Cory:
Unlock Your AI Assistant Now!
The prevalence of non-valvular atrial fibrillation (NVAF) continues to rise at rates that exceed anticipated projections. The heightened stroke risk and common intolerance of anticoagulation in NVAF patients have catalysed the advent of left atrial appendage occlusion (LAAO). Following the first-in-human LAAO procedure in 2002, two LAAO devices − WATCHMAN (Boston Scientific) and Amplatzer Amulet (Abbott) − received European conformity (CE) marking in Europe in 2005 and 2008 and U.S. Food and Drug Administration (FDA) approval in the USA in 2015 and 2019, respectively. Recently, LAAO literature and volumes have surged, showcasing field progress as well as sparking debates on its appropriate use and best practices.
As organisational structure shapes behaviour, clinical practices in adopting new technology frequently follow guidelines established by professional societies. Current US and European guidelines assign a class IIb recommendation for LAAO in high-risk NVAF patients who have contraindications to long-term anticoagulation. Embedded in these guidelines are (1) the limited literature guiding LAAO use, and (2) the necessity to forecast ischaemic and bleeding risks when assessing a patient’s candidacy for LAAO. In the USA, healthcare payers mandate shared decision-making with non-LAAO implanters, along with the tracking of procedural outcomes through a national registry for reimbursement eligibility. It appears all necessary safeguards are in place for the rational dissemination of this promising technology. Why should we then question whether we are targeting the right patients? The reality is more nuanced and complex.
Risk prediction
Identifying patients at risk of stroke and bleeding is crucial for selecting the most appropriate stroke prevention strategy (Figure 1A). However, our current risk prediction tools are outdated, simplistic, and overlook essential factors known to influence both ischaemic and bleeding risks. For example, the widely used CHADS2VASC score has a modest predictive performance and does not account for the duration of atrial fibrillation (AF), left atrial (LA)/left atrial appendage (LAA) size and function, high-risk conditions (e.g., hypertrophic cardiomyopathy), or the presence of competing risks1. Indeed, the discrimination power of CHADS2VASC in predicting ischaemic stroke was found to be similar in the presence or absence of NVAF2. Similarly, the HAS-BLED score fails to account for prevalent pathologies in the NVAF population that significantly increase bleeding risks (e.g., cerebral amyloid angiopathy) or amplify the consequences of potential bleeds (e.g., frailty and fall risk).
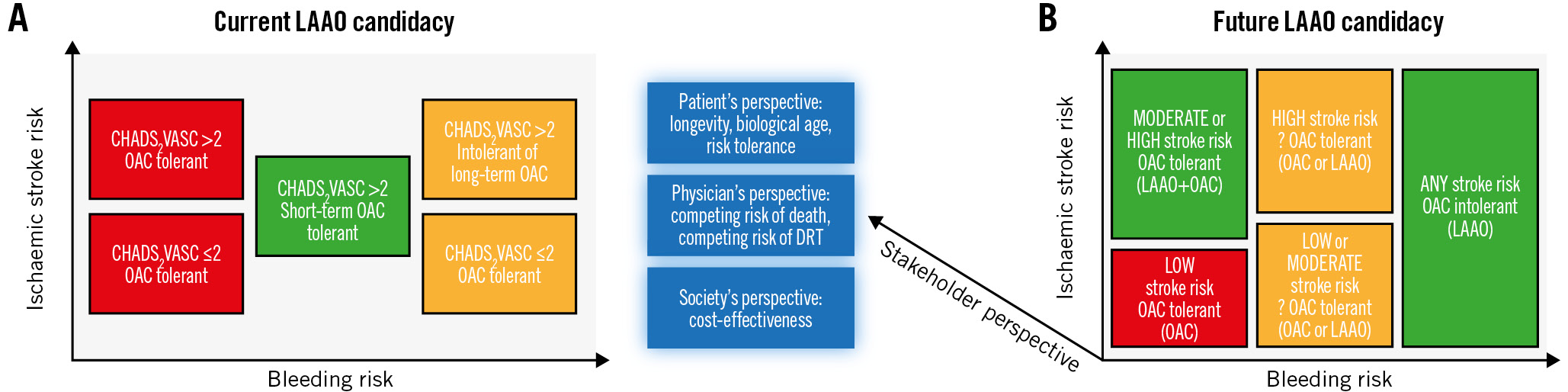
Figure 1. Current and future models of patient selection for left atrial appendage occlusion DRT: device-related thrombus; LAAO: left atrial appendage occlusion; OAC: oral anticoagulant
Stakeholder perspectives
Shared decision-making − designed to facilitate collaborative deliberation on diverse stroke prevention strategies − often fails to fully account for the values and preferences of all the involved parties3. The primary concern for patients is stroke risk, while physicians primarily prioritise considerations related to bleeding events and mortality. This complicates the elusive labelling found in the guidelines on “anticoagulation tolerance or contraindication”. Physicians and healthcare payers often view age as a crucial factor in determining eligibility for LAAO. However, relying solely on chronological age, rather than considering biological age and/or predicted longevity, may result in the exclusion of many suitable candidates4. Conversely, the reassurance derived from the reported acute safety of LAAO in national registries may overlook signals indicating futility, as evidenced by several longer-term reports showing high mid- and long-term mortality rates after the procedure. Similarly, while physicians exhibit modest concern regarding the risk of device-related thrombus, which affects at least 3-4% of patients post-LAAO, patients may find this risk considerably worse because of its associated risk of stroke and its significant management implications5.
The future
Technologies advance through cycles of progress and setbacks. In medicine, setbacks often arise from unaddressed knowledge gaps and utilisation issues rather than inherent shortcomings of the devices themselves. To ensure LAAO’s continued pivotal role in stroke prevention, several steps must be taken:
First, risk prediction schemes should evolve into comprehensive models that consider the physiological, electrical, anatomical, and comorbid factors that contribute to stroke and bleeding risks (Figure 1B).
Second, our appraisal of the utility versus futility of LAAO should be critically re-evaluated. Elderly patients with lone NVAF and a few comorbidites should not be denied the procedure based on their age alone. On the other hand, patients with multimorbidity and a high competing risk of short- and mid-term death should be counselled about the potential futility of a preventative procedure.
Third, similar to other emerging implantable devices, LAAO is currently applied in high-risk patients usually with advanced AF as an “alternative” to anticoagulation. However, if the highly anticipated CHAMPION-AF and CATALYST trials yield positive results, they may extend the eligibility for LAAO to include anticoagulation-tolerant patients. This potential expansion of indications will bring forth both opportunities and challenges, including the evaluation of the combined upfront utilisation of LAAO alongside anticoagulation as the primary strategy for stroke prevention in appropriate patients.
Fourth, stakeholder perspectives should be incorporated as a third dimension into our decision tree, alongside stroke and bleeding risks (Figure 1B). Proper accounting for the values of the main stakeholder (the patient) while considering others requires additional research on the patient’s perspective, competing risks, and cost-effectiveness.
Significant strides have been made in stroke prevention, with LAAO representing a substantial advancement in this domain. However, ensuring the future success of LAAO necessitates a retrospective examination to address lingering knowledge gaps that were overlooked during the hurried development of the procedure.
Conflict of interest statement
M. Alkhouli is on the advisory board for Abbott and Boston Scientific. D.R Holmes has no conflicts of interest to declare.

