Cory:
Unlock Your AI Assistant Now!
As transcatheter aortic valve implantation (TAVI) is increasingly used in younger populations with fewer comorbidities, the long-term durability of transcatheter heart valves (THVs) has become a major issue. Each type of THV features distinct expansion mechanisms, stent frame designs, and leaflet configurations that may influence valve haemodynamic performance, leaflet mechanical stress, and long-term valve durability1. However, direct comparisons of durability beyond 5 years between balloon-expandable (BE) and self-expanding (SE) THVs remain scarce and largely indirect1. Nonetheless, current data suggest that SE THV devices with a supra-annular design may be associated with better durability compared to BE THV devices12.
The Myval/Octacor THV (Meril Life Sciences) is a new balloon-expandable valve that has recently demonstrated non-inferiority to both the SAPIEN 3 BE THV (Edwards Lifesciences) and the Evolut SE THV (Medtronic) at 30-day and 1-year follow-up in the head-to-head LANDMARK and COMPARE-TAVI randomised trials345.
In this issue of EuroIntervention, Jain et al6 assessed the 4-year durability of the Myval/Octacor THV. Although this study shares several limitations with previous studies, including (i) the competing risk of death was not accounted for and (ii) the study was not randomised, it has several important strengths. First, an independent echocardiography core lab was used. Second, bioprosthetic valve deterioration (BVD) and bioprosthetic valve failure (BVF) were defined according to Valve Academic Research Consortium-3 criteria. Lastly, the follow-up period was substantial, extending to 4 years.
A key finding of this study is that moderate or greater paravalvular leak (PVL) – previously identified as an independent predictor of mortality – was relatively frequent, occurring in 5.9% of patients at 30 days. This rate is notably higher than the 2-3% reported with the same device in two recent randomised trials (Figure 1A)345. While the authors suggest this may be partly due to implantation in patients with large annuli, the majority of cases occurred in patients receiving valves in the standard size range (23-26 mm) and rather suggest that the higher PVL rate is more likely related to the difference in the populations: selected patients in randomised controlled trials versus real-world all-comers populations. Similarly, Silva et al2 recently reported significantly higher rates of PVL with both the SAPIEN 3 BE THV and Evolut SE THV in real-world settings compared to those seen in the PARTNER (n=1,000; ClinicalTrials.gov: NCT02675114) EVOLUT Low Risk (n=1,468; NCT02207569) (Figure 1A).
More intriguing is the progressive increase in aortic regurgitation over time, reaching 9.2% at 4 years. This trend is consistent with findings from the COMPARE-TAVI trial, which showed a rise in aortic regurgitation from 2% at 30 days to 4% at 1 year with the Myval/Octacor THV5. However, further investigation is needed to clarify whether this progression results from worsening PVL or the development of new transvalvular regurgitation due to valve thrombosis or early structural valve deterioration (SVD).
While SAPIEN 3 BE THV devices have been associated with a relatively high incidence of moderate or severe patient-prosthesis mismatch (PPM), a notable finding of the present study is the low rate of severe PPM observed with the Myval/Octacor BE THV: 3.6% for moderate PPM and 2.0% for severe PPM (Figure 1B). As with other outcomes, this may be, at least in part, attributed to the inclusion of a substantial proportion of patients with very large annuli.
The main finding of this study is that BVD ≥stage 2 at 4 years was high, reaching 10.4% – twice the rate reported at 5 years with the SAPIEN 3 BE THV and 5 times higher than with the Evolut SE THV1 (Figure 1C). This was primarily driven by stage 2 BVD, observed in 9.7% of patients, largely due to SVD, as no cases of endocarditis were reported and overt valve thrombosis occurred in only 0.5%. Despite the elevated incidence of ≥stage 2 BVD likely related to SVD, the all-cause BVF rate remains relatively low at 3.3%, which is comparable to rates previously reported with the SAPIEN 3 BE THV and may be considered reassuring (Figure 1D).
Overall, the findings of the study by Jain et al6 have two major implications. First, they underscore the need for long-term outcome and durability data from prospective randomised trials directly comparing SE THVs and BE THVs. Notably, the SMART Trial (n=716; NCT04722250), the BEST trial (n=2,000; NCT05454150), the LANDMARK trial, and the COMPARE-TAVI trial, which are comparing the Evolut, SAPIEN 3, and Myval/Octacor THVs have all completed enrolment, and patients will be followed for up to 10 years. Second, these findings highlight the importance of continued research and development to enhance THV durability, particularly by targeting valve calcification, a key contributor to bioprosthetic valve degeneration.
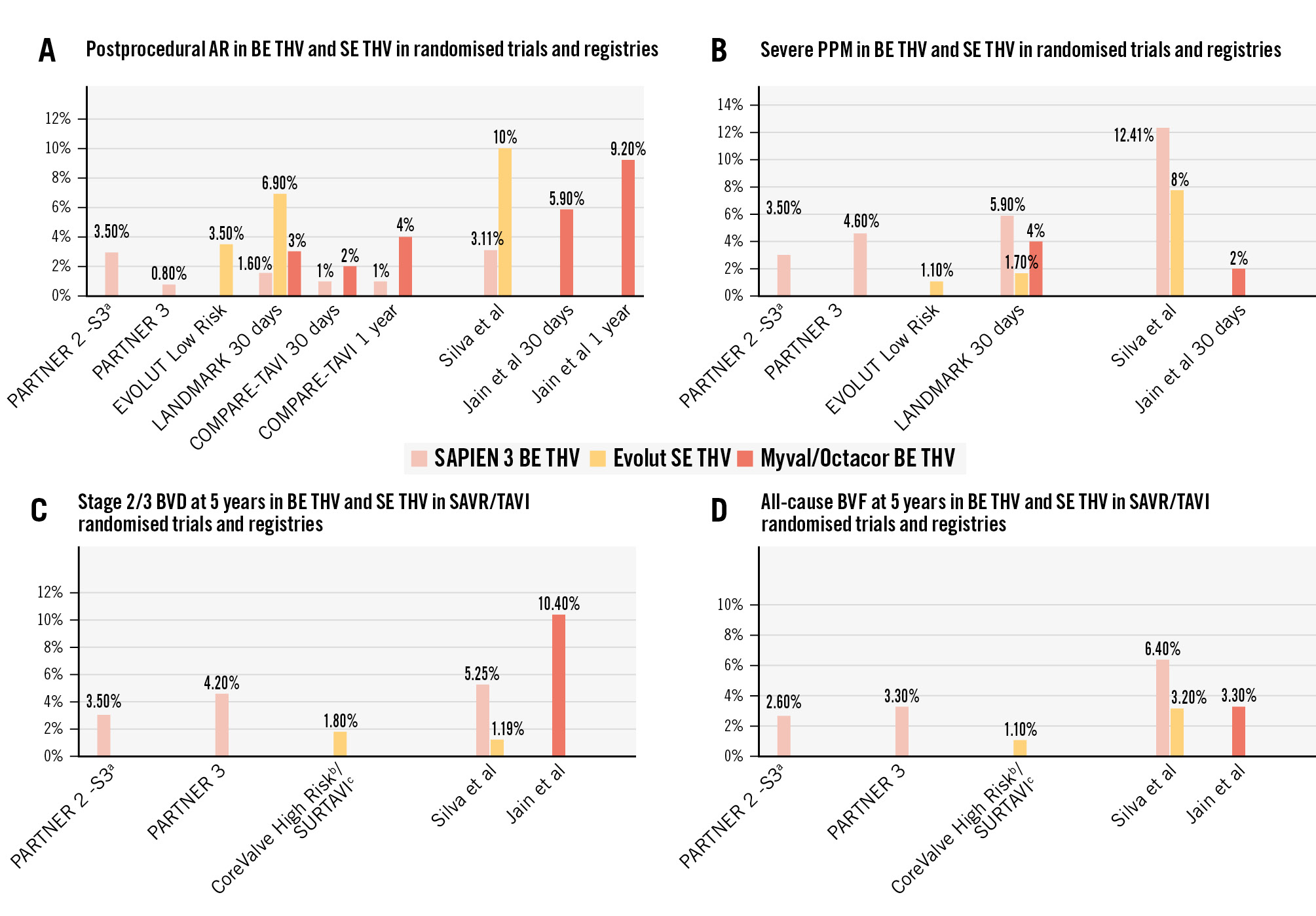
Figure 1. Rates of aortic regurgitation, PPM, bioprosthetic valve deterioration and failure in the Myval balloon-expandable valve versus other types of transcatheter heart valves in randomised trials and registries. aClinicalTrials.gov: NCT01314313; bNCT01240902; cNCT01586910. Moderate/severe postprocedural AR (A), severe PPM (B), bioprosthetic valve deterioration (BVD) stage 2/3 at 5 years (C), and bioprosthetic valve failure (BVF) at 5 years (D) in BE THV and SE THV in randomised trials and registries. AR: aortic regurgitation; BE: balloon-expandable; PPM: patient-prosthesis mismatch; SAVR: surgical aortic valve replacement; SE: self-expanding; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
Conflict of interest statement
P. Pibarot has received institutional funding from Edwards Lifesciences, Medtronic, Pi-Cardia, and Cardiac Success for echocardiography core laboratory analyses, blood biomarker analyses, and research studies in the field of interventional and pharmacological treatment of valvular heart diseases, for which he received no personal compensation. E. Van Belle has no conflicts of interest to declare.

