Introduction
Coronary angiography has long been used to guide percutaneous coronary intervention (PCI). However, it has limitations in accurately assessing atherosclerotic burden, vessel and lumen dimensions, stent morphology, and plaque characteristics. Intravascular imaging (IVI), including intravascular ultrasound (IVUS) and optical coherence tomography (OCT), has been associated with improved assessment of lesion significance, optimised stent implantation and superior stent-related outcomes. IVUS and OCT have distinct characteristics, such as penetration depth and axial resolution, that may make each of them more suitable for specific scenarios. Current evidence on PCI guidance mainly consists of studies comparing IVI to angiography, with direct comparisons of IVUS and OCT with respect to clinical endpoints being quite limited. As such, whether the use of either IVUS or OCT to guide PCI leads to better post-PCI outcomes remains an area of uncertainty.
Pros
Compared with OCT, IVUS can visualise the full thickness of the vessel wall and provide information on the appropriate device size and landing zone, even in PCI for diffuse disease. The major strength of IVUS is that it is technically easier to use, without the need for blood clearance, whereas OCT requires blood clearance. Thus, IVUS is more useful for complex lesion subsets including ostial lesions (where blood clearance is impossible), left main coronary artery lesions (multiple pullbacks are typically required and stents are often extended to the aortic ostium), severely calcified lesions (frequent in patients with renal insufficiency), and chronic total occlusions (contrast injection is frequently prohibited, because it may extend the subintimal space). Because of advances in drug-eluting stent technology, resulting in lower adverse event rates, IVI guidance for PCI has been focused on complex lesions.
The first major randomised trial to compare IVUS- versus angiography-guided PCI in complex lesions was IVUS-XPL (Impact of IntraVascular UltraSound Guidance on Outcomes of Xience Prime Stents in Long Lesions), which included 1,400 patients with an anticipated stent length ≥28 mm (Table 1)1. The primary endpoint was target lesion failure (TLF), including cardiac death, target lesion myocardial infarction, or target lesion revascularisation (TLR), at 1 year. IVUS guidance reduced the target lesion failure rate by about 50%, mainly driven by the lower TLR rate. Subsequently, the ULTIMATE Trial (Intravascular Ultrasound Guided Drug-Eluting Stents Implantation in “All-comers” Coronary Lesions), including 1,448 patients with more complex lesions, showed almost identical results, and a recent patient-level pooled analysis including both IVUS-XPL and ULTIMATE showed a reduction of 3-year cardiac mortality.
Conversely, ILUMIEN IV (the largest, and truly multicountry, trial to compare OCT versus angiography-guided PCI for complex patients [defined as those with diabetes mellitus] or complex lesions) failed to show a reduction of 2-year target vessel failure, mainly due to an almost identical rate of target vessel revascularisation in each arm. Furthermore, a recent network meta-analysis including 15,489 patients from 24 randomised trials showed more consistent benefits to support IVUS guidance, compared to OCT guidance2. This may be explained by the inclusion of a more complex lesion subset in IVUS trials compared with OCT trials, regional differences (IVUS trials in mainly Asian countries versus OCT trials in North America or Europe), or the COVID-19 pandemic; however, further clarification of which complex lesions would derive more benefit from intravascular imaging is necessary. Based on a subgroup analysis of randomised trials, complex lesion subsets may include unprotected left main disease, ostial lesions, chronic total occlusions, or severely calcified lesions, for many of which IVUS is more suitable for PCI guidance than OCT.
Though not a randomised trial, the IVUS-TRONCO-ICP study, which included 1,010 propensity-matched patients from 4 Spanish registries who underwent unprotected left main PCI, showed a clinical benefit of IVUS in reducing mortality and definite stent thrombosis, especially in patients with distal left main bifurcation lesions treated with 2-stent techniques3. Subsequently, the British Cardiovascular Intervention Society's national PCI database, which included 11,264 unprotected left main PCIs, showed a clear reduction of early mortality by intravascular imaging guidance (>90% of IVUS usage)4. Finally, the results of the OPTIMAL Trial (OPtimizaTIon of Left MAin PCI with IntravascuLar Ultrasound) including 800 patients with unprotected left main lesions randomised to IVUS versus angiography guidance will be available in 2025 and may provide a definitive answer on the impact of IVUS guidance in unprotected left main PCI5. The evidence for unprotected left main lesions (the most complex and high-risk lesions) is lacking in OCT guidance.
In summary, in the current, complex, contemporary PCI era, IVUS has more robust evidence to support its clinical impact than OCT.
Table 1. Summary of key randomised trials and network meta-analyses.
| Trial name | No. of patients | IVUS or OCT | TLF or TVF | TLR or TVR | TVMI | Cardiac death | Definite/ probable ST | Rate of complex lesions* |
|---|---|---|---|---|---|---|---|---|
| RESET | 543 | IVUS | 0.59 (0.28-1.24) | 0.66 (0.31-1.41) | 0% vs 0.7% | 0% vs 0.4% | 0.4% vs 0.4%† | Stent length ≥28 mm (100) |
| CTO-IVUS | 402 | IVUS | 0.35 (0.13-0.97) | 0.48 (0.17-1.42) | 0% vs 1.0% | 0% vs 1.0% | 0% vs 1.5% | CTO (100) |
| IVUS-XPL | 1,400 | IVUS | 0.50 (0.34-0.75) | 0.54 (0.33-0.89) | 0.67 (0.19-2.36) | 0.43 (0.17-1.12) | 1.00 (0.14-7.10) | Stent length ≥28 mm (100) |
| ULTIMATE | 1,448 | IVUS | 0.60 (0.42-0.87) | 0.64 (0.41-1.00) | 0.46 (0.19-1.14) | 0.68 (0.34-1.38) | 0.12 (0.02-0.99) | Mean stent length of 66 mm per patient, moderate-severe calcium (25), ULM (13), 2-stent bifurcation (13), CTO (12) |
| RENOVATE-COMPLEX PCI | 1,639 | IVUS (73%)OCT (26%) | 0.64 (0.45-0.89) | 0.69 (0.40-1.18) | 0.74 (0.45-1.22) | 0.47 (0.24-0.93) | 0.25 (0.02-2.75)‡ | Stent length ≥38 mm (55), true bifurcation (22), CTO (20), severe calcium (14), ostial lesion (15), ISR (14), ULM (12) |
| Network meta-analysis¶ | 7,189 | IVUS | 0.67 (0.56-0.80) | 0.69 (0.54-0.87) | 0.91 (0.69-1.19) | 0.57 (0.37-0.90) | 0.60 (0.35-1.05) | Not provided |
| ILUMIEN IV | 2,487 | OCT | 0.90 (0.67-1.19) | 0.99 (0.71-1.40) | 0.77 (0.48-1.22) | 0.57 (0.25-1.29) | 0.36 (0.14-0.91) | Stent length ≥28 mm (67), severe calcium (11), ISR (11), CTO (7), 2-stent bifurcation (3) |
| OCTOBER | 1,201 | OCT | 0.70 (0.50-0.98) | 0.61 (0.32-1.13) | 0.90 (0.60-1.34) | 0.53 (0.22-1.25) | 0.75 (0.17-3.34)‡ | True bifurcation lesion (100) with ULM involvement (19) |
| Network meta-analysis¶ | 4,976 | OCT | 0.77 (0.63-0.94) | 0.83 (0.63-1.09) | 0.82 (0.62-1.09) | 0.58 (0.36-0.94) | 0.49 (0.26-0.92) | Not provided |
| Data are HRs (95% confidence interval) using the longest follow-up data or Kaplan-Meier estimates if an HR was not available. HRs in bold indicate statistical significance. *The percentage is shown in parentheses; †including definite, probable, or possible ST; ‡definite ST only; ¶frequentist random-effect network meta-analysis data. CTO: chronic total occlusion; CTO-IVUS: Impact of IVUS-guided Chronic Total Occlusion InterVention With DrUg-eluting Stents on Mid-term Angiographic and Clinical Outcomes; HR: hazard ratio; ILUMIEN IV: OPtical Coherence Tomography Guided Coronary Stent IMplantation Compared to Angiography: A Multicenter Randomized TriaL in PCI; ISR: in-stent restenosis; IVUS: intravascular ultrasound; IVUS-XPL: Impact of IntraVascular UltraSound Guidance on Outcomes of Xience Prime Stents in Long Lesions; OCT: optical coherence tomography; OCTOBER: European Trial on Optical Coherence Tomography Optimized Bifurcation Event Reduction; RENOVATE-COMPLEX-PCI: Randomized Controlled Trial of Intravascular Imaging Guidance Versus Angiography-Guidance on Clinical Outcomes After Complex Percutaneous Coronary Intervention; RESET: Real Safety and Efficacy of a 3-month Dual Antiplatelet Therapy Following Zotarolimus-eluting Stents Implantation; ST: stent thrombosis; TLF: target lesion failure; TLR: target lesion revascularisation; TVF: target vessel failure; TVMI: target vessel myocardial infarction; TVR: target vessel revascularisation ULM: unprotected left main disease; ULTIMATE: Intravascular Ultrasound Guided Drug Eluting Stents Implantation in “All-comers” Coronary Lesions | ||||||||
Conflict of interest statement
A. Maehara has been a consultant for Boston Scientific, Abbott, and Philips; has been on an advisory board for SpectraWAVE; and has received honoraria from Nipro.
Cons
In this comparison of IVUS and OCT, is there equipoise? Are we providing a level playing field in terms of advantages/disadvantages for both imaging modalities? Can one really compare them? We will discuss these points below.
IS THERE EQUIPOISE?
Equipoise is defined as “... a state of genuine uncertainty on the part of the clinical investigator regarding the comparative merits of each arm in a trial”6. This can be achieved in head-to-head trials. Direct comparisons in 3,324 patients show that there is no difference between OCT and IVUS for all clinical endpoints7. Further, meta-analysis also showed that “indirect” network comparisons are consistent with direct appraisals7. Thus, at this point, one can conclude that IVUS does not have more robust data than OCT to inform PCI guidance. Yet, there is more to this discussion.
ARE WE PROVIDING A LEVEL PLAYING FIELD IN TERMS OF THE ADVANTAGES FOR BOTH IMAGING MODALITIES?
In Figure 1, several clinical scenarios are shown in which there is no equipoise, and, therefore, comparative “robust data” cannot be generated. There are imaging modality-specific trials that leverage on each technology’s advantages. For example, in the context of acute coronary syndrome with an ambiguous culprit lesion location, OCT has been assigned a Class IIb indication8. Nobody can say, therefore, that, in this context, IVUS has less robust data since its radial/axial resolution does not enable a head-to-head comparison with OCT in a clinical trial. Conversely, a zero-contrast imaging-guided PCI may be easier to set up using IVUS rather than OCT because of the intrinsic need for blood clearance (saline could be an option) with OCT. In Figure 1, we also display other potential clinical scenarios and lesion subsets in which one can reasonably consider that the data are robust and provide “clinical” equivalence and some others in which one is preferred over the other. Needless to say, hybrid intravascular imaging systems (Novasight [Convavi Medical]), which are already U.S. Food and Drug Administration/Pharmaceuticals and Medical Agency approved, avoid the unnecessary discussion on which one to use, since the systems include both technologies in the same catheter. Thus, as more hybrid systems become available, and providing they claim some of the market share, this comparison will no longer be necessary.
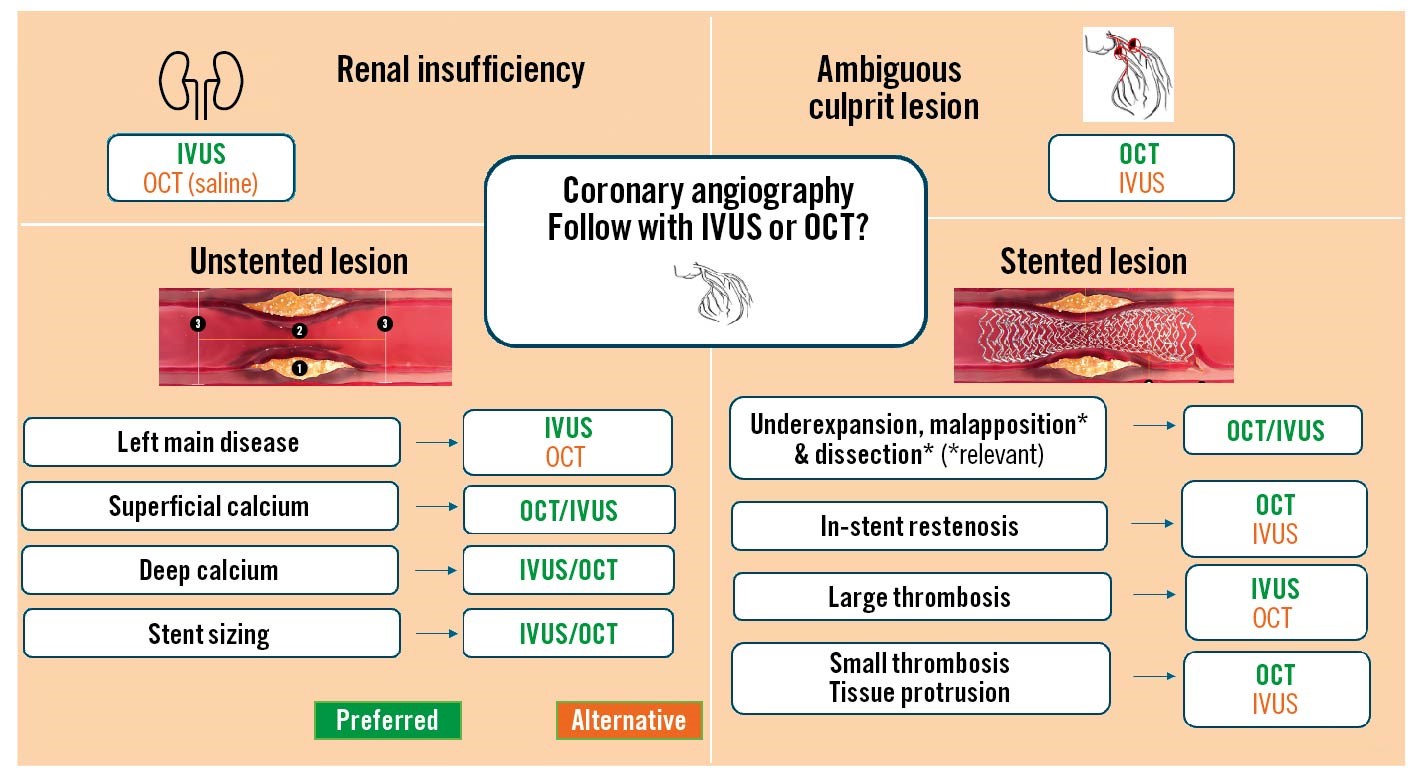
Figure 1. Clinical and morphology-based intravascular imaging selection. Intravascular ultrasound (IVUS) and optical coherence tomography (OCT) are often used to evaluate patients with renal insufficiency (upper left); since IVUS does not require contrast use, it is shown as the preferred option (green). Guidelines assigned OCT a Class IIb indication in patients with ambiguous culprit lesions (upper right). In non-stented lesions, the indication for IVUS is Class IIb9 for evaluating left main disease and, therefore, shown in green; OCT is a good alternative in this setting. IVUS is better at characterising deep calcium, but OCT can fully evaluate superficial calcium, as it often portrays the complete area and thickness (unlike IVUS, which only provides the arc of calcium). For stent sizing, there are several options: for the method that uses the diameter of the external elastic membrane, IVUS is better because of its excellent penetration; yet, for the options in which lumen diameters are used, lumen dimensions are readily and quickly available with OCT and, therefore, equivalent to IVUS. The stented lesion evaluation is also illustrated for relevant malapposition (>0.4 mm) and dissections (>60 degrees); both imaging modalities provide reasonably equivalent information, as is true for the detection of underexpansion. For in-stent restenosis, the neointima with lipid or early calcification is better evaluated with OCT. Lastly, in the presence of a large (flow-limiting) thrombus, IVUS provides good insight, but OCT is preferred for smaller thrombi and tissue protrusion because of its high resolution.
CAN ONE REALLY COMPARE IVUS TO OCT IN TERMS OF ALL AVAILABLE DATA?
For PCI guidance, the first robust OCT clinical trial took place in 2015; at that point in time, there were at least 15 years of IVUS clinical trials. Thus, undoubtedly, IVUS data contribute many more patients and studies to the totality of the evidence. Does the antiquity of IVUS make it a better clinically validated technology? Or just a good older instrument?
In conclusion, both OCT and IVUS are important in guiding PCI, and the choice between them should be individualised based on the clinical context and specific goals of the procedure. Both modalities offer unique advantages and limitations, and their complementary use can enhance the safety and effectiveness of PCI.
Conflict of interest statement
H.M. Garcia-Garcia has had grants or contracts from Medtronic, Biotronik, Abbott, Neovasc, Corflow, Alucent Biomedical, Philips, and Chiesi (paid to the institution); and has received consulting fees from Boston Scientific, Medis, Abbott, and ACIST. J. Sanz-Sánchez has received institutional/speaking fees from Boston Scientific, Medtronic, Biosensors, and Terumo.

