromised LV function who are not suitable for surgery. ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; ICU: intensive care unit; LV: left ventricular; MCS: mechanical circulatory support; PCI: percutaneous coronary intervention |
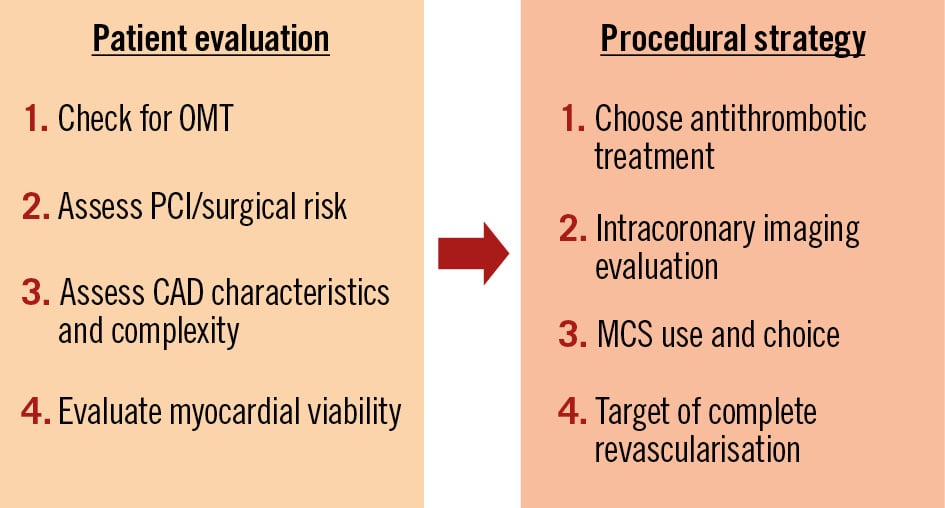
Figure 2. Stepwise clinical decision-making for high-risk PCI in patients with reduced left ventricular ejection fraction. Decision-making includes patient evaluation and procedural strategies in heart failure patients with reduced left ventricular function requiring revascularisation while being at increased risk for bypass surgery. CAD: coronary artery disease; MCS: mechanical circulatory support; OMT: optimal medical therapy; PCI: percutaneous coronary intervention
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD, PhD; Department of Cardiology and Angiology, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
A. Schäfer received honoraria from Zoll Circulation, AstraZeneca, Amgen, BMS, Pfizer, Daiichi Sankyo, and Boehringer Ingelheim; as well as an institutional grant from Abiomed. C. Collet received honoraria from HeartFlow, Pie Medical Imaging, Boston Scientific, Siemens Healthineers, CathWorks, and Abbott; as well as clinical research grants from HeartFlow and Abbott. T.K. Rudolph received honoraria from Abbott and Philips. A. Roguin received honoraria from Sanofi-Aventis, Novo Nordisk, Novartis, Biotronik, Amgen, and Boehringer Ingelheim. R. Colleran received honoraria from Medtronic; educational grants from Boston Scientific and Abbott; as well as research grants from Biosensors. G. Stefanini received honoraria from Abbott, Boston Scientific, and Pfizer/Bristol-Myers Squibb. T. Lefèvre received honoraria from Edwards Lifesciences, Abbott, Boston Scientific, Terumo, and Medtronic. N. Van Mieghem received honoraria from Abbott, Boston Scientific, Biotronik, Edwards Lifesciences, Medtronic, Abiomed, PulseCath BV, Amgen, Daiichi Sankyo, and JenaValve; as well as research grants from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, PulseCath BV, and Daiichi Sankyo. G. Cayla received honoraria from Medtronic, Edwards Lifesciences, MicroPort, Amgen, AstraZeneca, Abbott, Biotronik, Bristol-Myers Squibb, Sanofi-Aventis, and Pfizer. A. Baumbach received honoraria from Faraday, JenaValve, MicroPort, Pi-Cardia, and AstraZeneca. A. Witkowski received honoraria from Boston Scientific and Abbott. F. Burzotta received honoraria from Abiomed, Abbott, Medtronic, and Terumo. D. Capodanno received honoraria from Bayer, AstraZeneca, Amgen, Daiichi Sankyo, Boehringer Ingelheim, Biotronik, Medtronic, and Menarini; he is a minor shareholder with CERC. R. Al-Lamee received honoraria from Abbott, Menarini, and Philips. A. Banning received honoraria from Miracor, Boston Scientific, Shockwave Medical, and Abbott. P. MacCarthy received research grants from Boston Scientific; and honoraria from Edwards Lifesciences. R. Gottardi is a minor shareholder with Tevar Ltd. F.S. Schoenhoff received a research grant from Three Hearts Foundation; and honoraria from Vascular International. M. Czerny received honoraria from Terumo Aortic, Medtronic, and Endospan; and is a shareholder with TEVAR Ltd and Ascense Medical. M. Thielmann received honoraria from Cytosorbents. N. Werner received honoraria from AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Boston Scientific, Abiomed, Daiichi Sankyo, and Shockwave Medical; as well as research grants from Shockwave Medical and Abiomed. G. Tarantini received honoraria from Abbott, Abiomed, Medtronic, Boston Scientific, Edwards Lifesciences, MicroPort, and GADA. The other authors have no conflicts of interest to declare. The Guest Editor reports consultancy fees from Novartis and Meril Life Sciences; speaker honoraria from Boston Scientific, Amgen, Daiichi Sankyo, and Meril Life Sciences; speaker honoraria paid to his institution from BMS/Pfizer, Daiichi Sankyo, Boston Scientific, Siemens, and Amgen; and research grants paid to his institution from Boston Scientific and Abbott.
Supplementary data
To read the full content of this article, please download the PDF.

