Abstract
Transcatheter aortic valve implantation (TAVI) is a safe and effective procedure for the treatment of aortic stenosis. With the recently broadened indications, there is a larger cohort of patients likely to outlive their first transcatheter heart valve (THV). This review discusses relevant lifetime planning considerations, focusing on the utility of preprocedural computed tomography imaging to help implanters future-proof their patients who are likely to outlive their first valve. The initial priority is to optimise the index procedure by maximising THV haemodynamic function and durability. This involves maximising the effective orifice area, minimising the risk of new pacemaker implantation, reducing paravalvular regurgitation, and preventing coronary obstruction and annular rupture. In patients requiring a second valve procedure, a significant proportion will require a TAVI-in-TAVI, and implanters should consider the key priorities for a redo procedure, including the increased risks of patient-prosthesis mismatch and conduction abnormalities, promoting coronary reaccessibility, and preventing coronary obstruction and sinus sequestration. Careful planning can identify potential hurdles as well as predict the feasibility and likely outcomes of redo-TAVI, to help individualise care over the lifetime of each patient.
Transcatheter aortic valve implantation (TAVI) is a safe and effective procedure for symptomatic severe aortic stenosis (AS) that is increasingly offered to patients who are younger and at low surgical risk. The long-term safety and efficacy of TAVI compared to surgical aortic valve replacement (SAVR) has been demonstrated in the PARTNER 3 (5 years), Evolut Low Risk (4 years) and NOTION (10 years) studies123, whilst the recently published DEDICATE study is the first non-industry-sponsored randomised controlled trial to show non-inferiority of TAVI compared to SAVR in low- and intermediate-risk patients up to 1 year4.
The most recent American College of Cardiology/American Heart Association guidelines recommend TAVI for patients aged >80 years and suggest shared decision-making to determine the choice between TAVI and SAVR in those aged 65-80 years5. In contrast, the European Society of Cardiology/European Association of Cardio-Thoracic Surgery (ESC/EACTS) guidelines suggest TAVI in patients aged 75 years or more and SAVR in younger patients at low surgical risk, with shared decision-making in other patients according to clinical, anatomical and procedural factors6. These broadened indications have resulted in a larger cohort of patients with fewer comorbidities and longer life expectancy who are likely to outlive their first transcatheter heart valve (THV) and require a second procedure.
There is now increasing appreciation of the need for carefully planned lifetime management of AS. Whilst seemingly obvious, lifetime planning begins with optimisation of the first procedure to maximise THV haemodynamic function and durability. Precise preprocedural measurements derived from computed tomography (CT) can help implanters to choose an optimal THV to maximise the effective orifice area (EOA), minimise the need for pacemaker implantation, reduce paravalvular regurgitation, and avoid coronary obstruction and annular rupture.
In patients expected to outlive their first THV, the choice of valve as well as the depth of implantation are key to the feasibility of a redo procedure. This review discusses relevant planning considerations prior to TAVI to help implanters future-proof their patients who are likely to outlive their first valve (Central illustration). Part 1 summarises the key priorities to optimise the first implant, whilst Part 2 discusses specific TAVI-in-TAVI considerations.
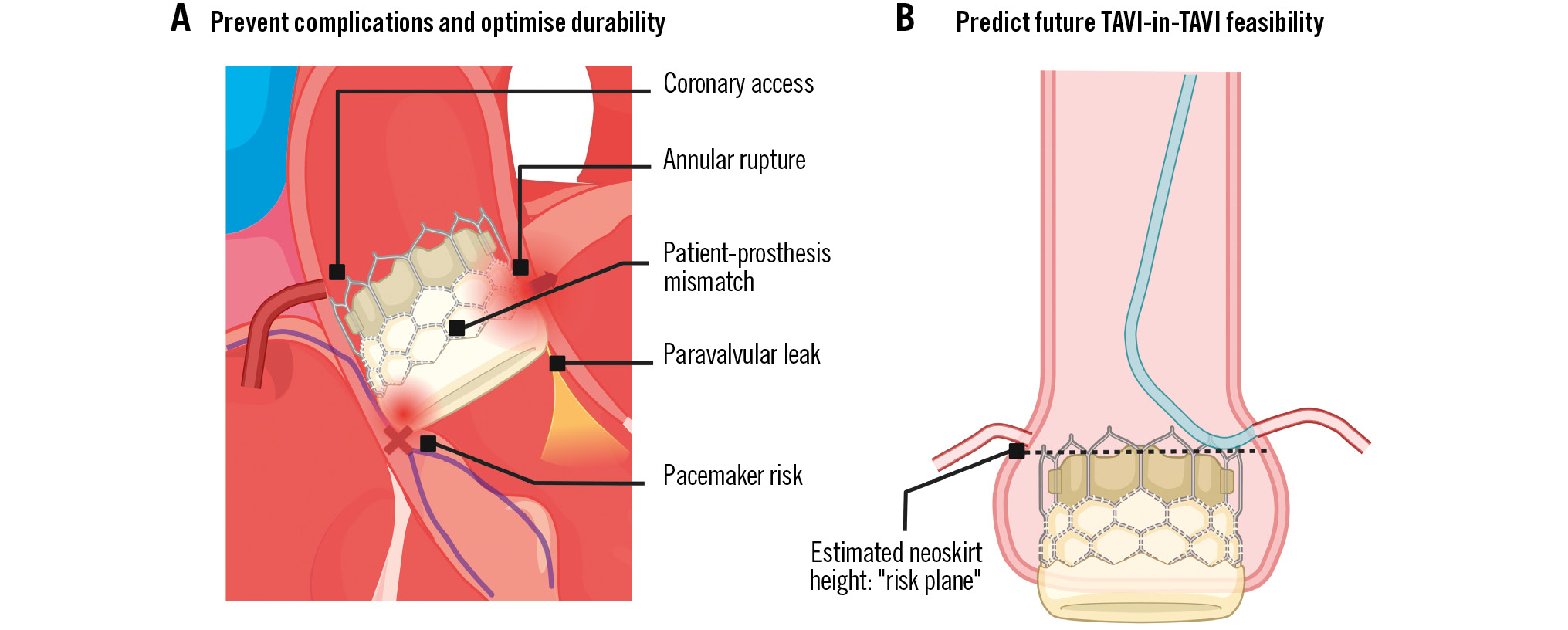
Central illustration. Index TAVI lifetime planning considerations. A) Priorities for optimising the index TAVI; (B) considerations for future TAVI-in-TAVI feasibility. TAVI: transcatheter aortic valve implantation.
Part 1: optimising the initial procedure
Patient-prosthesis mismatch
Patient-prosthesis mismatch (PPM) occurs when the prosthesis orifice area is too small for a given patient’s body size and is evaluated by indexing the EOA to the patient’s body surface area (BSA). PPM is generally defined by an indexed EOA (iEOA) <0.85 cm2/m2 (severe PPM ≤0.65 cm2/m2).
With improved THV design and more accurate CT-guided valve sizing, the incidence of severe PPM has steadily dropped, with a rate of 6.3% in current-generation devices7. Severe PPM is associated with all-cause and cardiac mortality related to poorer valve haemodynamics and elevated afterload with reduced regression of left ventricular mass8. In contrast, mild or moderate PPM does not appear to increase mortality after TAVI9.
The highest risk of PPM is in patients at extremes of body size or with very small aortic annuli10. The recently published SMART randomised control trial11 showed superiority of self-expanding valves (SEV) compared to balloon-expandable valves (BEV) in annuli ≤430 mm2 with respect to bioprosthetic valve dysfunction, although there were no reported clinical differences at 1 year.
Hahn et al (2019)12 published THV design- and size-specific post-TAVI haemodynamics, generated from core laboratory-adjudicated values for both BEV and SEV. To date, this serves as the most comprehensive reference for implanters to predict PPM after TAVI, as well as guidance on the most reproducible measurement technique. These prediction models were assessed by a large prospective Swiss registry, which found that predicted iEOA corresponded to a lower severity of PPM compared to measured iEOA but found no increased risk of death over a median follow-up of 429 days13. Other key takeaways from Hahn et al’s paper include recommendations on assessing longitudinal valve function via comparison to the patient’s prior haemodynamics rather than absolute change alone.
In smaller annuli, implanters may be influenced to choose a SEV, but in low surgical risk patients with greater life expectancy, careful consideration should also be given to SAVR with root enlargement which may help facilitate future valve-in-valve procedures14.
Paravalvular leak
The incidence of paravalvular leak (PVL) has dropped steadily over the past decade due to the use of multiplanar CT and developments in valve engineering that have improved annular sealing15. Insufficient oversizing, underexpansion, annular ellipticity as well as total or asymmetric aortic valve complex calcium have been linked with clinically significant PVL, whereas with improvements in valve skirt design, implantation depth no longer appears to be a significant predictor16. With regard to valve choice, BEV appear to have a lower incidence of PVL compared to SEV16.
Whilst there is consensus regarding reduced 5-year mortality with moderate or greater PVL, the long-term outcomes of mild PVL are unclear. Whilst some studies suggest increased mortality and rehospitalisation, others have shown no differences compared to patients with no PVL17.
Minimising conduction abnormalities
Avoidance of a pacemaker is a priority in patients with greater life expectancy, since pacemaker implantation independently increases all-cause mortality and heart failure rehospitalisation18, likely as a consequence of pacemaker-induced cardiac dyssynchrony and lead-related tricuspid regurgitation.
Careful review of the preprocedural CT allows accurate annular sizing and provides information on the risks and benefits of relative oversizing, which is associated with post-TAVI abnormalities. The membranous septum forms the non-muscular superior portion of the interventricular septum within the interleaflet triangle at the base of the non- and right coronary cusps. The bundle of His is exposed as it traverses and bifurcates along its inferior margin, and the infra-annular membranous septum length (IA-MSL) has consequently been described as a surrogate marker which may be measured on CT. Two other anatomical variants of the atrioventricular (AV)-His complex have also been described, with potential ramifications. The first (affecting 30% of individuals) was described by Kawashima and Sasaki in 200519 and may be protective since the His bundle is shielded, as it extends inferiorly into the muscular ventricular septum. In contrast, the second, less common variation (affecting 20%) may result in a perilously exposed His bundle traversing under a thin layer of subendocardium in the membranous septum – the so-called “naked” His bundle.
The IA-MSL can be accurately and reliably measured on CT, and whilst various methods exist, a reproducible technique20 involves placing the crosshair between the non- and right coronary cusp on an axial view of the aortic root in an appropriately gated systolic phase image to isolate the interleaflet triangle and visualise the membranous septum on the stretched-vessel plane (Figure 1). Absolute IA-MSL <3 mm20 and an implant depth greater than the IA-MSL21 are published predictors of pacemaker implantation after TAVI.
Valve implant depth along with left coronary cusp calcification at the left ventricular outflow tract (LVOT) level have been described as predictors of pacemaker dependency22. More than half of the patients requiring an acute permanent pacemaker after TAVI are not dependent at 1 year, secondary to recovery of the conduction pathway. The benefit of preventing pacemaker dependency is amplified in patients with longer life expectancy, as dependency has been associated with significantly increased mortality at extended follow-up23. In pacemaker-dependent patients, conduction system pacing, particularly through left bundle branch pacing, may be superior to other techniques and reduce the risk of pacemaker-induced cardiomyopathy24. SEV have historically resulted in comparatively higher pacemaker rates compared to BEV, but this gap appears to be closing with the cusp-overlap technique that facilitates an accurate and higher implantation depth25. Novel methods using preprocedural computed simulation may further assist in predicting the risk of post-TAVI conduction abnormalities as well as PVL. Existing studies have simulated future THV force on the LVOT (maximum contact pressure) and the proportional area subjected to that force (contact pressure index), with good correlation between these indices and the likelihood of pacemaker implantation and appropriate valve sealing26.
By incorporating the IA-MSL, calcium distribution and valve oversizing with established electrocardiographic predictors (such as pre-existing right bundle branch block), operators can make an informed decision on initial valve choice and implantation depth to help reduce pacemaker rates and dependency.
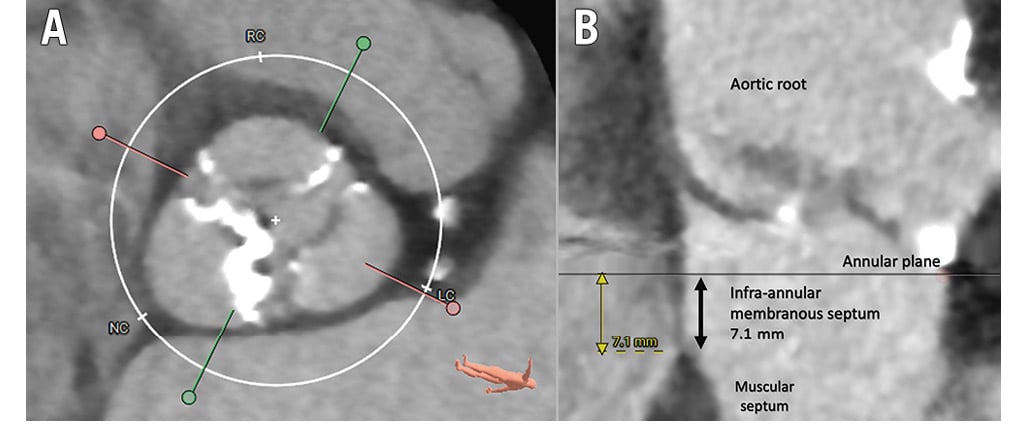
Figure 1. Identification and measurement of the infra-annular membranous septum. A) Axial view of the aortic root showing three leaflets with a crosshair between the non-coronary cusp (NC) and right coronary cusp (RC). B) Corresponding “stretched-vessel” view demonstrating the interleaflet triangle and infra-annular membranous septum. LC: left coronary cusp
Risk of coronary obstruction and feasibility of coronary access
Coronary obstruction is a rare but serious complication of TAVI that arises in 0.6% of native valve procedures27 with a 30-day mortality of up to 50%. Obstruction occurs as a result of displacement of the pre-existing native leaflet or, less commonly, occlusion by the inferior THV skirt and may be a direct consequence of leaflet-induced coronary obstruction or indirect, via sealing of the sinotubular junction (STJ) and sequestration of the coronary sinus. Anatomical risk factors include low-lying coronaries, a shallow STJ or coronary sinus, and bulky calcified leaflets, whilst procedural risk factors include high implantation depth and valve-in-valve implantation within a prior surgical bioprosthesis28. The current literature is mixed with regard to valve choice to prevent coronary obstruction2729.
Reliable prediction of coronary obstruction is nuanced and requires careful preprocedural CT planning to understand the unique interplay between the coronary ostia, sinus, leaflets and STJ in each patient (Figure 2). Furthermore, it requires appreciation of the dimensions, design, and implantation depth of the intended THV. A recently published score validated the use of three CT-derived predictors of coronary obstruction, with >90% specificity and sensitivity in patients with a coronary cusp height greater than the coronary ostial height, and a virtual valve-to-coronary (VTC) distance ≤4 mm or culprit leaflet calcification >600 mm330.
Supra-annular valves, with higher commissural posts that have a greater risk of reaching the coronary ostia and STJ, pose an increased risk of coronary obstruction compared to annular valves29. Whilst misaligned supra-annular valves (such as the Evolut [Medtronic] and ACURATE neo2 [Boston Scientific] platforms) pose the greatest danger of coronary obstruction, this risk may be partly offset via reliable commissural alignment31. Commissural alignment may be challenging in patients with eccentric coronary ostia or using the current-generation SAPIEN (Edwards Lifesciences) or Portico (Abbott) platforms. However, it may be feasible with the upcoming SAPIEN X4, which is not yet commercially available but under current evaluation in the ongoing ALLIANCE safety and efficacy study (ClinicalTrials.gov: NCT05172960).
The “snorkel” (or “chimney”) stent technique or Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) leaflet modification technique should be considered in patients with high-risk features for coronary obstruction. The “snorkel” technique maintains coronary flow via a stent protruding from the coronary ostium back into the aorta, whereas BASILICA uses electrocautery to lacerate and splay the associated aortic leaflet. Preliminary head-to-head data suggest high efficacy rates of both techniques with comparable outcomes at 1 year, but long-term data are not yet available32.
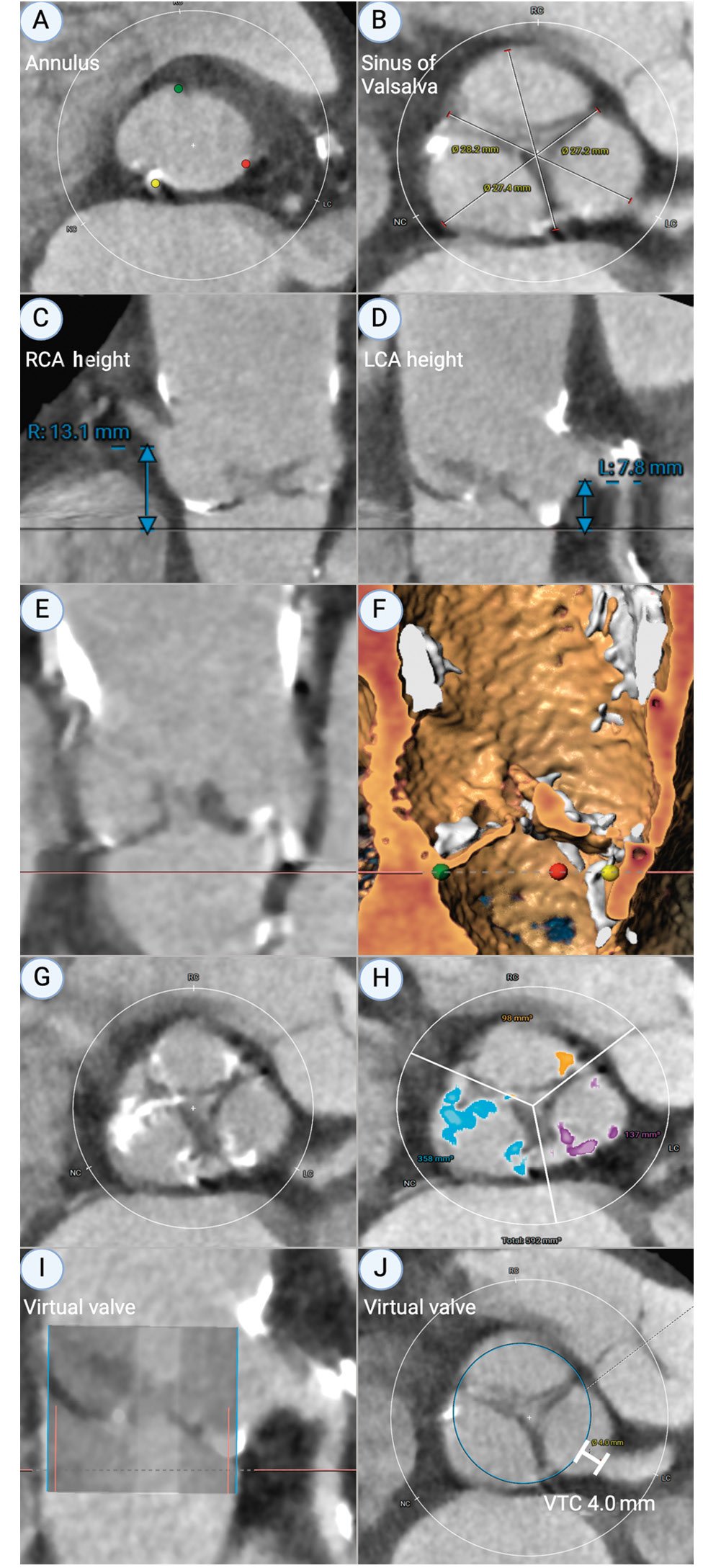
Figure 2. Relevant CT-based measurements (performed on 3Mensio [Pie Medical Imaging]) to aid implanters to determine the risk of coronary obstruction. A) Annular dimensions for valvular sizing; (B) sinus of Valsalva measurement; (C) right coronary artery (RCA) ostial height; (D) left coronary artery (LCA) ostial height; (E) stretched-vessel view of aortic root and calcium; (F) reconstruction of corresponding stretched-vessel view to visualise calcium; (G) + (H) leaflet distribution of calcium; (I) stretched-vessel view of virtual valve; (J) axial view of aortic root demonstrating virtual valve ring and VTC (valve-to-coronary) distance. CT: computed tomography; R: right; L: left
Annular rupture
Annular rupture is a rare but potentially fatal complication of TAVI that is more common with BEV33. CT sizing and anatomical characterisation with precise annular measurement have assisted in reducing its incidence.
The aortic apparatus is a dynamic structure that fluctuates in size over the course of the cardiac cycle, and the largest measurements are therefore obtained in systole. BEV are routinely sized based upon annular area and oversized by 0-10%, whereas SEV employ a perimeter-based sizing algorithm and are oversized by 10-25%. Whilst reducing paravalvular leak and optimising iEOA, excessive valve oversizing increases the risk of annular rupture.
Subannular LVOT calcification presents the greatest risk of annular rupture, especially when nodular calcification is located adjacent to the left fibrous trigone and left/right commissure34. The greatest risk is with BEV35, whereas SEV are rarely associated with annular rupture unless aggressive pre- or post-dilatation is undertaken. In this situation, balloon sizes should not exceed the mean diameter of the LVOT or STJ (whichever is smaller) with a balloon-to-artery ratio of 1 for semicompliant and <1 for non-compliant balloons35.
Figure 3 summarises a basic framework for valve preference in commonly experienced patient and anatomical scenarios.
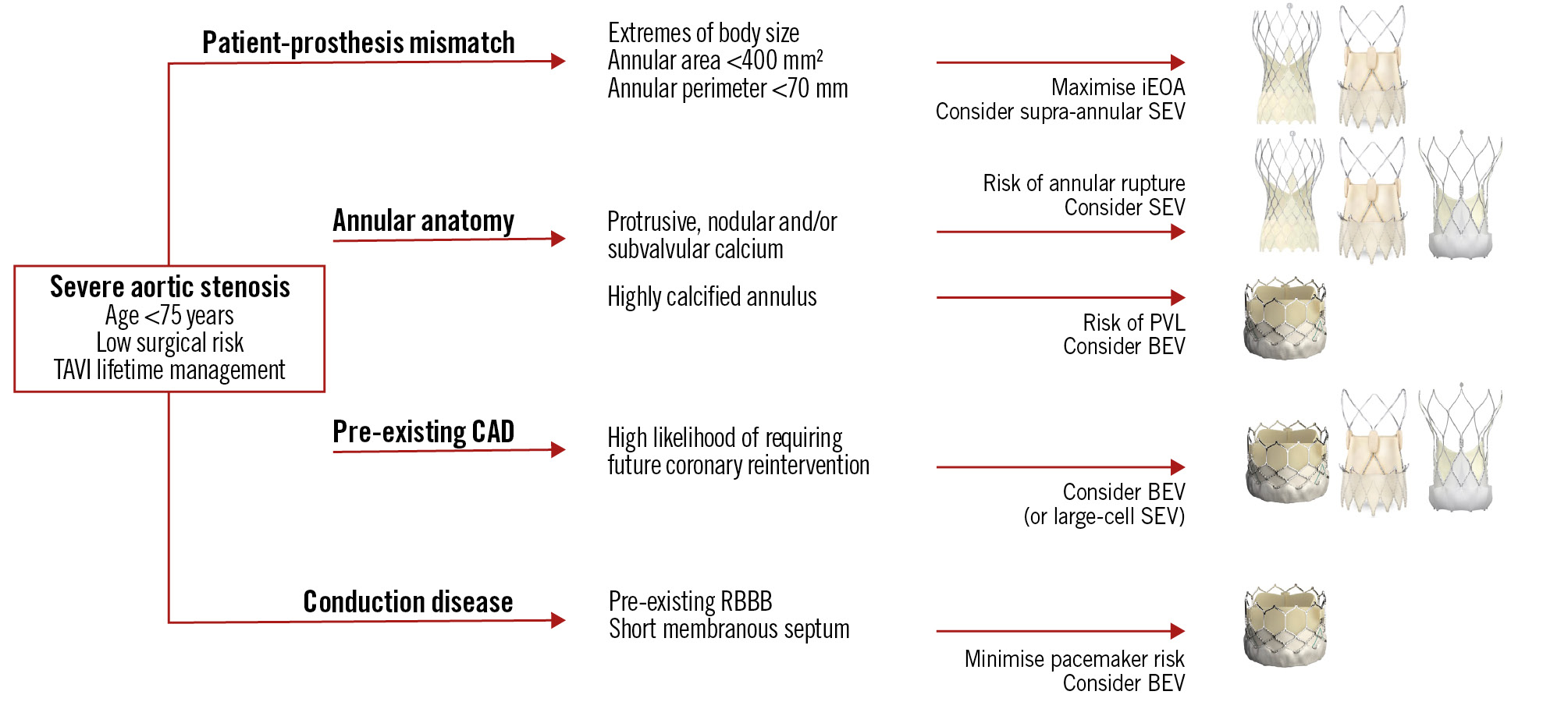
Figure 3. Commonly experienced TAVI clinical and anatomical scenarios, with the preferred valve option for each. BEV: balloon-expandable valve; CAD: coronary artery disease; iEOA: indexed effective orifice area; PVL: paravalvular leak; RBBB: right bundle branch block; SEV: self-expanding valve; TAVI: transcatheter aortic valve implantation
Leaflet thrombosis
Leaflet thrombosis is a reversible and dynamic phenomenon. Initial subclinical changes comprise hypoattenuated leaflet thickening (HALT), which may progress to restricted leaflet motion and bioprosthetic valve failure. A triad of hypercoagulability on the bioprosthetic surface, leaflet endothelial damage during device deployment, and stasis and turbulent flow may lead to HALT, which may occur in up to 12% of all TAVI cases36. A 2021 meta-analysis37 found intra-annular valves were associated with a 2-fold greater risk of subclinical leaflet thrombosis compared to supra-annular platforms. Untreated leaflet thrombosis was associated with a 2.6-fold increase in stroke, whilst a switch to anticoagulation resulted in resolution in 99% of cases. There are currently no data advocating for prophylactic anticoagulation to prevent HALT.
Predicting feasibility of TAVI-in-TAVI
In patients with extended life expectancy, it is paramount to predict the feasibility of TAVI-in-TAVI using the index CT. As Figure 4 outlines, we propose a simple framework which incorporates the key components.
As discussed earlier, accurate CT-derived annular and LVOT sizing aids in predicting PPM. Patients with a small annulus (<400 mm2) are at a particularly high risk of PPM, which may be exacerbated by TAVI-in-TAVI. In patients at low surgical risk, implanters may strongly consider a SAVR with root enlargement, to prevent the “Russian doll” effect with future TAVI-in-TAVI. Otherwise, the results of the recently published SMART study may sway implanters towards the initial use of SEV; however, an understanding of the implication on future coronary access is essential.
A future TAVI-in-TAVI will create a “neoskirt” (also known as “tube-graft”) due to pinning of the index THV leaflets. Therefore, the top of the index THV leaflets denotes a risk plane, which helps to determine the feasibility of a second THV with respect to accessing the coronary arteries. The estimated risk plane can be derived by subtracting the implantation depth from the THV leaflet height, which will differ based on valve choice, as detailed in Figure 5. The valve leaflets extend to the top of the balloon-expandable SAPIEN 3 (S3) commissure tab (top of the stent frame in SAPIEN XT) and to the top of the commissural post of self-expanding valves (Evolut R/PRO/PRO+/FX, ACURATE neo/neo2 and Portico Navitor). The self-expanding prostheses are generally taller, whilst the Evolut and ACURATE neo2 valves are supra-annular with leaflets that extend higher than the intra-annular S3 and Portico systems.
Ideally, implanters should aim to keep the risk plane below the coronary heights, or at least below the STJ, to optimise future coronary access. Figure 6 contains the highest recommended implantation depths to optimise the risk plane relative to the STJ and coronary heights when using the SAPIEN and Evolut platforms. The interaction of the planned valve with the CT-derived coronary and STJ measurements is integral in evaluating overall feasibility, which has been described by Medranda et al (2022)38 using post-TAVI CT simulation, and is further detailed in Figure 7. Depending on the software used for CT analysis, implanters can utilise a virtual circle or valve to visualise the relationship with surrounding aortic root structures.
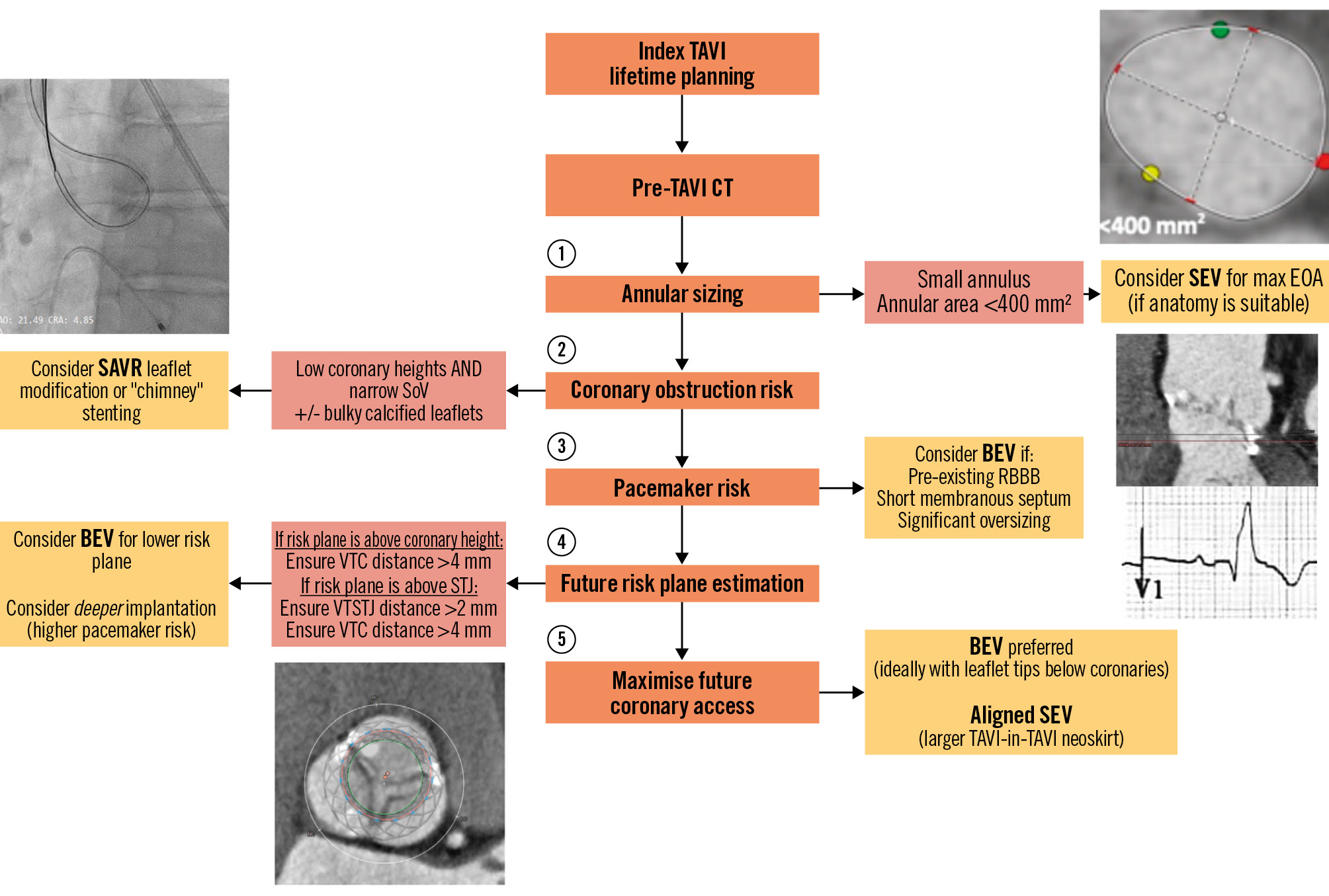
Figure 4. Stepwise approach to address factors that direct procedural planning when considering lifetime planning for patients undergoing TAVI. BEV: balloon-expandable valve; CT: computed tomography; EOA: effective orifice area; RBBB: right bundle branch block; SAVR: surgical aortic valve replacement; SEV: self-expanding valve; SoV: sinus of Valsalva; TAVI: transcatheter aortic valve implantation; VTSTJ: valve-to-sinotubular junction; VTC: valve-to-coronary
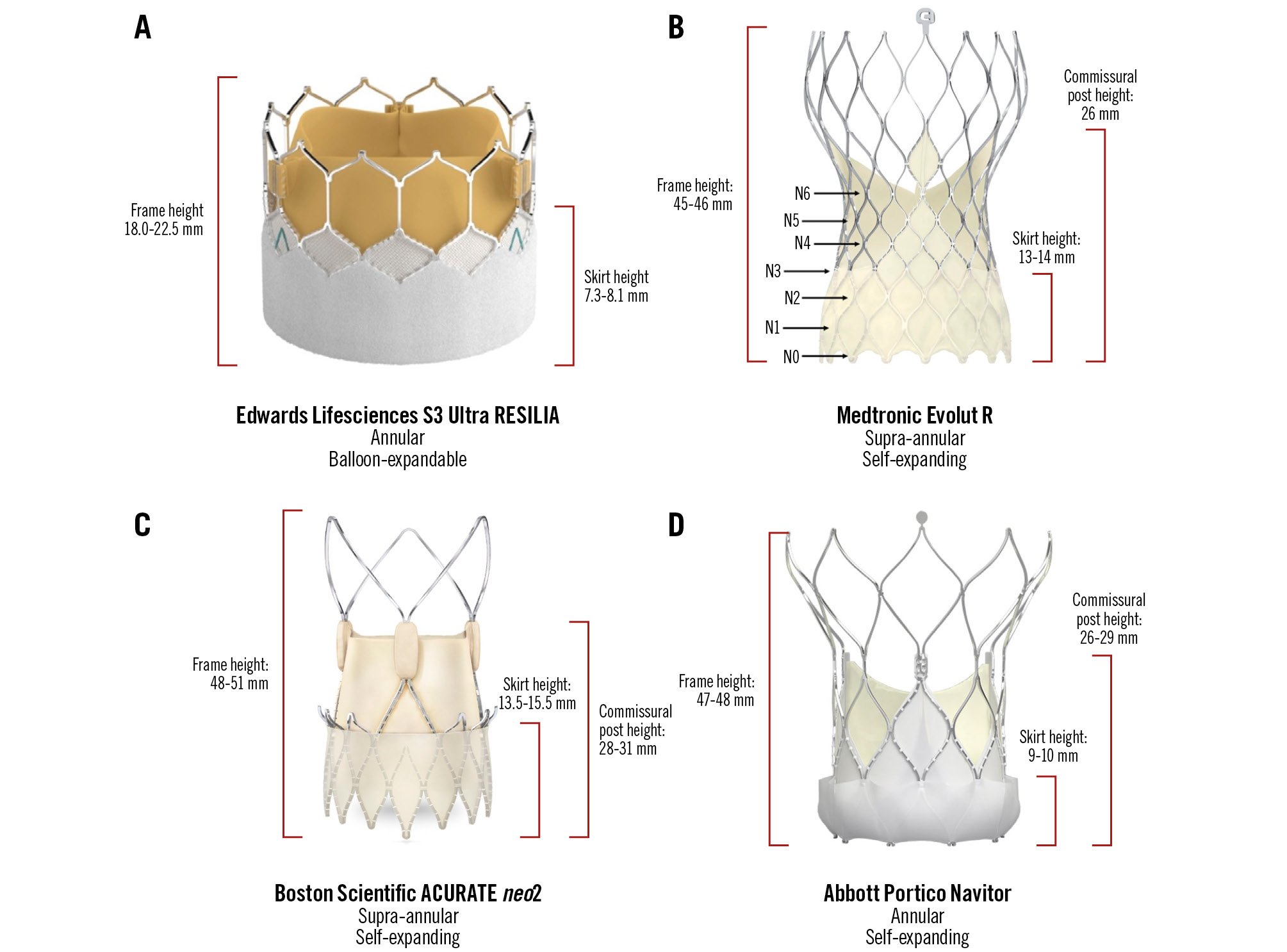
Figure 5. Modern transcatheter heart valve systems with relevant dimensions. A) Edwards Lifesciences S3 Ultra Resilia; (B) Medtronic Evolut R; (C) Boston Scientific ACURATE neo2; (D) Abbott Portico Navitor. N0-N6 refer to nodes 0-6 on the Medtronic Evolut valve, which has been used as a reference point for implant depth and second valve implant height. S3: SAPIEN 3
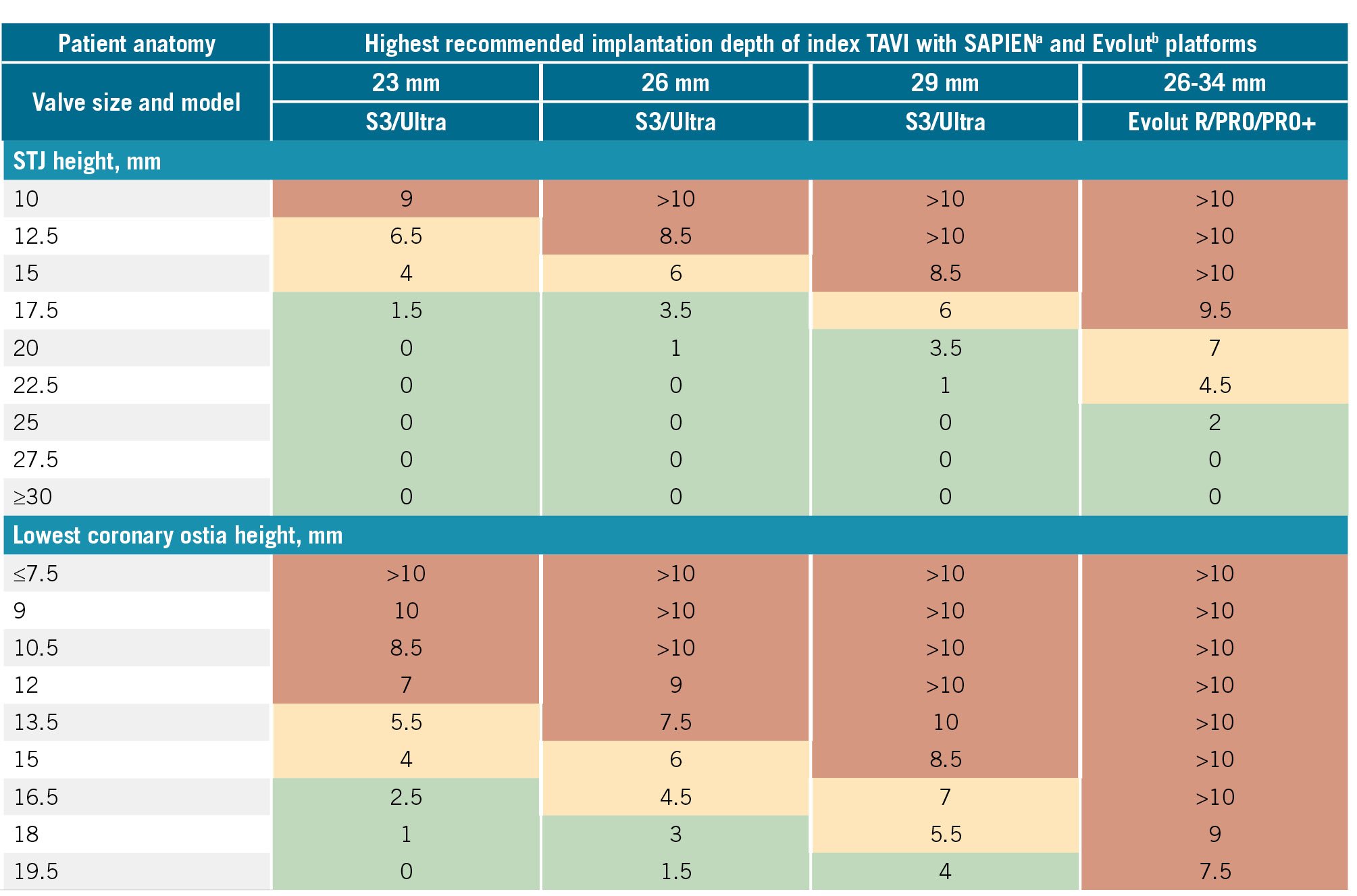
Figure 6. Recommended highest implantation depths to ensure that the index valve risk plane remains below the STJ and the coronary height. The risk plane is equal to the depth of implantation subtracted from the commissural height. Green squares indicate a relatively safe implantation depth in relation to conduction disease (<4 mm). Orange squares indicate the need for caution due to a moderately increased risk of conduction abnormalities (implant depth 4-8 mm). Red squares indicate a high risk of conduction abnormalities and/or an unfeasible implant depth (>8 mm). For orange and red squares, a VTSTJ >2 mm and VTC distance >4 mm are required to confirm the feasibility of TAVI-in-TAVI. aBy Edwards Lifesciences; bby Medtronic. S3: SAPIEN 3; STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation; VTC: valve-to-coronary; VTSTJ: valve-to-STJ; Ultra: SAPIEN Ultra
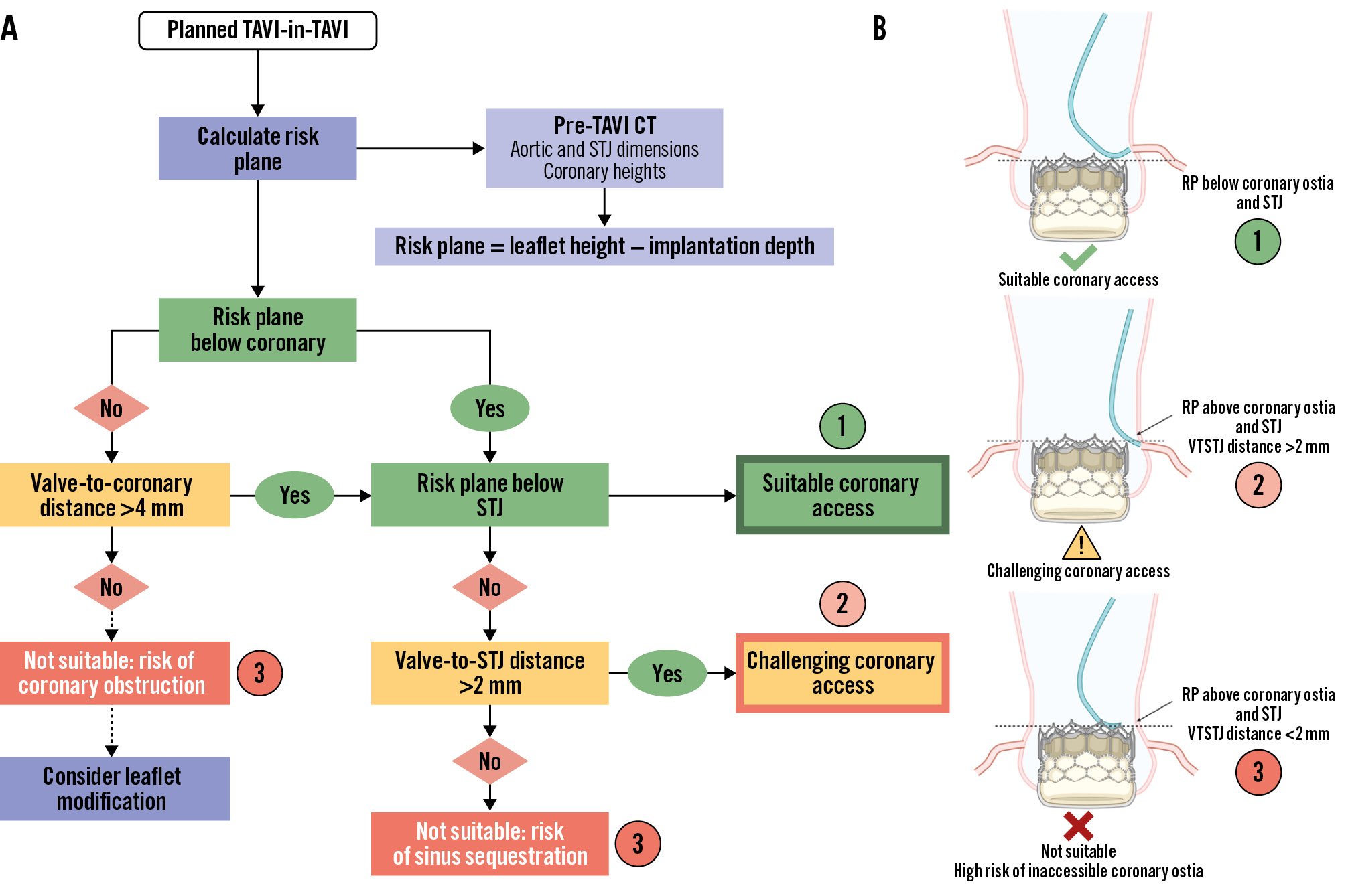
Figure 7. Framework for determining the feasibility of coronary access after TAVI-in-TAVI. A) Flowchart with relevant anatomical considerations to factor into planning TAVI-in-TAVI and coronary access. B) Illustration of various scenarios after TAVI-in-TAVI corresponding to feasibility of coronary access. CT: computed tomography; RP: risk plane; STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation; VTSTJ: valve-to-STJ
Part 2: TAVI-in-TAVI considerations
The anticipated durability of the index THV is a key consideration when planning the lifetime treatment of AS. The Valve Academic Research Consortium 3 (VARC-3) definitions of bioprosthetic valve failure include structural and non-structural valve deterioration, as well as thrombosis and endocarditis39.
The NOTION study compared the Medtronic CoreValve to SAVR and demonstrated a lower incidence of severe structural valve deterioration (SVD) following TAVI (1.5% vs 10%; p=0.02) at 10-year follow-up3. Separately, a British registry reported a 6% incidence of severe SVD 8 years after TAVI, although this figure was likely driven by a higher incidence in BEV40. Importantly, these studies assessed earlier-generation THV and are likely to overestimate the incidence of SVD associated with modern-generation THV.
Treatment options in patients with a failed TAVI include redo-TAVI or TAVI explant with subsequent SAVR. Risks associated with the latter strategy are relatively high, with a 30-day mortality of 11.9% in the EXPLANT-TAVR registry (2009-2020)41, even though the enrolled cohort were relatively complex (median Society of Thoracic Surgeons Predicted Risk of Mortality [STS-PROM] score 5.0%, urgent procedures 54%, concomitant unrelated procedure [e.g., bypass grafting or mitral valve replacement] 55%). Nevertheless, 30-day mortality was significantly higher compared with TAVI-in-TAVI in the EXPLANTTOREDO-TAVR registry42 (13.6% vs 3.4%; p<0.001), although landmark analysis beyond 30 days showed no difference at extended follow-up.
A separate consideration is that the surgical risk of TAVI explant may be magnified when SEV are extracted due to neoendothelialisation of the taller frame within the ascending aorta41. Despite growing experience and improved operative technique, the risks associated with TAVI explant and SAVR should be carefully balanced against the feasibility of successful TAVI-in-TAVI.
Despite its relative safety, redo-TAVI is not always the optimal treatment strategy. For example, redo-TAVI is not recommended in patients with valve failure secondary to active endocarditis, although one large study has confirmed the feasibility of TAVI as a salvage procedure in healed aortic valve endocarditis43. Similarly, implantation of a second annular valve in patients with severe PPM will further reduce iEOA and is therefore relatively contraindicated. Finally, patients who need concurrent cardiac surgery or are likely to require a third THV on account of their young age may be better served by TAVI explant and SAVR.
TAVI-in-TAVI: patient-prosthesis mismatch
The risk of PPM is increased following any valve-in-valve procedure due to the “Russian doll” effect of multiple bioprosthetic valves. This risk is magnified in patients with a large BSA (particularly those with a small annulus), since an initially suboptimal iEOA will be exacerbated after implantation of a second THV. Use of an appropriately sized supra-annular THV as the second valve will provide the maximum iEOA in this situation but may risk future coronary compromise.
TAVI-in-TAVI: managing the risk plane for coronary reaccess
Failure to acknowledge the risk plane can lead to two possible adverse outcomes. The most dangerous is if the neoskirt seals or sequesters the sinus, completely occluding coronary flow. Whilst rare, this can be fatal. The other scenario is indirect coronary obstruction, whereby extension of the neoskirt above the STJ or coronary ostia creates a high risk of compromised coronary flow and future difficulty in accessing the coronary ostia44.
The optimal scenario to prevent these outcomes is to ensure the risk plane lies below the midpoint of the lowest coronary ostium. In cases where the risk plane lies above the coronary height but below the STJ, a VTC distance >4 mm is recommended. If the risk plane extends above the STJ, then a VTC distance >4 mm and a valve-to-STJ distance >2 mm are required. If these criteria are not met, there is a high risk of future coronary inaccessibility44.
To manage the risk plane and facilitate second valve choice, Grubb et al (2023)44 utilised post-TAVI CT analysis to demonstrate that the highest likelihood of preserving coronary access following redo-TAVI within an initial Evolut valve was achieved by implanting an S3 in a low position (Evolut node 4), where only 20% of cases were deemed at high risk of coronary compromise. In those with an index S3, Fukui et al (2023)45 demonstrated that second valve choice had little influence on coronary access or flow, although the use of two S3 valves resulted in a substantially higher risk of at least moderate PPM (21% vs 1%).
Ochiai et al (2023)46 compared high (1-3 mm) and low (3-5 mm) depth implantation in both SEV and BEV using postprocedural CT and confirmed that a high implant increased the risk of sinus sequestration and difficult coronary access but lowered rates of left bundle branch block, pacemaker implantation and paravalvular leak. These conflicting priorities associated with implantation depth therefore require a considered and individualised approach for each patient. For example, in certain high-risk anatomies, a slightly deeper implant may mitigate the catastrophic outcome of sinus sequestration or coronary obstruction whilst increasing the risk of conduction abnormalities. However, it must be conceded that measurement of implantation depth is often imprecise, relying on fluoroscopic estimation that is usually performed in a two-cusp view from the nadir of the non-coronary cusp to the inferior valve frame.
Although commissural alignment can help to prevent coronary obstruction during the index TAVI, reliable alignment of the second implant to the initial THV is not yet possible. In one ex vivo model, strut misalignment reduced the dimensions of the accessible cell by up to 22%, thereby increasing the difficulty of future coronary catheterisation47. Whilst ACURATE neo implantation in an S3 resulted in the largest accessible cell sizes, a misaligned Evolut-in-Evolut was associated with the smallest accessible cell sizes. The authors also highlighted the differences in neoskirt height with various valve combinations, a duo of taller-frame THV predictably producing the highest risk plane.
TAVI-in-TAVI: other considerations
A separate implication of a deeper second THV implant is leaflet overhang, with one ex vivo study demonstrating up to 94% leaflet overhang in S3 THV implanted low (node 4 – see Figure 5) within Evolut valves48. Whilst leaflet overhang was not shown to impair redo THV function ex vivo, long-term outcomes and valve durability data are not yet available.
Implanters should also consider the variable re-expansion of the index THV following a second valve implant, which may cause an inadvertent increase in the diameter of the neoskirt. By widening and drawing the index THV closer to the coronary ostia and STJ, re-expansion may exponentially increase the risk of sinus sequestration and coronary obstruction. This phenomenon seems to be most prominent when an S3 is implanted within an Evolut THV, resulting in an increase of up to 2.5 mm in the waist diameter of the initial Evolut in bench studies48.
As an additional tool for implanters, the Redo TAV application (Bapat et al, KRUTSCH) was released in April 2024, and is available for free download on all smartphone devices. It serves as an educational step-by-step walkthrough for planning and executing TAVI-in-TAVI procedures.
TAVI-in-TAVI leaflet modification
Leaflet modification has been proposed as a bailout strategy in patients with aortic valve anatomy at high risk of coronary obstruction requiring a TAVI-in-TAVI procedure. Whilst the BASILICA technique has relied on standard “off-the-shelf” equipment, specific leaflet-splitting devices may streamline the procedure, and preliminary results from a study of 60 patients validating the safety and effectiveness of the ShortCut (Pi-Cardia) device has demonstrated no mortality at 30 days, with successful leaflet splitting in all patients49.
The success of TAVI-in-TAVI BASILICA is strongly dependent on the index THV’s commissural alignment and depth of implantation. Bench testing using current-generation platforms, such as the S3 and Evolut, suggest less reliable leaflet splaying after laceration50. Furthermore, there is a risk that the index THV leaflets could be pinned or “jailed” against their frame by the second, inner THV. Unfortunately, if the THV are initially misaligned, then the original commissural posts may continue to obstruct the coronary ostia, even after successful leaflet laceration. Cautionary use of TAVI-in-TAVI BASILICA is therefore recommended, and the procedure should only be considered in highly selected patients who are unsuitable for alternative treatment.
Conclusions
The Heart Team faces several competing priorities when planning TAVI procedures that must account for individual patient anatomy and lifetime management. Whilst use of SEV may promote greater iEOA, improved haemodynamics and reduced risk of annular rupture, it may also increase the incidence of pacemaker implantation and paravalvular leak. Furthermore, initial use of a taller-framed supra-annular valve in patients likely to require TAVI-in-TAVI may create a higher risk plane that increases the subsequent risk of sinus sequestration or coronary obstruction. Conversely, whilst BEV have shorter frames, they may increase the risk of annular rupture and PPM, the latter being compounded by a subsequent TAVI-in-TAVI procedure.
As TAVI is used increasingly in younger, low surgical risk patients, there is greater onus on implanters to carefully review the index CT, with the goals of optimal initial valve haemodynamics, reducing the risk of conduction abnormalities, prevention of coronary obstruction, and facilitating acute and long-term coronary access. CT can identify potential hurdles, predict the feasibility and likely outcomes of redo-TAVI, and help implanters to individualise care over the lifetime of each patient.
Conflict of interest statement
B. Prendergast has received institutional educational and research grants from Edwards Lifesciences; and has received speaker/consultancy fees from Abbott, Edwards Lifesciences, and Medtronic. V.N. Bapat has received institutional, educational and research grants, and is a consultant to Edwards Lifesciences, Medtronic, and Boston Scientific. N. Piazza is a consultant and proctor for Medtronic. The other authors have no conflicts of interest to report.

