Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Valve durability is a key consideration as the patient population eligible for transcatheter aortic valve implantation (TAVI) expands to include lower-risk and younger individuals who are expected to live many years after the procedure.
Aims: This registry aimed to assess the incidence of long-term structural valve deterioration (SVD) beyond 5 years post-TAVI.
Methods: Consecutive living patients who underwent TAVI up until 2014 using any commercially available transcatheter heart valve (THV) at 22 participant centres were enrolled in the European Valve Durability TAVI Registry. All patients underwent comprehensive echocardiographic assessments (61% were evaluated independently by a central core laboratory) within 6 months of enrolment and at least 5 years post-TAVI; SVD was defined according to Valve Academic Research Consortium 3 definitions.
Results: A total of 597 patients (aged 79.6±7.1 years at the time of TAVI; 47.2% male, mean Society of Thoracic Surgeons score 5.0%) were included. At a median of 6.1 years of follow-up (interquartile range 5.2-7.3 years), the crude incidence of moderate/severe SVD was 9.5% (n=57; moderate: 6.2%, n=37; severe: 3.4%, n=20). Predictors of SVD identified by Cox regression analysis were use of an intra-annular THV (hazard ratio [HR] 38.44, 95% confidence interval [CI]: 10.8-136.3; p<0.001), a small THV size (HR 4.82, 95% CI: 2.42-9.60; p<0.001) and moderate/severe postprocedural paravalvular leak (HR 3.64, 95% CI: 1.59-8.32; p=0.002).
Conclusions: The incidence of moderate/severe SVD during long-term follow-up after TAVI is low, with severe SVD being even rarer than moderate SVD. SVD occurs more frequently in patients treated with older-generation intra-annular valves and in those with small-sized THVs.
Valve durability is a critical concern as the population eligible for transcatheter aortic valve implantation (TAVI) expands to younger, lower-risk individuals with longer life expectancies1234. As the number of younger patients undergoing TAVI increases, it becomes essential to have reliable, long-term durability data for various types of transcatheter heart valves (THVs) to guide lifetime management decisions effectively.
While studies show preserved valve function up to 5 years after TAVI5678910, limited data exist on long-term durability beyond this period, primarily because of the competing risk of death111213 and the generally elderly patient population in earlier studies14. Inconsistent definitions of structural valve deterioration (SVD) in past trials have created uncertainty about its true incidence, and retrospective analyses often lacked crucial evaluations.
The European Valve Durability TAVI Registry was established to address these issues, with the primary aim of prospectively assessing SVD prevalence in a living patient cohort who had undergone TAVI at least 5 years previously. The registry incorporated the findings of echocardiography performed at the time of enrolment to ensure accurate and comprehensive assessment of THV function and detailed characterisation of SVD.
Methods
Study population
The European Valve Durability TAVI Registry was an international, longitudinal, multicentre, observational study conducted in European countries with the aim of collecting long-term data on the durability of TAVI from all high-volume centres in Europe that started TAVI procedures before 2014 (utilising devices available at that time). The focus of the study was on patients who had undergone TAVI at least 5 years before the launch of the registry. Inclusion was limited to those still alive at the time of enrolment, allowing a representative real-time snapshot of the TAVI population with prospective assessment of long-term echocardiographic valve function (Figure 1). These selection criteria enabled contemporary evaluation of THV performance via clinical evaluation and echocardiographic assessment during the 6-month period following enrolment of individual centres in the registry. Patients with a prior history of aortic valve intervention were excluded from the analysis.
Figure 1. Flowchart of the study population. TAVI: transcatheter aortic valve implantation
Data collection
All data were managed by the EURObservational Research Programme (EORP), which is overseen by the European Society of Cardiology (ESC). Demographic, procedural, and in-hospital outcome data were extracted from the European Valve Durability TAVI Registry database, and baseline echocardiographic data were acquired from the initial transthoracic study conducted within 1 month after the index TAVI procedure. All contributing centres were then invited to contact patients by phone to arrange a clinical visit and updated follow-up echocardiogram within 6 months of enrolment. Subsequent assessment of both baseline and follow-up echocardiograms by a central core laboratory (Rennes University Hospital Centre, France) ensured consistent reporting and allowed a more comprehensive evaluation of THV function.
Definitions
The joint ESC/European Association of Percutaneous Cardiovascular Interventions/European Association for Cardio-Thoracic Surgery (EAPCI/EACTS) definitions of SVD were the most widely used at the time of study design but were subsequently replaced by Valve Academic Research Consortium (VARC)-3 definitions that incorporate the ESC/EAPCI/EACTS principles and introduce additional criteria for more effective differentiation between SVD, non-structural valve deterioration (NSVD), and prosthesis-patient mismatch (PPM)1415. VARC-3 definitions were therefore used for the primary analysis, with secondary comparison and sensitivity analyses carried out using the original ESC/EAPCI/EACTS definitions.
The original, complete VARC-3 definition of SVD includes a concomitant decrease in Doppler velocity index. However, velocity indices were not systematically calculated in our study, and we therefore employed the modified “haemodynamic” definition, as previously applied in the NOTION trial12. The description of annular calcification on computed tomography prior to TAVI was site reported as none, mild, moderate or severe based upon the circumferential extent and the depth and thickness of calcification projecting into the left ventricular outflow tract16. Small device sizes were characterised according to THV type: SAPIEN/XT ≤23 mm (Edwards Lifesciences), CoreValve/Evolut R ≤26 mm (Medtronic), Portico ≤25 mm (Abbott), Lotus ≤23 mm (Boston Scientific), Direct Flow ≤25 mm (Direct Flow Medical)17.
Statistical analysis
Univariable analysis was applied to both continuous and categorical variables, with continuous variables reported as mean±standard deviation (SD) or median with interquartile range (IQR), and categorical variables as counts and percentages. Group comparisons used the Kruskal-Wallis test, chi2 test, or Fisher’s exact test for expected cell counts <5. Monte-Carlo estimates of exact p-values were used where necessary. For echocardiography comparisons between timepoints or investigator and core lab data, McNemar’s, Bowker’s, or the signed-rank test were applied. Cumulative incidence of SVD and 95% confidence intervals (CI) were calculated with the Kaplan-Meier method. Univariate Cox analysis of demographic, clinical, and procedural variables preceded the multivariable Cox model for SVD occurrence. Multicollinearity was checked before proceeding with the multivariable model; variables with multicollinearity issues were excluded. A backward Cox regression identified SVD predictors (p<0.05). Analysis was carried out for all devices and separately for those currently available. Model fit was assessed using concordance, the goodness-of-fit test, and Schoenfeld residuals18. SAS software version 9.4 (SAS Institute) was used.
Results
Study population
Living patients who had undergone TAVI in 2014 or earlier at 1 of 22 participating centres using any commercially available THV were included in this multicentre European registry between February 2019 and February 2020. Those who were unable to attend the reference centre for comprehensive follow-up echocardiography within 6 months of enrolment were excluded (Figure 1). The final study population with echocardiographic data extending at least 5 years after TAVI consisted of 597 patients (mean age 79.6±7.1 years at the time of the procedure; mean age 86.1±7.1 years at enrolment; 47.2% male; mean Society of Thoracic Surgeons score 5.0±3.9%). Baseline characteristics at the time of TAVI are presented in Table 1.
Table 1. Baseline characteristics of the overall population and of patients with or without moderate or severe (stage 2-3) SVD.
| Baseline characteristics | Total (n=597) | SVD (n=57) | No SVD (n=540) |
|---|---|---|---|
| Age, yrs | 79.6±7.1 | 79.2±6.6 | 79.6±7.1 |
| Male | 282 (47.2) | 21 (36.8) | 261 (48.3) |
| Logistic EuroSCORE, % | |||
| Mean±SD | 15.14±10.71 | 16.27±10.47 | 15.03±10.74 |
| Median [IQR] | 12.0 [8.0-18.7] | 12.0 [9.0-19.5] | 12.0 [8.0-18.7] |
| STS-PROM score, % | |||
| Mean±SD | 4.98±3.89 | 5.63±4.82 | 4.90±3.74 |
| Median [IQR] | 4.0 [2.7-6.0] | 4.3 [2.6-6.9] | 4.0 [2.7-5.8] |
| EuroSCORE II, % | |||
| Mean±SD | 5.17±4.62 | 4.69±4.26 | 5.21±4.65 |
| Median [IQR] | 3.7 [2.3-6.0] | 2.9 [2.1-4.9] | 3.7 [2.4-6.0] |
| Concomitant diseases at the time of TAVI | |||
| Diabetes mellitus | 157 (26.3) | 17 (29.8) | 140 (25.9) |
| Hypertension | 468 (78.5) | 49 (86.0) | 419 (77.7) |
| Dyslipidaemia | 350 (59.5) | 35 (62.5) | 315 (59.2) |
| Hyperparathyroidism | 9 (1.7) | 0 (0) | 9 (1.9) |
| GFR <30 ml/min/1.73 m2 | 57 (9.9) | 8 (15.1) | 49 (9.4) |
| Kidney failure requiring dialysis | 2 (0.3) | 1 (1.8) | 1 (0.2) |
| Chronic pulmonary disease | 94 (16.0) | 6 (10.5) | 88 (16.5) |
| Peripheral artery disease | 91 (15.4) | 9 (15.8) | 82 (15.3) |
| Coronary artery disease | 264 (44.5) | 24 (42.1) | 240 (44.8) |
| Patient history | |||
| Prior MI | 88 (15.2) | 6 (11.1) | 82 (15.6) |
| Prior TIA | 16 (2.7) | 1 (1.8) | 15 (2.8) |
| Prior stroke | 36 (6.0) | 4 (7.0) | 32 (5.9) |
| Prior TIA+stroke | 51 (8.6) | 4 (7.0) | 47 (8.7) |
| Prior pacemaker | 67 (11.2) | 6 (10.5) | 61 (11.3) |
| Prior atrial fibrillation | 120 (20.4) | 8 (14.0) | 112 (21.1) |
| NYHA Class III-IV | 346 (66.7) | 38 (70.4) | 308 (66.2) |
| Previous intervention before TAVI | |||
| Prior CABG | 106 (17.8) | 10 (17.5) | 96 (17.8) |
| Prior other cardiac surgery | 26 (4.4) | 3 (5.3) | 23 (4.3) |
| Prior PCI | 160 (27.3) | 10 (17.5) | 150 (28.3) |
| Pre-TAVI CT scan | 417 (71.0) | 41 (71.9) | 376 (70.9) |
| Mean annulus diameter, mm | 23.45±2.33 | 22.30±1.69 | 23.57±2.36 |
| Annulus perimeter, mm | 76.5±13.1 | 72.7±17.7 | 76.8±12.6 |
| Annulus area, mm² | 435.2±99.2 | 444.1±156.4 | 434.4±92.7 |
| Severe aortic valve calcification | 76 (32.2) | 13 (50.0) | 63 (30.0) |
| Eccentric valve calcification | 24 (21.1) | 0 (0) | 24 (24.0) |
| Data are presented as n (%) or mean±SD unless otherwise indicated. CABG: coronary artery bypass graft; CT: computed tomography; EuroSCORE: European System for Cardiac Operative Risk Evaluation; GFR: glomerular filtration rate; IQR: interquartile range; MI: myocardial infarction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack | |||
Procedural data and in-hospital outcomes
TAVI was performed via the transfemoral approach in 90.3% of cases, with THV distribution as follows: CoreValve (n=305, 51.2%), SAPIEN/XT (n=238, 39.9%), Lotus (n=30, 5.0%), Direct Flow (n=16, 2.7%), Portico (n=4, 0.7%), and Evolut R (n=3, 0.5%) (Table 2, Supplementary Table 1). In-hospital major stroke or transient ischaemic attack (TIA) occurred in 1.5%, major vascular complications in 3.7%, major or life-threatening bleeding in 5.5%, stage 2/3 acute kidney injury in 1.3%, and new pacemaker implantation in 11.3% (Supplementary Table 2).
Table 2. Procedural characteristics of the overall population and of patients with or without moderate or severe (stage 2-3) SVD.
| Procedural characteristics | Total (n=597) | SVD (n=57) | No SVD (n=540) |
|---|---|---|---|
| Valve type | |||
| SAPIEN/XTa | 238 (39.9) | 35 (61.4) | 203 (37.7) |
| CoreValveb | 305 (51.2) | 16 (28.1) | 289 (53.6) |
| Lotusc | 30 (5.0) | 4 (7.0) | 26 (4.8) |
| Porticod | 4 (0.7) | 0 (0) | 4 (0.7) |
| Direct Flowe | 16 (2.7) | 2 (3.5) | 14 (2.6) |
| Evolut Rb | 3 (0.5) | 0 (0) | 3 (0.6) |
| Size of the device | |||
| ≥26 mm | 466 (78.2) | 27 (47.4) | 439 (81.4) |
| Small | 233 (39.1) | 36 (63.2) | 197 (36.5) |
| Large | 363 (60.9) | 21 (36.8) | 342 (63.5) |
| Intra-annular prosthesis | 288 (48.3) | 41 (71.9) | 247 (45.8) |
| Access | |||
| Femoral | 537 (90.3) | 49 (86.0) | 488 (90.7) |
| Transapical | 23 (3.9) | 4 (7.0) | 19 (3.5) |
| Other | 35 (5.9) | 4 (7.0) | 31 (5.8) |
| General anaesthesia | 266 (48.0) | 30 (52.6) | 236 (47.5) |
| Predilatation | 429 (73.5) | 47 (85.5) | 382 (72.2) |
| Post-dilatation | 119 (21.0) | 11 (20.4) | 108 (21.0) |
| Valve malpositioning | 7 (1.2) | 0 (0) | 7 (1.3) |
| Final angiographic implantation depth, mm | 5.27±3.17 | 8.50±0.71 | 5.21±3.17 |
| Final angiographic AR | |||
| ≥Moderate | 30 (6.1) | 5 (10.0) | 25 (5.6) |
| Postprocedural echocardiographic data | |||
| Peak gradient, mmHg | 18.48±8.46 | 20.81±7.52 | 18.26±8.52 |
| Mean gradient, mmHg | 9.82±4.26 | 11.07±4.21 | 9.69±4.25 |
| Effective orifice area, cm² | 1.74±0.50 | 1.74±0.48 | 1.74±0.50 |
| PVL | |||
| None-trivial | 248 (49.9) | 22 (45.8) | 226 (50.3) |
| Mild | 218 (43.9) | 19 (39.6) | 199 (44.3) |
| Moderate | 30 (6.0) | 6 (12.5) | 24(5.3) |
| Severe | 1 (0.2) | 1 (2.1) | 0 (0) |
| Moderate or severe PVL | 31 (6.2) | 7 (14.6) | 24 (5.3) |
| Type of hospital | |||
| University | 410 (68.7) | 43 (75.4) | 367 (68.0) |
| Community or district | 187 (31.3) | 14 (24.6) | 173 (32.0) |
| Data are presented as n (%) or mean±SD. aBy Edwards Lifesciences; bby Medtronic; cby Boston Scientific; dby Abbott; eby Direct Flow Medical. AR: aortic regurgitation; PVL: paravalvular leak; SD: standard deviation; SVD: structural valve deterioration | |||
Postprocedural versus long-term follow-up echocardiography in the overall population
The median echocardiographic follow-up was at 6.1 years (IQR 5.2-7.3 years) and extended to ≥7, 8, 9 or 10 years after the TAVI procedure in 189 (31.7%), 102 (17.1%), 48 (8.0%) and 23 (3.9%) patients, respectively. Compared to postprocedural assessment, the proportion of patients with no or trivial paravalvular leak (PVL) at long-term follow-up increased (50.9% vs 59.4%; p<0.001), while the percentage with mild PVL fell (43.2% vs 34.9%; p=0.05) (Supplementary Table 3). The incidence of moderate or severe PVL remained unchanged (5.9% vs 5.7%; p=0.88), whereas the incidence of moderate or severe valvular aortic regurgitation (AR) increased over time (1.3% vs 3.4%; p=0.03), accompanied by a fall in valve area (1.8±0.5 cm2 vs 1.6±0.5 cm2; p<0.001), increased frequency of moderate to severe mitral regurgitation (11.5% vs 18.2%; p<0.001), and elevated estimated systolic pulmonary artery pressure (37.5 mmHg vs 39.9 mmHg; p=0.02).
Postprocedural versus long-term follow-up echocardiography according to THV type
Our second analysis focused exclusively on previous generations of currently available THVs, specifically comparing intra-annular (SAPIEN/XT or Portico) with supra-annular (CoreValve or Evolut R) devices, and excluded valve systems that are no longer commercially available (Lotus and Direct Flow). Regarding baseline characteristics, patients treated with supra-annular devices presented with a greater burden of comorbidities, including a higher incidence of coronary artery disease (48.4% vs 38.3%; p=0.019) and a history of myocardial infarction (17.5% vs 10.4%; p=0.02). They also experienced more cerebrovascular events (7.8% vs 3.7%; p=0.05) and had smaller native annuli as measured by computed tomography (perimeter: 75.2 mm vs 78.1 mm; p=0.006; area: 407.8 mm² vs 454.7 mm²; p<0.001). However, severe renal impairment was more prevalent in patients with intra-annular devices (14.7% vs 7.4%; p=0.006) (Supplementary Table 4).
Compared to postprocedural measurements, the peak gradient at long-term follow-up was slightly lower in patients treated with a supra-annular THV (15.6 mmHg vs 15.0 mmHg; p=0.02), while not significantly different in those with an intra-annular device (20.6 mmHg vs 23.6 mmHg; p=0.65). Consistent with these findings, the effective orifice area (EOA) remained stable in patients who received a supra-annular THV (p=0.20) but fell significantly in those with an intra-annular THV (1.9±0.5 cm2 vs 1.6±0.5 cm2; p<0.001). There was a significant increase in the frequency of moderate or severe valvular AR (p=0.03) in patients treated with an intra-annular THV that was not observed in those with a supra-annular valve (p=0.66).
The frequency of mild PVL fell over time in patients with a supra-annular THV (54.2% vs 39.6%; p<0.001), accompanied by an increase in the incidence of no ÂÂÂÂÂÂÂÂÂor trivial PVL (39.6% vs 54.6%; p<0.001), while PVL severity remained unchanged in those with an intra-annular THV (p=0.66). Finally, systolic pulmonary artery pressure increased significantly in patients with a supra-annular THV (37 mmHg vs 41 mmHg; p=0.019), while the frequency of accompanying moderate to severe mitral regurgitation (MR) increased significantly with both valve types (9.5% vs 15.2%; p=0.03, and 12.5% vs 22.5%; p<0.001, for intra-annular and supra-annular THVs, respectively) (Table 3).
Table 3. Postprocedural and long-term follow-up echocardiography according to THV type (intra-annular vs supra-annular) in patients with both evaluations.
| Intra-annular | Supra-annular | |||||
|---|---|---|---|---|---|---|
| Post-procedure (n=242) | Follow-up (n=242) | p-value | Post-procedure (n=308) | Follow-up (n=308) | p-value | |
| Peak gradient, mmHg | 20.6±7.0 | 23.6±15.8 | 0.65 | 15.6±7.3 | 15.0±9.8 | 0.021 |
| Mean gradient, mmHg | 11.3±4.0 | 13.2±9.2 | 0.35 | 8.0±3.6 | 8.1±6.5 | 0.089 |
| Effective orifice area, cm² | 1.9±0.5 | 1.6±0.5 | <0.001 | 1.7±0.4 | 1.7±0.5 | 0.20 |
| PVL | ||||||
| None-trivial | 129 (62.0) | 128 (61.5) | 0.66 | 95 (39.6) | 131 (54.6) | 0.009 |
| Mild | 68 (32.7) | 68 (32.7) | 130 (54.2) | 95 (39.6) | ||
| Moderate | 10 (4.8) | 12 (5.8) | 15 (6.3) | 13 (5.4) | ||
| Severe | 1 (0.5) | 0 (0) | 0 (0) | 1 (0.4) | ||
| Moderate or severe PVL | 11 (5.3) | 12 (5.8) | 0.78 | 15 (6.3) | 14 (5.8) | 0.85 |
| Intraprosthetic AR | ||||||
| None-trivial | 189 (92.2) | 171 (83.4) | 0.046 | 219 (91.6) | 230 (96.2) | 0.10 |
| Mild | 12 (5.9) | 21 (10.2) | 18 (7.5) | 6 (2.5) | ||
| Moderate | 3 (1.5) | 10 (4.9) | 2 (0.8) | 3 (1.3) | ||
| Severe | 1 (0.5) | 3 (1.5) | 0 (0) | 0 (0) | ||
| Moderate or severe intraprosthetic AR | 4 (2.0) | 13 (6.3) | 0.029 | 2 (0.8) | 3 (1.3) | 0.66 |
| PPM | 2 (0.9) | 6 (2.7) | 0.10 | 1 (0.4) | 2 (0.8) | 0.32 |
| Severe PPM | 1 (50) | 0 (0) | 0 (0) | 0 (0) | ||
| Late embolisation | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| LV ejection fraction, % | 57±12 | 57±10 | 0.19 | 56±11 | 54±11 | 0.08 |
| LV ejection fraction <30% | 6 (2.9) | 2 (1.0) | 0.16 | 5 (2.5) | 8 (3.9) | 0.37 |
| End-diastolic volume, ml | 96±34 | 97±40 | 0.83 | 101±36 | 91±35 | 0.013 |
| End-diastolic diameter, mm | 49±8 | 50±9 | 0.22 | 49±8 | 47±7 | 0.007 |
| End-systolic volume, ml | 43±25 | 44±24 | 0.71 | 48±35 | 41±28 | 0.11 |
| End-systolic diameter, mm | 35±14 | 34±10 | 0.89 | 33±7 | 35±10 | 0.16 |
| MR | ||||||
| None-trivial | 107 (50.7) | 81 (38.4) | 0.012 | 83 (34.6) | 80 (33.3) | 0.039 |
| Mild | 84 (39.8) | 98 (46.4) | 127 (52.9) | 106 (44.2) | ||
| Moderate | 17 (8.1) | 30 (14.2) | 28 (11.7) | 48 (20.0) | ||
| Severe | 3 (1.4) | 2 (0.9) | 2 (0.8) | 6 (2.5) | ||
| Moderate or severe MR | 20 (9.5) | 32 (15.2) | 0.028 | 30 (12.5) | 54 (22.5) | <0.001 |
| sPAP, mmHg* | 38±14 | 39±14 | 0.41 | 37±10 | 41±13 | 0.019 |
| Data are presented as n (%), n/N (%) or mean±SD. *n=90 for intra-annular THV; n=106 for supra-annular THV. AR: aortic regurgitation; LV: left ventricular; MR: mitral regurgitation; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; SD: standard deviation; sPAP: systolic pulmonary artery pressure; THV: transcatheter heart valve | ||||||
Moderate or severe SVD in the overall population
The crude incidence of moderate or severe SVD was 9.5% (n=57; moderate: 6.2%, n=37; severe: 3.4%, n=20).
The cumulative incidence of moderate or severe SVD after TAVI was as follows: 2.4% (95% CI: 1.0-3.8) at 6 years, 5.4% (95% CI: 3.0-7.7) at 7 years, 13.2% (95% CI: 8.5-17.6) at 8 years, 25.9% (95% CI: 18.2-32.9) at 9 years and 33.2% (95% CI: 23.6-41.6) at 10 years (Central illustration, Supplementary Table 5). The corresponding incidence of moderate or severe SVD according to ESC/EAPCI/EACTS definitions was 12.1% (n=72; moderate: 8.7%, n=52; severe 3.4%, n=20) (Supplementary Table 6).
Baseline clinical variables associated with moderate or severe SVD included older age at the time of TAVI (per 10-year increase: hazard ratio [HR] 1.48, 95% CI: 0.99-2.20; p=0.058), no prior percutaneous coronary revascularisation (28.3% vs 17.5%; HR 0.47, 95% CI: 0.24-0.94; p=0.034), and a small annulus dimension (mean annulus diameter 22.30±1.69 mm vs 23.57±2.36 mm; HR 0.74, 95% CI: 0.59-0.93; p=0.009) (Table 1, Supplementary Table 7).
Procedural factors included a small THV size (52.6% vs 18.6%; p<0.001), use of an intra-annular prosthesis (71.9% vs 45.8%; p<0.001) and the presence of moderate or severe PVL (14.6% vs 5.3%; p=0.008) after THV implantation (Table 2, Supplementary Table 7).
Cox regression analysis identified the use of an intra-annular THV as a strong predictor of SVD (HR 38.44, 95% CI: 10.84-136.30; p<0.001), along with the presence of moderate or severe PVL (HR 3.47, 95% CI: 1.52-7.90; p=0.003) and a small device size (HR 1.90, 95% CI: 1.04-3.49; p=0.038) (Supplementary Table 8). When comparing currently available THVs, specifically intra-annular (SAPIEN/XT or Portico) versus supra-annular (CoreValve or Evolut R) devices, the analysis confirmed intra-annular THV use as a significant predictor of SVD (HR 4.82, 95% CI: 2.42-9.60; p<0.001), accompanied by moderate or severe PVL (HR 3.64, 95% CI: 1.59-8.32; p=0.002) (Supplementary Table 9, Supplementary Table 10).
In terms of clinical outcomes after TAVI, patients who developed moderate or severe SVD had a higher rate of hospitalisation for heart failure compared to those without SVD (15.8% vs 7.1%; p=0.034). No differences were found for other major cardiovascular events, such as stroke and myocardial infarction (Supplementary Table 2).
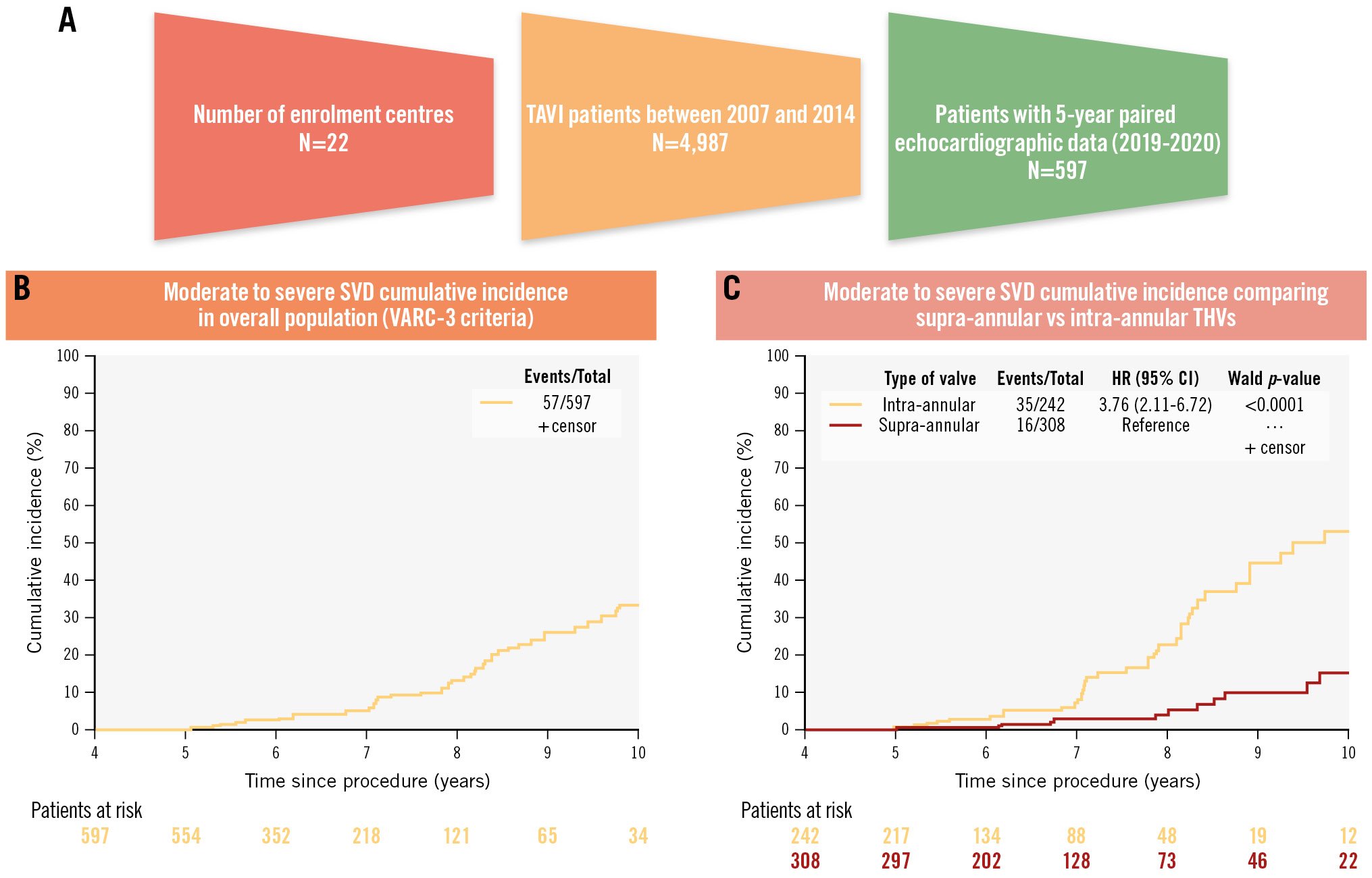
Central illustration. Cumulative incidence of moderate or severe SVD over 10-year follow-up. A) Study population. B) Cumulative incidence of moderate or severe SVD over 10-year follow-up for the entire cohort. Over a median follow-up of 6.1 years, the overall incidence of moderate to severe SVD was 9.5% (moderate: 6.2%; severe: 3.4%). C) Cumulative incidence of moderate or severe SVD over 10-year follow-up according to transcatheter heart valve design (supra-annular vs intra-annular). Moderate to severe SVD was more frequent with intra-annular than with supra-annular THVs. P-value is based on Cox regression. CI: confidence interval; HR: hazard ratio; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; VARC: Valve Academic Research Consortium
Moderate or severe SVD according to THV type and size
Our next analysis compared intra-annular (SAPIEN/XT, Portico) with supra-annular (CoreValve, Evolut R) devices. The proportion of patients with moderate or severe SVD was significantly higher in those with an intra-annular THV compared to those with a supra-annular valve (14.4% vs 5.2%; HR 3.76; p<0.0001) (Central illustration). This difference was confined to patients who received a small valve (SAPIEN/XT ≤23 mm [n=92] or Portico ≤25 mm [n=4] vs CoreValve/Evolut R ≤26 mm [n=119: 25.0% vs 5.0%; p<0.001]), and was not observed in patients with larger valves (SAPIEN/XT ≥26 mm [n=146], Portico ≥27 mm [n=0] vs CoreValve/Evolut R ≥29 mm [n=189]: 7.5% vs 5.3%; p=0.40) (Table 4, Figure 2, Supplementary Table 11).
Table 4. Echocardiography at long-term follow-up by THV size (large vs small) and type (supra-annular vs intra-annular).
| Large | Small | |||||
|---|---|---|---|---|---|---|
| Supra-annular (n=189) | Intra-annular (n=146) | p-value | Supra-annular (n=119) | Intra-annular (n=96) | p-value | |
| Peak gradient, mmHg | 15.42±10.80 | 20.10±12.75 | <0.001 | 15.43±11.48 | 28.90±17.46 | <0.001 |
| Mean gradient, mmHg | 7.97±6.34 | 11.02±7.41 | <0.001 | 8.69±8.18 | 16.27±10.30 | <0.001 |
| Effective orifice area, cm² | 1.69±0.51 | 1.74±0.49 | 0.38 | 1.71±0.48 | 1.31±0.39 | <0.001 |
| PVL | ||||||
| None-trivial | 110 (59.5) | 95 (68.8) | 0.22 | 59 (51.3) | 44 (51.2) | 0.71 |
| Mild | 64 (34.6) | 35 (25.4) | 50 (43.5) | 35 (40.7) | ||
| Moderate | 10 (5.4) | 8 (5.8) | 6 (5.2) | 7 (8.1) | ||
| Severe | 1 (0.5) | 0 (0) | 0 (0) | 0 (0) | ||
| Moderate or severe PVL | 11 (5.9) | 8 (5.8) | 0.95 | 6 (5.2) | 7 (8.1) | 0.40 |
| Intraprosthetic AR | ||||||
| None-trivial | 175 (95.6) | 127 (90.7) | 0.024 | 109 (94.8) | 61 (71.8) | <0.0001 |
| Mild | 4 (2.2) | 11 (7.9) | 5 (4.3) | 11 (12.9) | ||
| Moderate | 4 (2.2) | 1 (0.7) | 1 (0.9) | 11 (12.9) | ||
| Severe | 0 (0) | 1 (0.7) | 0 (0) | 2 (2.4) | ||
| Moderate or severe intraprosthetic AR | 4 (2.2) | 2 (1.4) | 0.70 | 1 (0.9) | 13 (15.3) | <0.001 |
| PPM | 2 (1.1) | 1 (0.7) | >0.99 | 1 (0.8) | 5 (5.4) | 0.089 |
| Severe PPM | 0 (0) | 0 (0) | 0 (0) | 1 (20.0) | ||
| LV ejection fraction, % | 52.6±11.3 | 55.4±10.1 | 0.034 | 56.6±8.9 | 58.3±9.8 | 0.14 |
| LV ejection fraction <30% | 10 (5.6) | 2 (1.4) | 0.054 | 1 (0.9) | 1 (1.1) | |
| End-diastolic volume, ml | 93.9±34.8 | 106.1±41.3 | 0.032 | 80.5±29.7 | 82.4±29.2 | 0.61 |
| End-diastolic diameter, mm | 47.8±8.1 | 49.7±7.8 | 0.057 | 45.4±6.5 | 47.4±10.3 | 0.39 |
| End-systolic volume, ml | 42.4±22.9 | 50.6±25.8 | 0.036 | 37.2±21.9 | 36.7±20.5 | 0.69 |
| End-systolic diameter, mm | 34.1±8.5 | 34.0±8.6 | 0.97 | 32.6±7.8 | 31.8±11.6 | 0.22 |
| MR | ||||||
| None-trivial | 63 (34.1) | 61 (43.3) | 0.062 | 40 (34.5) | 28 (31.8) | 0.87 |
| Mild | 78 (42.2) | 62 (44.0) | 52 (44.8) | 43 (48.9) | ||
| Moderate | 41 (22.2) | 17 (12.1) | 21 (18.1) | 16 (18.2) | ||
| Severe | 3 (1.6) | 1 (0.7) | 3 (2.6) | 1 (1.1) | ||
| Moderate or severe MR | 44 (23.8) | 18 (12.8) | 0.012 | 24 (20.7) | 17 (19.3) | 0.81 |
| sPAP, mmHg | 38.6±12.0 | 36.2±13.5 | 0.061 | 39.8±15.1 | 41.2±15.8 | 0.71 |
| Crude incidence of moderate or severe SVD | 10 (5.3) | 11 (7.5) | 0.40 | 6 (5.0) | 24 (25.0) | <0.001 |
| Data are presented as n (%) or mean±SD. AR: aortic regurgitation; LV: left ventricular; MR: mitral regurgitation; PPM: prosthesis-patient mismatch; PVL: paravalvular leak; SD: standard deviation; sPAP: systolic pulmonary artery pressure; SVD: structural valve deterioration; THV: transcatheter heart valve | ||||||
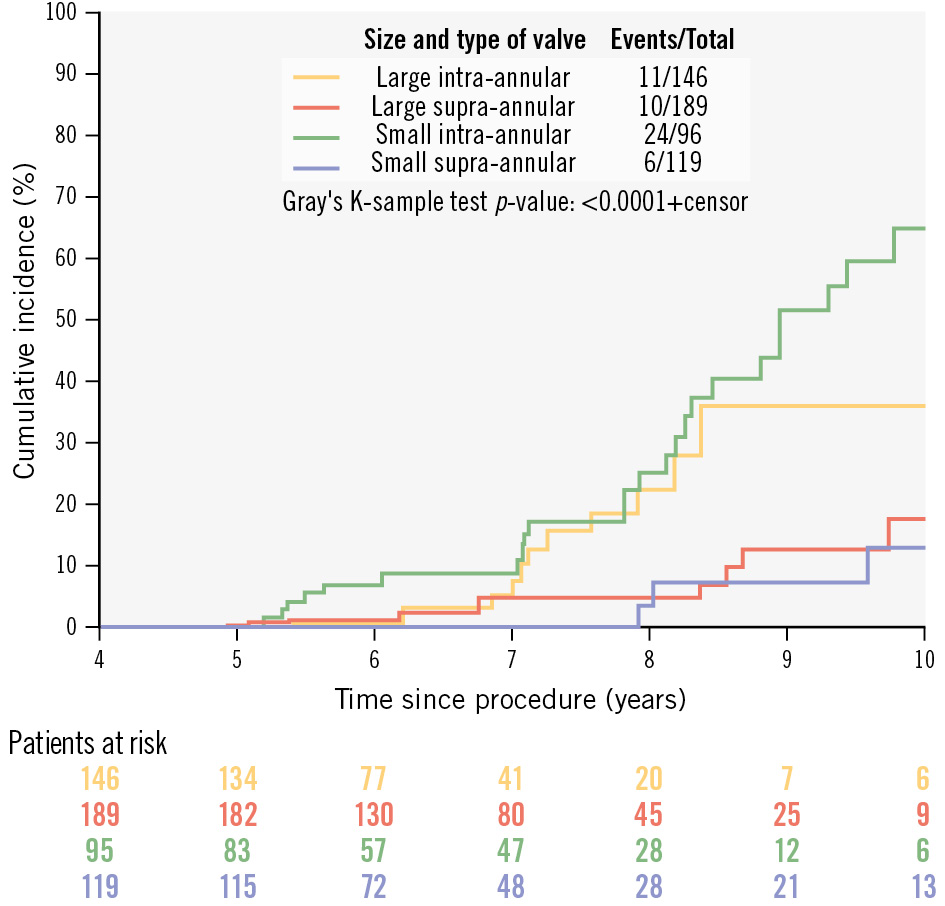
Figure 2. Cumulative incidence of moderate or severe structural valve deterioration according to transcatheter heart valve design (supra-annular vs intra-annular) and size (large vs small).
Other mechanisms of THV dysfunction
The crude incidences of thrombosis and PPM were 0.2% and 1.5%, respectively. There were no patients with infective endocarditis, cusp entrapment by pannus, aortic root dilatation, prosthesis erosion or embolism.
Core lab analysis
Independent echocardiographic analysis undertaken at the central core laboratory in a subset of 364/597 patients (61.0% of the original cohort) demonstrated a high rate of concordance (94.8%) with investigator-derived data (Supplementary Table 12).
Discussion
The durability of THVs has become a significant focus as TAVI indications extend to younger patients with extended life expectancies. The present study provides novel findings concerning the long-term durability of first- and second-generation THVs over a follow-up period of more than 5 years, based upon data from 603 patients enrolled in the European Valve Durability TAVI Registry.
The main findings of this study, based on living patients assessed at least 5 years after TAVI (with a median echocardiographic follow-up of 6.1 years [IQR 5.2-7.3 years]), are as follows:
(1) The overall long-term performance of THVs was excellent, as highlighted by the absence of a significant increase in the average peak and mean gradients, and an overall reduction in PVL. However, there was an increase in the incidence of moderate or severe AR.
(2) The crude incidence of moderate or severe SVD was relatively low (9.5%) and severe SVD was even rarer (3.4%).
(3) Moderate or severe SVD was more common in patients treated with intra-annular THVs and small device sizes, as well as those with postprocedural moderate or severe PVL.
(4) The higher incidence of SVD observed with intra-annular THVs was most notable in those patients with a small valve (with no significant difference in those with a larger device).
Comparative data
In recent years, data from various randomised studies and registries have consistently shown low rates of SVD after TAVI within a 5-year time frame. For instance, the PARTNER 1 trial assessed the first generation of balloon-expandable (intra-annular) THVs and revealed no evidence of SVD at 5-year follow-up6. The FRANCE-2 Registry (the largest midterm TAVI durability registry including over 4,000 patients with both supra-annular and intra-annular THVs) reported severe SVD in 2.5% and moderate or severe SVD in 13.3% (VARC-2 criteria) of surviving patients at 5 years19. Similarly, rates of irreversible severe SVD (according to VARC-3 definitions) were comparable at the 5-year follow-up in both treatment arms of the PARTNER 3 trial (balloon-expandable [intra-annular]: TAVI 1.1%; surgery: 1.0%)10.
Although the studies used varying definitions of SVD, its low incidence suggests that durability concerns may arise later, highlighting the need for long-term data to fully understand the implications of THV use.
However, data on THV function beyond 5 years after TAVI are very scarce, especially beyond 10 years20. Most available data come from relatively small registries and involve first-generation THVs, which report an incidence of severe SVD ranging from 2.4% to 5.9% (according to ESC/EAPCI/EACTS definitions)513212223. Specifically, the UK TAVI registry reported long-term outcomes in 221 patients (median echocardiographic follow-up of 7.0 years [IQR 5-13 years]; >10 years in 43 patients [19.5%]) with severe SVD identified in 13 (5.9%) of them at a median of 7.8 years after TAVI13. The NOTION trial included 145 patients who underwent TAVI with self-expanding (supra-annular) THVs between 2010 and 2013 and has thus far been the only randomised controlled study to provide follow-up data beyond 5 years. In this trial, the incidence of severe SVD (according to ESC/EAPCI/EACTS definitions) was lower after TAVI compared with surgical aortic valve replacement (SAVR; 13.9% vs 28.3%; p=0.0017) at 8-year follow-up with no significant difference in the incidence of bioprosthetic valve failure (BVF; 8.7% vs 10.5%; p=0.61)11. These findings remained consistent at a recently reported 10-year follow-up after application of the VARC-3 definitions, with a lower incidence of severe SVD after TAVI compared with SAVR (1.5% vs 10%; HR 0.2, 95% CI: 0.04-0.70; p=0.02) and an equivalent incidence of BVF in both treatment arms (9.7% vs 13.8%; HR 0.7, 95% CI: 0.4-1.5; p=0.4)12.
The reported incidence of SVD varies significantly across different studies despite the widespread adoption of the ESC/EAPCI/EACTS definitions. This may be due to the frequent absence of an external echocardiographic core laboratory, which increases the risk of interobserver variability, or the lack of systematic echocardiographic follow-up. These limitations potentially compromise the precise characterisation of THV dysfunction, particularly if evaluation relies upon the retrospective assessment of echocardiograms by a single individual. Furthermore, the limited number of patients available for long-term follow-up in these studies is largely due to the competing risk of death among the elderly and frail population that underwent TAVI during the early pivotal trials. This reduction in the at-risk cohort over time can bias the long-term durability data, as fewer individuals are available to assess the true longevity of the valves.
Given these considerations, our registry is unique in exclusively enrolling living patients who underwent TAVI at least 5 years previously and employing comprehensive echocardiographic evaluation to assess THV performance using VARC-3 criteria, thereby ensuring an accurate and real-time representation of SVD incidence in the TAVI population15. Furthermore, use of a centralised echocardiographic core laboratory (which analysed 61% of the studies and demonstrated a concordance rate of 95% with investigator data in defining SVD) substantially bolsters the reliability and validity of our findings.
Incidence of SVD according to type and size of THV
The incidence of moderate or severe SVD in our registry was significantly higher in patients treated with an intra-annular rather than a supra-annular THV (14.4% vs 5.2%; p<0.001). One potential explanation for this difference may be the inherent design features of the two valve systems. Supra-annular THVs generally have a greater EOA and reduced transvalvular gradients compared to intra-annular devices, with potential implications for sustained durability.
Our findings are consistent with those of the previously mentioned French registry, which demonstrated rates of moderate and severe SVD of 8.9% and 0%, respectively, at 5-year follow-up for self-expanding (supra-annular) THVs, compared with 13.8% and 4.1%, respectively, for balloon-expandable (intra-annular) devices19. In a single-centre German registry, at 7 years, the overall crude cumulative incidence of moderate or severe SVD, according to the ESC definition, was even higher at 14.9%. However, it was once again more frequent in balloon-expandable THVs compared to self-expanding THVs (22.6% vs 11.8%, respectively)23.
Similarly, in the head-to-head CHOICE trial, moderate or severe SVD was observed more frequently in patients with balloon-expandable (intra-annular) valves at 5-year follow-up (6.6% vs 0%; p=0.018)24. Finally, severe SVD was more frequent in patients with intra-annular THVs (SAPIEN/SAPIEN XT) compared with supra-annular devices (CoreValve) at a median follow-up of 7.0 years in the UK TAVI registry (11.9% vs 3.5%; p=0.02)13.
However, these observations unveil several unresolved questions regarding potential underlying mechanisms. The size of the aortic annulus is a crucial anatomical feature that significantly influences valve haemodynamics and clinical outcomes after both TAVI and SAVR, and previous research has shown that the risk of PPM is significantly higher after SAVR in patients with small annuli (and associated with adverse midterm clinical outcomes)25. Several studies have confirmed that TAVI offers an advantage in this setting, particularly when performed with a supra-annular THV262728. In our analysis, the significantly higher incidence of moderate/severe SVD affecting intra-annular THVs (SAPIEN/XT and Portico) compared to supra-annular devices (CoreValve/Evolut R) was restricted to patients treated with smaller valves (25.0% vs 5.0%; p<0.001), with no significant difference in those receiving larger devices. These observations are consistent with findings in the UK TAVI registry (severe SVD with small SAPIEN/XT 28.6% vs small CoreValve 3.6%). The recently published SMART trial randomised patients with small annuli to TAVI using either the Evolut PRO/PRO+/FX or SAPIEN 3/3 Ultra THVs and demonstrated superior haemodynamics at 1 year in those who received a supra-annular Evolut device (EOA 1.99 cm2 vs 1.50 cm2; p<0.001), suggesting an important association between haemodynamic performance and long-term durability1317.
Several other factors warrant consideration, including the impact of oversizing, which can lead to underexpansion, pinwheeling, and increased bending stress on the valve leaflets. Balloon post-dilation may also cause leaflet injury, potentially accelerating SVD. Additionally, THV design plays a critical role, as the outflow orifice of the prosthetic valve leaflets may be the primary determinant of haemodynamics, rather than the intra-annular or supra-annular positioning2729.
Accurate device sizing, increasing operator expertise and advances in THV design (including novel biomimetic platforms, improved skirt technology, anticalcification treatments and acellular leaflets) may address these concerns but will also require rigorous evaluation in future randomised trials and registries.
Limitations
Our study has several limitations. Firstly, long-term echocardiographic data were available for only a limited proportion of the total patients who underwent TAVI in participating centres between 2007 and 2014 (654/4,987; 13%). The patient population during this period was primarily elderly with various comorbidities, and many did not survive beyond 5 years post-procedure. The cause of death was unknown for most cases, so we cannot exclude the possibility that some succumbed to SVD-related complications. Thus, our registry lacks crucial data on 5-year survival after TAVI. Secondly, our inclusion criteria required patients to be alive at enrolment, preventing us from determining the incidence of BVF (according to VARC-3 definitions) without data on valve-related deaths. Thirdly, all TAVI procedures occurred before 2015, making our findings specific to first- and second-generation THVs. Finally, the COVID-19 pandemic hindered many patients from travelling to their TAVI centre for follow-up echocardiography, complicating enrolment and leading to the decision to halt recruitment at 654 subjects.
Conclusions
The European Valve Durability TAVI Registry assessed nearly 600 patients at least 5 years post-TAVI, demonstrating that the haemodynamic function of first-generation THVs remains stable up to 10 years. The incidence of moderate or severe SVD was relatively low (9.5%) at a median 6.1-year follow-up, with severe SVD even rarer (3.4%). However, rates were higher in patients with older-generation intra-annular THVs and smaller-sized devices.
Further research is needed to address the mechanisms behind accelerated SVD, and larger ongoing studies with newer THV designs will enhance generalisation. Our findings underscore the importance of imaging-guided and anatomically tailored device selection in clinical practice. However, due to potential confounding factors, future randomised trials are necessary to compare the durability of current intra-annular and supra-annular valves, especially in younger patients with long life expectancies.
Impact on daily practice
The European Valve Durability TAVI Registry highlights the long-term performance of transcatheter aortic valve implantation (TAVI), which is crucial as its use expands to younger and lower-risk populations. The low incidence of moderate to severe structural valve deterioration (SVD) beyond five years post-TAVI indicates that first-generation transcatheter heart valves (THVs) demonstrate promising durability for extended use. However, caution is advised when using intra-annular valves or small THVs, as these are associated with a higher risk of SVD.
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD, PhD; Department of Cardiology and Angiology, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Acknowledgements
The following colleagues made a significant contribution to the initial study design: Michael Haude (Germany), Andreas Baumbach (UK), Alec Vahanian (France), Thomas Modine (France), Jeroen Bax (the Netherlands), Peter Ludman (UK), Aldo Maggioni (Italy), Guillaume L’Official (France), Erwan Donal (France), Pilar Jiménez Quevedo (Spain), Stefan Stortecky (Switzerland), Jeroen Bax (the Netherlands), Alberto Rodrigues (Spain), Rui Campante Teles (Spain), Corrado Tamburino (Italy), Jose Maria de la Torre Hernandez (Spain), Antoine Guédès (Belgium), David Hildick-Smith (UK), Katia Orvin (Israel), and Bernhard Reimers (Italy).
The EORP Oversight Committee and the Registry Executive Committee of the EURObservational Research Programme members are all listed in Supplementary Appendix 1. Data collection was conducted by the EORP Department from the ESC by Yue Song and Carole Toulouse as Clinical Project Managers, Emanuela Fiorucci and Patti-Ann McNeill as Project Officers, and Gagan Chhabra and Maryna Andarala as Data Managers. Statistical analyses were performed by Cécile Laroche and Stateo.
All investigators are listed in Supplementary Appendix 1.
Funding
Since the start of the EORP, the following companies have supported the programme: Abbott Vascular Int. (2011-2021), Amgen Cardiovascular (2009-2018), AstraZeneca (2014-2021), Bayer AG (2009-2018), Boehringer Ingelheim (2009-2019), Boston Scientific (2009-2012), the Bristol-Myers Squibb and Pfizer Alliance (2011-2019), Daiichi Sankyo Europe GmbH (2011-2020), the alliance between Daiichi Sankyo Europe GmbH and Eli Lilly and Company (2014-2017), Edwards Lifesciences (2016-2019), Gedeon Richter Plc. (2014-2016), Menarini Int. Op. (2009-2012), MSD - Merck & Co. (2011-2014), Novartis Pharma AG (2014-2020), ResMed (2014-2016), Sanofi (2009-2011), SERVIER (2009-2021), and Vifor (2019-2022).
Conflict of interest statement
B. Prendergast discloses lecture fees from Edwards Lifesciences; is a member of a Medtronic research steering committee; received a consultancy fee from MicroPort; received personal fees as part of the Valvosoft data safety and monitoring committee; and received personal payment as part of the advisory board of Anteris. A. Chieffo declares honoraria for lectures from Abbott, Boston Scientific, Abiomed, Biosensors, Menarini, Medtronic, Shockwave Medical, and Penumbra. D.J. Blackman declares receiving an institutional research grant from Medtronic; received payment or honoraria for lectures, support for attending meetings/travels and consulting fees from Medtronic, Abbott, and Edwards Lifesciences; and participated on a data safety monitoring board or advisory board for Medtronic and Abbott. D. Capodanno declares consulting fees from Abbott; payment or honoraria from Sanofi (personal), Terumo (personal), Medtronic (institution), and Novo Nordisk (personal); and participation on a data safety monitoring board or advisory board for MedAlliance (personal). G.G. Toth declares consulting fees from Abbott, Medtronic, Abiomed, and Boston Scientific; payment or honoraria from Abbott, Terumo, and Biotronik; participation on an advisory board for Boston Scientific and Rede Optimus CRO; and has been a PCR board member. O. De Backer declares consulting fees from Abbott, Medtronic, and Boston Scientific; and payment or honoraria from Abbott, Medtronic, and Boston Scientific. S. Noble declares consulting fees from Medtronic (proctor); and support for attending meetings and/or travel from Abbott and Edwards Lifesciences. A.S. Petronio declares funds for research from Medtronic; consulting fees from Medtronic (through his institution), Abbott (personal), and Edwards Lifesciences (personal); speaker fees from Medtronic and Abbott; participation on an advisory board for Abbott; and participation on a steering committee for Medtronic. S. Windecker reports research, travel or educational grants to the institution from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, B. Braun, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardinal Health, Cardiovalve, Cordis Medical, CorFlow Therapeutics, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Farapulse Inc., Fumedica, Guerbet, Idorsia, Inari Medical, Infraredx, Janssen Cilag, Johnson & Johnson, MedAlliance, Medicure, Medtronic, Merck Sharp & Dohm, Miracor Medical, Novartis, Novo Nordisk, Organon, OrPha Swiss, Pharming Technologies, Pfizer, Polares, Regeneron, Sanofi‐Aventis, Servier, Sinomed, Terumo, Vifor, and V-Wave; he also served as an advisory board member and/or member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Boston Scientific, Biotronik, Bristol-Myers Squibb, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Recardio, Sinomed, Terumo, and V‐Wave, with payments to the institution but no personal payments; he is also a member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without impact on his personal remuneration; and he is ESC Vice President and Associate Editor of JACC CV Interventions. S. Schüpke declares participation on a data safety monitoring board or advisory board for the TARGET-FIRST study. C. Giannini declares speaker fees from Medtronic. The other authors have no conflicts of interest to declare. The Guest Editor reports consultancy fees from Novartis and Meril Life Sciences; speaker honoraria from Boston Scientific, Amgen, Daiichi Sankyo, and Meril Life Sciences; reports speaker honoraria paid to his institution from BMS/Pfizer, Daiichi Sankyo, Boston Scientific, Siemens, and Amgen; and research grants paid to his institution from Boston Scientific and Abbott.
Supplementary data
To read the full content of this article, please download the PDF.

