Introduction
Bivalirudin, a direct thrombin inhibitor, has been extensively studied as an alternative option for anticoagulation in patients with acute coronary syndromes (ACS) undergoing percutaneous coronary intervention (PCI). Studies comparing bivalirudin to heparin yielded mixed results, with signals of concern about acute stent thrombosis; in addition, the perceived lower bleeding risk associated with bivalirudin has been attributed to the increased use of glycoprotein IIb/IIIa inhibitors (GPI) in heparin arms, suggesting potential bias in the comparison. Nevertheless, more recent trials and meta-analyses showed that bivalirudin was associated with similar or even lower risks of death and ischaemic events – including stent thrombosis – as well as less bleeding compared to heparin. In the light of accumulating evidence, whether bivalirudin should be considered as the preferred option for anticoagulation in ACS patients undergoing PCI is an area of ongoing debate.
Pros
Gregg W. Stone, MD; Oludamilola Akinmolayemi, MD, MPH
The optimal anticoagulation regimen during PCI in ST-segment elevation myocardial infarction (STEMI) and non-STEMI (NSTEMI) patients would reduce the risks of death, stent thrombosis, and reinfarction while minimising bleeding complications. The most widely used agent during PCI is unfractionated heparin, a mixture of polysaccharides of varying length that potentiates antithrombin but has many “off-target” effects, including activating platelets and binding to platelet factor 4, which can cause heparin-induced thrombocytopenia (HIT). The biological effects of heparin are also unpredictable, making this agent difficult to titrate. In contrast, bivalirudin is a small molecule that only binds to thrombin; it inhibits platelets, has highly predictable pharmacokinetic and pharmacodynamic properties, a short half-life of 15-25 minutes, and no risk of HIT.
Individual randomised control trials of anticoagulation with bivalirudin versus heparin during PCI in NSTEMI and STEMI patients have reported conflicting results. Early studies compared bivalirudin use in the catheterisation laboÂratory only to heparin plus the routine use of a GPI with an extended post-PCI infusion. In NSTEMI patients, bivalirudin reduced major bleeding and demonstrated similar rates of mortality, stent thrombosis and reinfarction. In higher-risk STEMI patients, bivalirudin reduced mortality and major bleeding; however, stent thrombosis was increased in the first 24 hours after discontinuing bivalirudin, perhaps unsurprisingly, given the lack of antithrombin activity in this early high-risk period1. Subsequent studies independently suggested that 1) routine use of GPI with heparin was no longer necessary with dual antiplatelet therapy and only served to increase bleeding and 2) a 2- to 4-hour “high-dose” post-PCI infusion of bivalirudin could eliminate the greater risk of stent thrombosis in STEMI patients without increasing bleeding. The recent BRIGHT-4 trial was thus performed in which 6,016 Chinese patients with STEMI undergoing primary PCI (PPCI) were randomised to heparin with provisional GPI use (reserved for thrombotic complications) versus bivalirudin with a median 3-hour post-PCI infusion2. Bivalirudin reduced the rates of 30-day mortality (by 25%), major bleeding, and (unexÂpectedly) stent thrombosis. In retrospect, this latter finding is not surprising given platelet activation with unfractionated heparin, an undesirable consequence that was only mitigated in earlier trials with routine GPI use. As a result, the most recent European Society of Cardiology (ESC) guidelines recoÂgnise bivalirudin as an alternative to heparin during PPCI in STEMI (class IIa) patients but suggest that the BRIGHT-4 results be validated in a non-Asian population to ensure their generalisability3.
How do we reconcile these conflicting results? On reflection, clear answers were already present in the earlier randomised trials but were not evident given the numerous different heparin and bivalirudin regimens allowed. We thus performed an individual patient data pooled analysis of all the large trials of bivalirudin versus heparin that had been completed prior to BRIGHT-4. In patients with non-STEMI (5 trials, 12,155 randomised patients), bivalirudin, compared with heparin, decreased major bleeding by 41%, with similar rates of mortality and ischaemic events (including stent thrombosis) and consistent outcomes regardless of the use of a post-PCI bivalirudin infusion or routine GPI use with heparin4. In patients with STEMI (4 trials, 6,244 randomised patients), bivalirudin with a post-PCI high-dose infusion for 2-4 hours decreased cardiac mortality by 44% and major bleeding by 44%, with similar rates of stent thrombosis and reinfarction, thus validating the BRIGHT-4 results5.
Bivalirudin is now generic and thus only marginally more costly than heparin. Bivalirudin is also simple to use: cath lab use only in NSTEMI patients and cath lab plus a 2- to 4-hour post-PCI infusion at the same dose in STEMI patients. Given its proven clinical advantages, the implications are clear: its routine use in all ACS will prevent major bleeding (even with radial artery access, by preventing non-access site-related bleeding) and, in STEMI patients, will save thousands of lives. The evidence is in – the time for debate has passed!
Conflict of interest statement
G.W. Stone has received speaker honoraria from Medtronic, Pulnovo, Infraredx, Abiomed, Amgen, and Boehringer Ingelheim; has served as a consultant to Abbott, Daiichi Sankyo, Ablative Solutions, CorFlow, Cardiomech, Robocath, Miracor, Vectorious, Apollo Therapeutics, Elucid Bio, Cardiac Success, Valfix, TherOx, HeartFlow, Neovasc, Ancora, Occlutech, Impulse Dynamics, Adona Medical, Millennia Biopharma, Oxitope, HighLife, Elixir, Remote Cardiac Enablement, and Aria; and has equity/options from Cardiac Success, Ancora, Cagent, Applied Therapeutics, Biostar family of funds, SpectraWAVE, Orchestra BioMed, Aria, Valfix, and Xenter; his employer, Mount Sinai Hospital, has received research grants from Shockwave Medical, Abbott, Abiomed, BioVentrix, Cardiovascular Systems Inc, Philips, Biosense Webster, Vascular Dynamics, Pulnovo, and V-Wave. O. Akinmolayemi has no conflicts of interest to declare.
Cons
Rod H. Stables, DM, FRCP
The publication of the BRIGHT-4 trial2 has reawakened interest in bivalirudin, particularly in the setting of emergent PPCI for STEMI. The use of a postprocedural high-dose infusion of bivalirudin appears to have avoided the problems with higher rates of stent thrombosis observed with bivalirudin in earlier trials.
Studies comparing bivalirudin and heparin for systemic anticoagulation at the time of PCI have been performed since the 1990s. It is perhaps surprising that it has taken this long to identify the optimal dose regimen for bivalirudin. It may be time to re-examine and refine our approach to the use of heparin, as there may be similar scope for gain.
Both drugs are systemic anticoagulants; if we achieve the desired therapeutic effect, over the full period of clinical risk, then it seems reasonable to assume that the efficacy and safety of the agents would be comparable. Too modest an effect (or therapy over too short a duration) risks thrombotic complications; failure in the opposite direction risks bleeding. Once an adverse event of any type occurs, then others may follow. Event clustering in a single patient is well recognised and drives the association between, for example, bleeding and mortality (Figure 1).
Both drugs have limitations that demand attention to detail in clinical application. Bivalirudin has a short half-life, and a continued infusion moderates thrombotic risk in the vulnerable post-PCI phase. Heparin has a less predictable therapeutic effect. Measurement of the individual patient response (usually with activated clotting time [ACT]) and dose adjustment is vital. There is a relationship between high ACT measurements and subsequent bleeding. The only way to avoid occasional initial overdosing is to start with a modest bolus – perhaps 70 units per kilogram of body weight (u/kg) – and then to re-bolus as required, with ACT checks after initial dosing and subsequently at regular intervals. In the MATRIX and HEAT-PPCI trials, the eventual average dose used was 80 u/kg, but variations in patient response and between drug batches mean that vigilance, with personalised dosing, during the procedure is important.
As with bivalirudin, it is important that systemic anticoagulation is maintained for some hours after the procedure, and hence, a final ACT measurement at completion, with potential additional dosing to full therapeutic levels, is important for moderating the risk of acute stent thrombosis.
Heparin therapy has advantages that justify the time and effort for dose adjustment. Bivalirudin is expensive, with even the best-value generic product costing about 150 times as much as heparin; there is an even bigger cost differential in some healthcare economies. There will be further, modest cost savings, derived from reduced nursing care costs, and gains in patient comfort and mobility, associated with the avoidance of a peri- and post-procedural infusion.
PPCI patients will often have received heparin therapy from first responder medical teams, and mixed agent treatment can be avoided. Heparin is also the default agent in other PCI activity, and there may be some benefit and safety gain in its consistent use – in terms of uniform protocols, staff training and confidence. Finally, the heparin effect can, if required, be reversed with protamine.
It is possible that a new study − using both agents according to their ideal dosing regimen − with equivalent adjunctive antiplatelet therapy intent is now indicated. Could this be HEAT-PPCI 2?
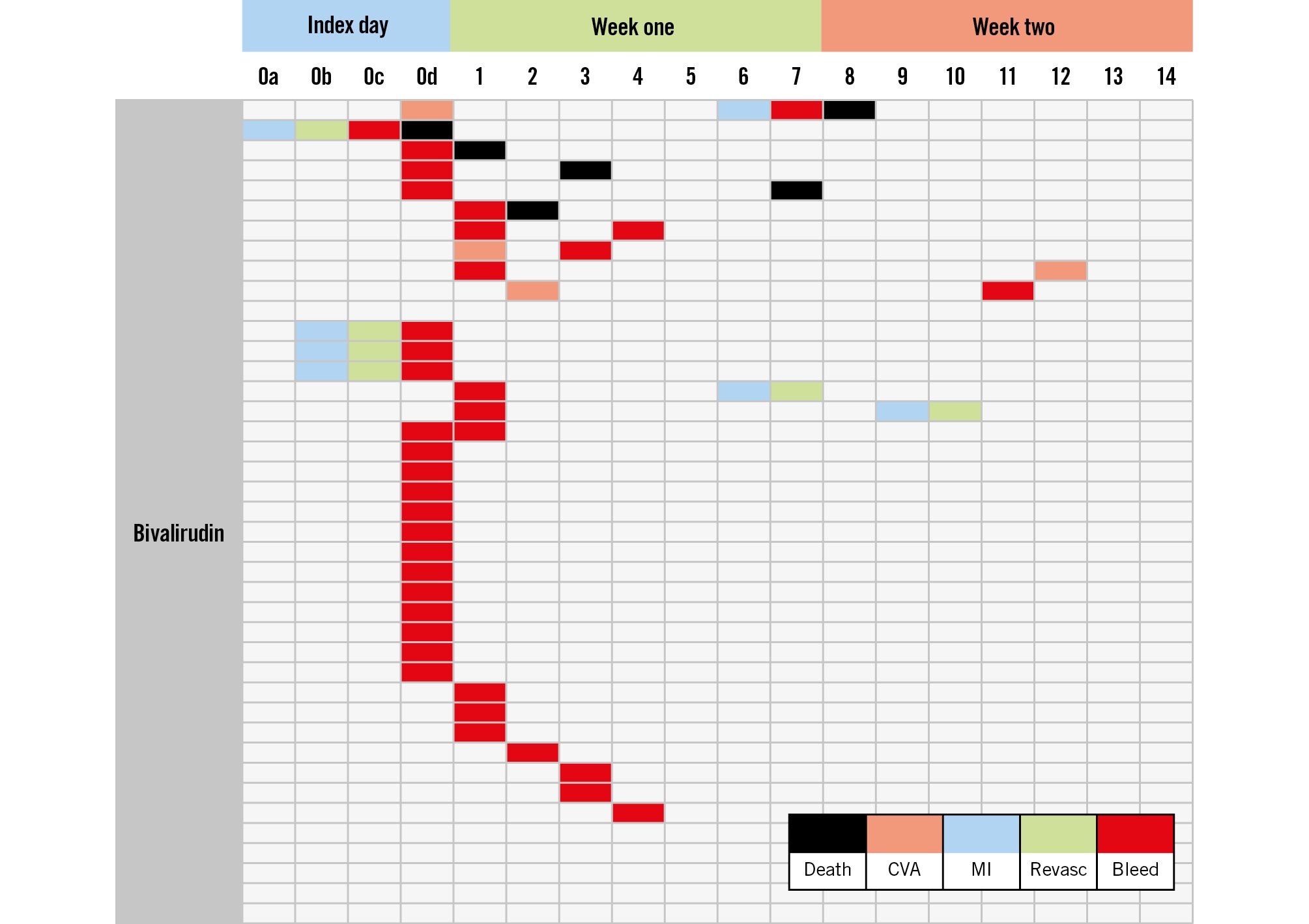
Figure 1. Adverse events up to 14 days, as observed in the bivalirudin arm of the HEAT-PPCI trial6. Some initial bleeding events are followed by ischaemic issues and vice versa. Mortality often follows another adverse event. A similar pattern is seen in the heparin arm. CVA: cerebrovascular accident; MI: myocardial infarction; Revasc: revascularisation
Conflict of interest statement
R.H. Stables has no conflicts of interest to declare.

