Cory:
Unlock Your AI Assistant Now!
We recently published, in EuroIntervention Volume 20, Number 5, the rationale and design of the One-Month DAPT in CABG Patients (ODIN) trial (ClinicalTrials.gov: NCT05997693)1. ODIN is designed to address a critical gap in knowledge: whether short-term dual antiplatelet therapy (DAPT) with low-dose aspirin and ticagrelor is more effective than aspirin alone to reduce the risk of ischemic events and graft failure after coronary artery bypass graft (CABG) surgery in patients with chronic coronary syndromes while minimising the risk of bleeding associated with longer DAPT durations. The trial was initially conceived as a prospective, randomised, double-blind, placebo-controlled, international, multicentre study.
Despite our best efforts over the past year, we have encountered significant challenges in securing a suitable placebo for ticagrelor. These challenges have been multifaceted and have included difficulties identifying a manufacturer, the prohibitive cost associated with placebo production, and the lengthy time frames required for manufacturing and regulatory approval. Ultimately, these obstacles have made it unfeasible to conduct the study as a placebo-controlled trial as initially planned. This experience is not unique to ODIN; the challenges faced by investigator-initiated drug trials in acquiring placebos have been well documented2. Non-industry sponsors often lack the financial and logistical resources to match the capabilities of industry-backed trials, which raises concerns about the ability of academic investigators to generate high-quality evidence in drug trials and the industry monopoly of placebo-controlled drug trials.
In response to these challenges, we modified the trial to an open-label design (Figure 1). As the primary outcome of ODIN (a hierarchical composite of all-cause death, myocardial infarction, stroke, revascularisation, and graft failure) comprises objective clinical events that are unlikely to be affected by the placebo effect3 and assessors will be blinded to treatment allocation, the change to an open-label design will not reduce the rigour of the trial.
Despite the change in trial design, ODIN remains poised to address a critical question in the management of post-CABG patients and has the potential to significantly impact clinical practice.
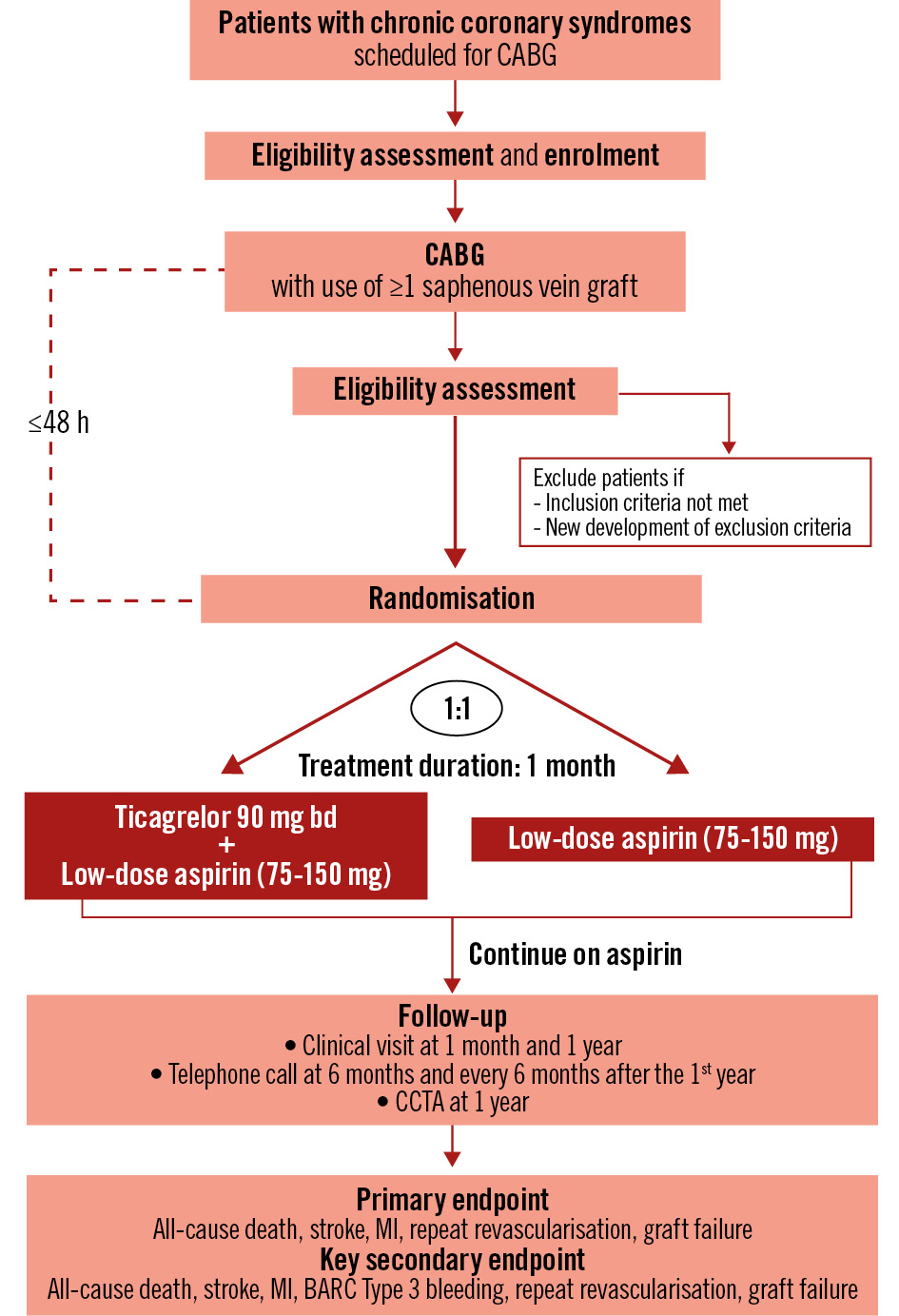
Figure 1. Study flowchart. BARC: Bleeding Academic Research Consortium; bd: twice daily; CABG: coronary artery bypass graft; CCTA: coronary computed tomography angiography; MI: myocardial infarction
Conflict of interest statement
The authors have no conflicts of interest to declare.

