Abstract
Background: In patients undergoing transcatheter aortic valve implantation (TAVI), the presence of a low-flow, low-gradient (LFLG) status has been associated with higher mortality at short-term follow-up.
Aims: We aimed to evaluate long-term survival after TAVI in patients with classical (cLFLG) and paradoxical LFLG (pLFLG) aortic stenosis (AS) compared to high-gradient (HG)-AS.
Methods: Patients undergoing TAVI at our centre with a hypothetical minimum 5-year follow-up were divided into 3 groups: (1) HG-AS (mean gradient [MG] >40 mmHg), (2) cLFLG-AS (MG <40 mmHg, ejection fraction [EF] <50%), and (3) pLFLG-AS (MG <40 mmHg, EF ≥50%). The primary endpoint of the study was all-cause mortality. Propensity score-weighted survival analysis was performed to adjust for possible baseline confounders.
Results: A total of 574 subjects were included (73% HG-AS, 15% pLFLG-AS, 11% cLFLG-AS). The median survival time was 4.8 years, with a maximum of 12.3 years. Patients with cLFLG-AS presented the highest baseline cardiovascular risk. At unadjusted survival analysis, patients with cLFLG-AS showed the worst long-term prognosis, with a rapid decrease in survival within the first year, while pLFLG- and HG-AS patients presented similar survival rates (p=0.023). At weighted long-term analysis, cLFLG- and HG-AS had similar survival rates. Baseline EF was not related to long-term mortality, while patients with a post-TAVI left ventricular ejection fraction (LVEF) improvement >10% lived significantly longer (p=0.02).
Conclusions: Classical LFLG-AS patients had lower long-term survival rates as compared to pLFLG-AS and HG-AS patients. However, after adjustment for possible baseline confounders, a low-flow status per se did not have an impact on long-term mortality after TAVI. Post-TAVI LVEF recovery was associated with improved long-term outcome.
Transcatheter aortic valve implantation (TAVI) has emerged as the preferred treatment for elderly patients with severe symptomatic aortic stenosis (AS)12. Up to one-third of patients with severe AS34 exhibit a low-flow, low-gradient (LFLG) condition, characterised by an aortic valve area (AVA) <1 cm2, a mean transvalvular gradient (MG) <40 mmHg, and a stroke volume index (SVi) <35 ml/m25. Low-flow, low-gradient status can be further categorised into classical LFLG (cLFLG) when associated with a left ventricular ejection fraction (LVEF) <50% and paradoxical LFLG (pLFLG) when the LVEF is ≥50%1. Despite a clear benefit of TAVI over conservative treatment in patients with LFLG-AS6789, cLFLG status has been related to worse post-TAVI outcomes at midterm follow-up compared to high-gradient (HG)-AS91011. Whether the worse outcome can be attributed to the low-flow status itself or is influenced by a more compromised baseline clinical condition remains a topic of debate1213. However, it is reasonable to assume that multiple factors may contribute to the worse prognosis in cLFLG-AS patients, who are typically frailer and affected by various comorbidities8914. On the contrary, patients with pLFLG-AS undergoing TAVI have shown survival rates at 1-year follow-up that are comparable to those of patients diagnosed with HG-AS1516. Notwithstanding, data on the long-term outcomes of LFLG-AS patients treated with TAVI are lacking. The aim of our study was to assess the long-term survival (up to 10 years) following TAVI in patients with cLFLG- or pLFLG-AS compared to those with HG-AS.
Methods
Study design
This study represents a retrospective analysis conducted using data from the Padua University REVALVing Experience (PUREVALVE) registry, which includes all consecutive patients who underwent TAVI for severe symptomatic AS (aortic valve area <1 cm2 or <0.6 cm2/m2 of body surface area) at our institution. For the purpose of the study, we included in the analysis all consecutive patients who underwent TAVI between June 2007 and December 2017. Indications for TAVI were based on a Heart Team decision. Patients were further divided into 3 different subgroups according to their baseline echocardiographic findings: (1) HG-AS: MG >40 mmHg; (2) cLFLG-AS: MG <40 mmHg, SVi <35 ml/m2, LVEF <50%; and (3) pLFLG-AS: MG <40 mmHg, SVi <35 ml/m2, LVEF ≥50%. We excluded from the current analysis (a) patients who underwent valve-in-valve interventions, (b) patients with preprocedural MG <40 mmHg associated with a normal SVi (>35 ml/m2), and (c) TAVI patients without technical success based on Valve Academic Research Consortium (VARC)-3 criteria17. Follow-up time was defined as the time from the procedure to the last documented contact with the patient (alive) or to the time of documented death. The primary endpoint was all-cause mortality. We also determined the percentage of patients who presented a significant improvement in LVEF within the first year after the procedure (>10%)1819. All patients provided written informed consent for the procedure and data collection. The study was approved by the Institutional Ethics Committee and conforms to the principles outlined in the Declaration of Helsinki.
Echocardiographic data
All patients underwent comprehensive baseline transthoracic echocardiography (TTE) by an experienced echocardiographer at our centre, in accordance with established guidelines2021. Follow-up TTE was performed during the index hospitalisation and subsequently during follow-up, generally at 3, 6, and 12 months. LVEF was derived from left ventricular (LV) end-diastolic and end-systolic volumes measured on the apical 2- and 4-chamber views, using the Simpson method. Aortic valve area was calculated using the continuity equation. To confirm the severity of AS, dobutamine stress echocardiography was performed in patients with LFLG status and reduced LVEF (cLFLG-AS), while the aortic valve calcium score was evaluated at preprocedural multidetector computed tomography (CT) in case of pLFLG-AS1.
Device and procedure
Transcatheter heart valve (THV) choice and TAVI access were based on a Heart Team decision. Five types of THV were implanted: (1) the balloon-expandable SAPIEN, SAPIEN XT and SAPIEN 3 (Edwards Lifesciences); (2) the mechanically expandable LOTUS Edge (Boston Scientific); (3) the self-expanding CoreValve, Evolut R and Evolut PRO (Medtronic); (4) the self-expanding ACURATE neo (Boston Scientific) and (5) the JenaValve Trilogy (JenaValve). Percutaneous coronary revascularisation was performed in case of severe coronary artery disease involving proximal vessel segments, and this approach was consistent over the study period2223. In the presence of other severe valvular diseases, patients were managed in accordance with international guidelines following the TAVI procedure. In the absence of recent coronary intervention, discharge therapy consisted of dual antiplatelet therapy for 6 months, or a combination of an oral anticoagulant and aspirin (up to 6 months) if anticoagulation was clinically indicated24. All patients were treated with guideline-directed optimal medical therapy after discharge.
Statistical analysis
Continuous variables are expressed as mean±standard deviation (SD) or median (interquartile range [IQR]) and were compared using the Student's t-test or the Mann-Whitney U test, as appropriate. Categorical variables are presented as counts (%) and were compared using the chi-square test or Fisher’s exact test as appropriate. Survival curves with 95% confidence intervals (CI) were estimated for the 3 considered groups using the Kaplan-Meier (KM) method and compared with the log-rank test.
To account for potential confounders, we performed a propensity score (PS)-weighted analysis to estimate the average treatment effect (ATE). The covariates used for calculating the PS were chosen according to their clinical significance or previously reported independent impact on mortality among LFLG patients10. The following parameters were included in the PS model used to compare pLFLG-AS and HG-AS patients: LVEF, age, sex, body mass index, renal function, the presence of diabetes mellitus, atrial fibrillation, or chronic obstructive pulmonary disease, and the access route. On the contrary, LVEF was not included in the PS model used to compare cLFLG-AS and HG-AS patients, as LVEF determined patient classification into 1 of these 2 groups. Multivariable Cox regression analyses, including all variables included in the PS, were performed as sensitivity analyses. To adjust for the possible interaction between LVEF and survival, we subsequently performed a weighted multivariable Cox regression analysis including LVEF and cLFLG status. A Cox proportional hazards model, taking into account LVEF measured at multiple timepoints during the first year of follow-up, was employed to evaluate the association between longitudinally assessed LVEF and mortality risk. To account for within-patient correlation, robust covariance estimation was used. To avoid bias due to incomplete case analyses, missing data in baseline characteristics were handled with Multivariate Imputation via Chained Equations using the mice package (v3.13.0; van Buuren & Groothuis-Oudshoorn, 2011). For all the analyses, a 2-sided p<0.05 was considered to be significant. Statistical analyses were performed using R software, version 4.1.2 (R Foundation for Statistical Computing). “WeightIt” (version 0.12.0; Noah Greifer, 2021; method “npcbps” and “energy”), “Cobalt”, “survival”, “RISCA”, “ggplot2”, and “adjustedCurves” R packages were used for weight estimation, assessing balance on covariate distributions, estimating log-rank adjusted p-values and plotting adjusted KM curves.
Results
Baseline characteristics and procedural data
Out of 681 patients who underwent TAVI at our institution, 574 subjects were included in the analysis (Figure 1, Central illustration). Of these, 419 (73%) fulfilled the criteria for HG-AS, 91 (15%) for pLFLG-AS and 64 (11%) for cLFLG-AS. Baseline clinical, echocardiographic, and procedural characteristics are summarised in Table 1. Compared to patients diagnosed with HG-AS, those with cLFLG-AS were more often male (62.5% vs 46.8%; p=0.022), younger (77 vs 81 years old; p<0.001) and characterised by higher surgical risk (European System for Cardiac Operative Risk Evaluation [EuroSCORE] II 5.92% vs 3.87%; p<0.001; Society of Thoracic Surgeons Predicted Risk of Mortality [STS-PROM] score 5.42% vs 4.62%; p=0.321). Compared to those diagnosed with HG-AS, patients with cLFLG-AS more often had concomitant coronary artery disease (68.8% vs 55.4%; p=0.049), previous myocardial infarction (26.6% vs 15.0%; p=0.003) and prior permanent pacemaker implantation (17.2% vs 6.7%; p=0.011). Conversely, pLFLG and HG-AS patients presented comparable baseline characteristics.
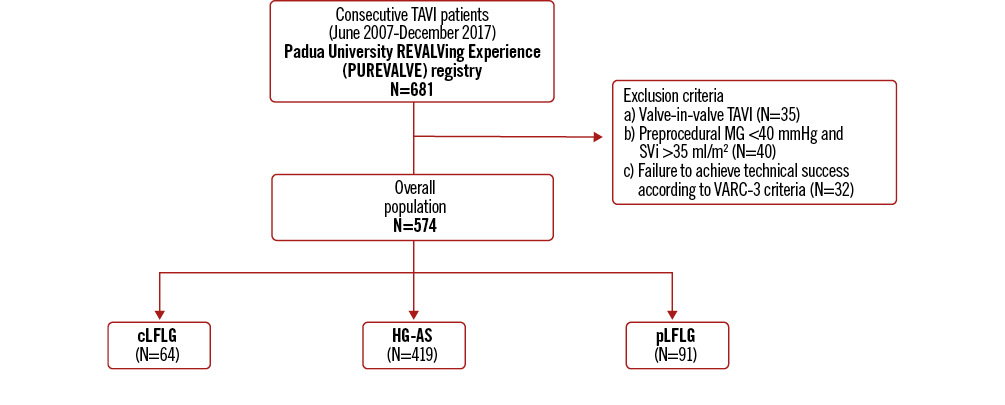
Figure 1. Study flowchart. cLFLG: classical low-flow, low-gradient; HG-AS: high-gradient aortic stenosis; MG: mean gradient; pLFLG: paradoxical low-flow, low-gradient; SVi: stroke volume index; TAVI: transcatheter aortic valve implantation; VARC: Valve Academic Research Consortium
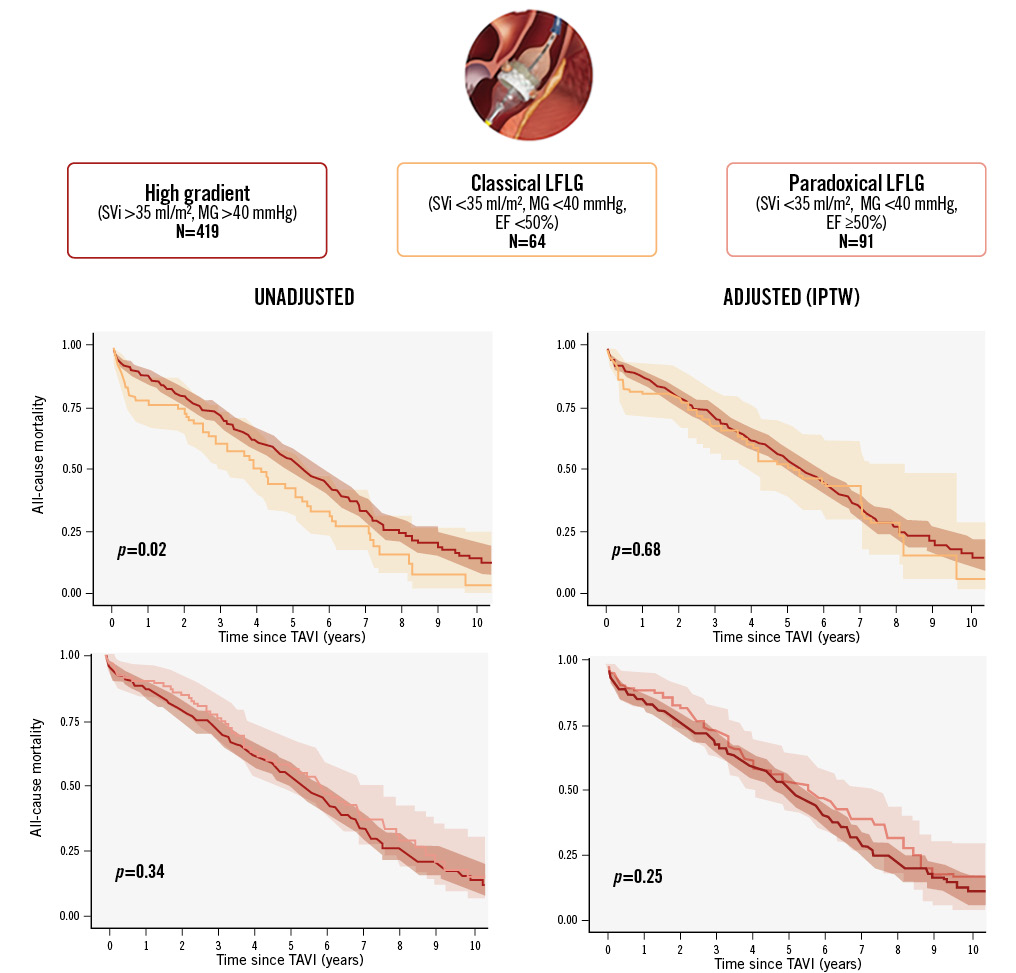
Central illustration. Long-term survival of patients with aortic stenosis undergoing TAVI according to valve flow status. Variables included in the propensity score models: age, sex, body mass index, renal function, diabetes mellitus, atrial fibrillation, chronic obstructive lung disease, access route and left ventricular ejection fraction (the latter only for paradoxical LFLG-AS vs HG-AS comparison). AS: aortic stenosis; EF: ejection fraction; HG: high-gradient; IPTW: inverse probability of treatment weighting; LFLG: low-flow, low-gradient; MG: mean gradient; SVi: stroke volume index; TAVI: transcatheter aortic valve implantation
Table 1. Baseline characteristics and procedural data.
| Variable | HG-AS (n=419) | cLFLG-AS (n=64) | p-value* | pLFLG-AS (n=91) | p-value# |
|---|---|---|---|---|---|
| Clinical characteristics | |||||
| Age, years | 81.00±5.91 | 77.00±11.06 | <0.001 | 81.00±4.80 | 0.359 |
| Male sex | 196 (46.8) | 40 (62.5) | 0.022 | 35 (38.5) | 0.167 |
| BMI, kg/m2 | 25.82 [14.87-45.03] | 24.50 [19.10-44.92] | 0.048 | 26.04 [17.97-38.86] | 0.973 |
| Hypertension | 381 (90.9) | 55 (85.9) | 0.254 | 82 (90.1) | 0.690 |
| Diabetes mellitus | 117 (27.9) | 23 (35.9) | 0.187 | 29 (31.9) | 0.445 |
| Dyslipidaemia | 257 (61.3) | 44 (68.8) | 0.271 | 54 (59.3) | 0.638 |
| Atrial fibrillation | 126 (30.1) | 26 (40.6) | 0.111 | 34 (37.4) | 0.218 |
| Previous TIA/stroke | 48 (11.5) | 9 (14.8) | 0.524 | 14 (15.4) | 0.492 |
| COPD | 100 (23.9) | 19 (29.7) | 0.350 | 24 (26.4) | 0.688 |
| CKD (eGFR <60 ml/min/1.73 m2) | 239 (57.0) | 39 (60.9) | 0.589 | 48 (52.7) | 0.357 |
| Permanent PM | 28 (6.7) | 11 (17.2) | 0.011 | 7 (7.7) | 0.823 |
| CAD | 232 (55.4) | 44 (68.8) | 0.049 | 48 (52.7) | 0.565 |
| Previous PCI | 72 (17.2) | 13 (20.3) | 0.597 | 22 (24.2) | 0.136 |
| Previous CABG | 46 (11.0) | 11 (17.2) | 0.150 | 11 (12.1) | 0.860 |
| Previous AMI | 64 (15.3) | 17 (26.6) | 0.003 | 19 (20.9) | 0.216 |
| EuroSCORE II, % | 3.87 [0.91-32.67] | 5.92 [0.99-50.07] | <0.001 | 4.41 [1.13-24.45] | 0.232 |
| STS-PROM, % | 4.62 [0.70-47.10] | 5.42 [1.03-57.20] | 0.321 | 4.78 [1.23-37.00] | 0.191 |
| Echocardiographic characteristics | |||||
| LVEF, % | 59.00 [22.72-78.00] | 34.50 [19.00-49.00] | <0.001 | 60.00 [50.00-76.00] | 0.002 |
| Max transaortic gradient, mmHg | 71.00 [57.00-132.00] | 50.00 [32.00-75.00] | <0.001 | 48.00 [33.00-75.00] | <0.001 |
| Mean transaortic gradient, mmHg | 49.00 [42.00-109.00] | 28.00 [6.00-39.00] | <0.001 | 34.00 [21.00-39.00] | <0.001 |
| AVA, cm2 | 0.74 [0.27-1.80] | 0.82 [0.49-1.56] | 0.003 | 0.84 [0.48-1.60] | <0.001 |
| AVAi, cm2/m2 | 0.43 [0.11-0.90] | 0.48 [0.26-1.03] | 0.008 | 0.48 [0.23-0.87] | <0.001 |
| LVEDVi, ml/m2 | 59.0 [22.8-135.0] | 68.0 [34.2-158.9] | 0.002 | 61.3 [21.5-102.4] | 0.189 |
| Moderate or severe MR | 10 (2.5) | 3 (4.6) | 0.213 | 5 (5.4) | 0.092 |
| Moderate or severe TR | 13 (3.1) | 2 (3.1) | 0.742 | 3 (3.2) | 0.453 |
| Procedural data | |||||
| Type of anaesthesia | 0.599 | 0.839 | |||
| Deep sedation | 272 (65.4) | 37 (59.7) | 62 (68.1) | ||
| General anaesthesia | 141 (33.9) | 25 (40.3) | 29 (31.9) | ||
| Access site | 0.054 | 0.981 | |||
| Transfemoral | 288 (68.7) | 37 (57.8) | 67 (73.6) | ||
| Trans-subclavian | 3 (0.7) | 1 (1.6) | 1 (1.1) | ||
| Transapical | 124 (29.6) | 23 (35.9) | 23 (25.3) | ||
| Transaortic | 4 (1.0) | 3 (4.7) | 0 (0) | ||
| THV model | 0.136 | 0.289 | |||
| CoreValve/Evolut R/PROa | 64 (15.2) | 12 (19.4) | 11 (12.1) | ||
| SAPIEN/SAPIEN XT/3b | 287 (68.4) | 45 (70.3) | 71 (78.0) | ||
| JenaValve Trilogyc | 4 (1.0) | 2 (3.2) | 1 (1.1) | ||
| LOTUS Edged | 47 (11.2) | 3 (4.8) | 4 (4.4) | ||
| ACURATE neod | 17 (4.1) | 0 (0) | 4 (4.4) | ||
| Early safety (at 30 days) | 344 (82.3) | 52 (81.4) | 0.471 | 75 (82.4) | 0.486 |
| Device success (at 30 days) | 404 (96.5) | 61 (95.8) | 0.204 | 88 (96.7) | 0.332 |
| Need for PM | 54 (13.1) | 9 (13.6) | 0.231 | 12 (13.4) | 0.763 |
| Discharge medications | |||||
| Aspirin | 350 (85.3) | 55 (85.9) | 0.658 | 80 (87.9) | 0.376 |
| Dual antiplatelet therapy | 270 (64.4) | 38 (59.3) | 0.263 | 55 (60.4) | 0.425 |
| Oral anticoagulant | 130 (31.0) | 24 (37.5) | 0.132 | 35 (38.4) | 0.125 |
| Beta blockers | 356 (85.4) | 57 (89.0) | 0.114 | 80 (87.9) | 0.274 |
| ACE inhibitors/ARBs | 314 (74.9) | 46 (71.8) | 0.165 | 69 (75.8) | 0.723 |
| MRA | 260 (62.0) | 55 (85.9) | 0.064 | 53 (58.2) | 0.521 |
| Values are presented as mean±standard deviation, n (%) or median [interquartile range]. *P-value refers to the comparison between the NFHG-AS group and the cLFLG group. #P-value refers to the comparison between the NFHG-AS group and the pLFLG group. aBy Medtronic; bby Edwards Lifesciences; cby JenaValve; dby Boston Scientific. ACE: angiotensin-converting enzyme; AMI: acute myocardial infarction; ARB: angiotensin II receptor blocker; AS: aortic stenosis; AVA: aortic valve area; AVAi: AVA index; BMI: body mass index; CABG: coronary artery bypass graft; CAD: coronary artery disease; CKD: chronic kidney disease; cLFLG: classical low-flow, low-gradient; COPD: chronic obstructive pulmonary disease; eGFR: estimated glomerular filtration rate; EuroSCORE: European System for Cardiac Operative Risk Evaluation; HG: high-gradient; LVEDVi: left ventricular end-diastolic volume index; LVEF: left ventricular ejection fraction; MR: mitral regurgitation; MRA: mineralocorticoid receptor antagonist; NF: no-flow; PCI: percutaneous coronary intervention; pLFLG: paradoxical low-flow, low-gradient; PM: pacemaker; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; THV: transcatheter heart valve; TIA: transient ischaemic attack; TR: tricuspid regurgitation | |||||
Procedural data and periprocedural outcomes
TAVI was performed through the transfemoral approach in 66.7% of the cases, without significant differences among groups. The balloon-expandable SAPIEN/SAPIEN XT/3 were the most frequently used prostheses, followed by the self-expanding CoreValve/Evolut R/PRO. Rates of device success and early safety at 30 days were similar among groups (Table 1).
Clinical outcomes
The median follow-up time was 4.8 years (IQR 2.3-6.2) with a maximum of 12.3 years. KM estimates for overall survival at 2, 4, 6, 8, and 10 years were 80% (95% CI: 77-83), 62% (95% CI: 58-66), 42% (95% CI: 38-46), 25% (95% CI: 21-30), and 12% (95% CI: 8-17), respectively. Unadjusted survival KM curves for the 3 groups are reported in Figure 2, Figure 3A and Figure 4A. Ten-year all-cause mortality was higher in patients with cLFLG-AS compared to those with HG-AS (p=0.02), while pLFLG-AS and HG-AS groups presented similar long-term survival (p=0.34). Among cLFLG-AS patients, the most significant decrease in mortality occurred within the first year after the procedure (KM estimates at 1 year: 75% [95% CI: 62-84] for cLFLG-AS, 89% [95% CI: 82-95] for pLFLG-AS, 88% [95% CI: 84-91] for HG-AS; p=0.009) (Supplementary Figure 1), with similar unadjusted survival curves from the 1-year mark onwards (p=0.3) (Supplementary Figure 2). Adjusted survival curves are reported in Figure 3B and Figure 4B. After performing propensity score weighting, no difference was found in terms of long-term mortality among the 3 different groups (the balance of covariates included in the PS analysis before and after the weighting is reported in Supplementary Figure 3). Classical LFLG status did not show a significant impact on weighted survival, even after adjusting for baseline LVEF (Supplementary Table 1). A sensitivity analysis, consisting of 2 multivariable Cox regression models with all covariates included in the PS, confirmed the absence of a significant impact of either cLFLG or pLFLG status on long-term survival (Supplementary Table 2, Supplementary Table 3).
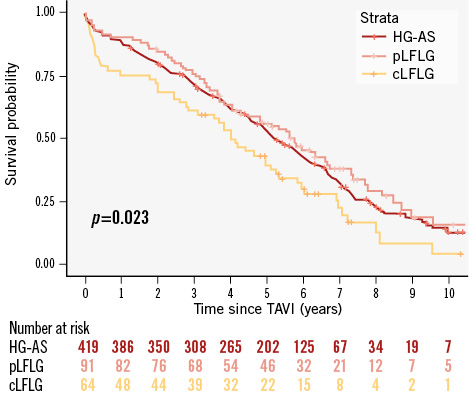
Figure 2. Unadjusted KM survival curves. cLFLG: classical low-flow, low-gradient; HG-AS: high-gradient aortic stenosis; KM: Kaplan-Meier; pLFLG: paradoxical low-flow, low-gradient; TAVI: transcatheter aortic valve implantation
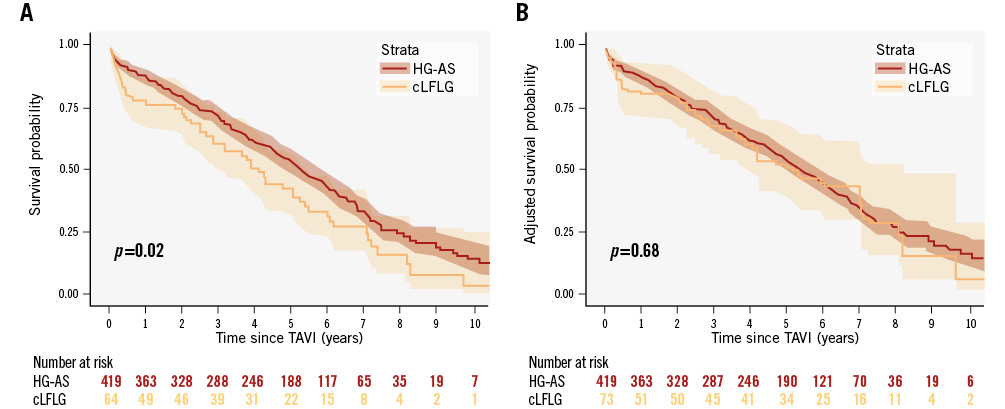
Figure 3. KM survival curves for cLFLG- and HG-AS. A) Unadjusted survival curves, and (B) adjusted KM survival curves. cLFLG: classical low-flow, low gradient; HG-AS: high-gradient aortic stenosis; TAVI: transcatheter aortic valve implantation
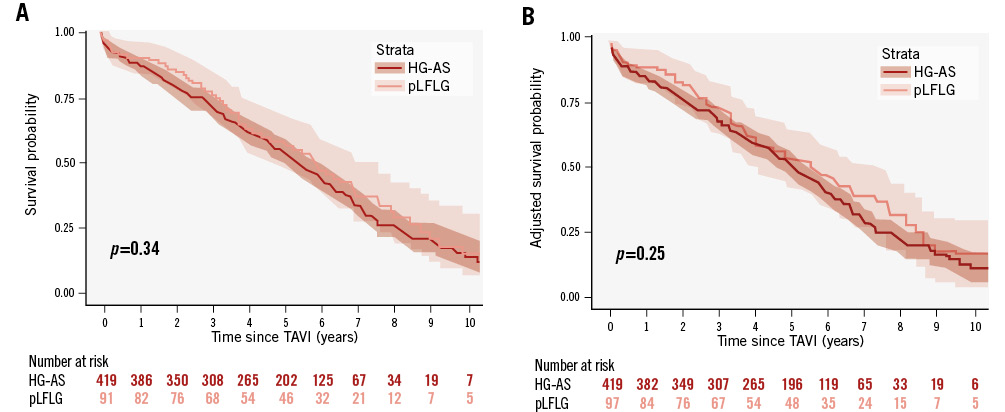
Figure 4. KM survival curves for pLFLG- and HG-AS. A) Unadjusted survival curves, and (B) adjusted KM survival curves. HG-AS: high-gradient aortic stenosis; pLFLG: paradoxical low-flow, low gradient; TAVI: transcatheter aortic valve implantation
LVEF improvement
Out of the 64 patients diagnosed with cLFLG-AS, echocardiographic follow-up throughout the first year after the procedure was available for 58 (91%) of them. Postprocedural improvement in LVEF >10% was common among patients with cLFLG-AS, occurring in approximately two-thirds of this subgroup (63%). As shown in Supplementary Figure 4, these patients experienced significantly longer survival compared to those with no or slighter (<10%) LVEF recovery (p=0.02). Moreover, by accounting for the longitudinal measures of LVEF throughout the first year of follow-up, the Cox proportional hazards model showed that an increase in LVEF during the follow-up period was associated with a lower likelihood of death (hazard ratio 0.9692, 95% CI: 0.9517-0.987; p<0.001).
Discussion
The main findings of our study − the first to examine long-term survival after TAVI in subjects with pLFLG- and cLFLG-AS versus HG-AS − can be summarised as follows: (1) patients with cLFLG-AS undergoing TAVI showed lower unadjusted 1-year survival rates as compared to those with pLFLG- or HG-AS, but no difference was observed from the 1-year mark onwards; (2) the worse long-term post-TAVI outcome of cLFLG-AS subjects seemed to be related more to the higher patient baseline risk rather than the low-flow status itself; (3) baseline LVEF did not appear to predict long-term survival in cLFLG-AS patients. Conversely, an early LVEF improvement post-TAVI, which was observed in two-thirds of cLFLG-AS subjects, yielded longer survival.
Low-flow, low-gradient status in the context of AS represents a challenging clinical setting both for diagnosis and therapeutical decision-making. As proven by a large amount of data in literature, TAVI has emerged as a suitable and effective treatment option also in this subset of patients91516. Specifically, in keeping with previous reports10, our patients with pLFLG-AS had similar post-TAVI outcomes at midterm follow-up compared to those with HG-AS. Furthermore, we extended these findings to a longer follow-up period. Conversely, subjects with cLFLG-AS have been shown to have the worst short-term post-TAVI survival among different AS flow statuses591011152526272829. Our study confirms the worse 1-year survival after TAVI of cLFLG patients (75%), as compared to that of patients with either pLFLG- or HG-AS (89% and 88%), that has already been observed in smaller single-centre registries510. Furthermore, it extends these findings to a longer follow-up period, highlighting a time-dependent mortality risk, which appeared to be concentrated in the first year after the procedure. Only one previous paper has reported on the outcomes of these patients beyond 1 year (with a median follow-up time of 3 years), observing lower post-TAVI survival rates in patients with cLFLG-AS11. However, the retrospective nature of the study along with the absence of a statistical adjustment to accommodate potential baseline confounders prevented further insights into the pathophysiological mechanism behind these findings. As in previous reports11151930, our cLFLG-AS patients presented higher baseline cardiovascular and surgical risks (median EuroSCORE II 5.92% for cLFLG-AS vs 4.41% for pLFLG-AS vs 3.87% for HG-AS) due to concomitant comorbidities and frailty. We performed a propensity-weighted survival analysis to mitigate this underlying bias, and we found that a cLFLG status per se does not seem to carry an increased all-cause mortality risk after TAVI. This is in contrast with the findings of another previous single-centre registry with limited (1-year) follow-up, which reported lower survival rates of cLFLG patients even after adjustment for baseline clinical characteristics. Possible explanations for these conflicting results could be the longer follow-up of our study, the relatively lower surgical risk of our cLFLG patients (median STS-PROM score 5.4% vs 8.2% reported in the paper by Puls et al11), and the different covariates included in our PS model10 (which did not include LVEF). Moreover, we could speculate that the similar outcome of pLFLG- and HG-AS patients (with similar baseline risk) supports the concept that the low-flow status itself might have a lesser impact on prognosis than a patient’s comorbidities. The impact of baseline LVEF on TAVI outcomes has been a matter of debate. Our results confirm the lack of association between baseline LVEF and survival after TAVI in cLFLG patients, consistent with findings from previous studies in the field111931. On the contrary, we found that an early post-TAVI increase in LVEF >10% yielded a 54% improvement in postprocedural median survival time (1,985 days vs 1,288 days)1819. While the latter finding seems reasonable from a pathophysiological standpoint, it remains to be explained why the presence of contractile reserve at pre-TAVI dobutamine stress echocardiography has failed to show a significant survival benefit323334. In conclusion, our results confirm the long-term efficacy and safety of TAVI in LFLG patients. Moreover, they suggest the importance of accurate preprocedural patient selection, in order to identify frailer patients for whom TAVI might be futile.
Limitations
This study is a single-centre, retrospective post hoc analysis of a prospective all-comers TAVI registry. The small sample size prevented further subanalyses to identify the subset of cLFLG patients who might demonstrate postprocedural LVEF improvement. Moreover, the small number of patients at risk beyond 5 years of follow-up prevents definite conclusions on post-TAVI outcomes in patients with LFLG-AS. In particular, due to the wide 95% CI obtained, we cannot definitively exclude the presence of a type II error. Nevertheless, our study population is one of the largest on the topic51135 and is the first to report preliminary results on the very late outcomes after TAVI in patients with LFLG-AS. Most of the patients had been referred to our centre after already completing preprocedural screening. Consequently, we were unable to provide data on preprocedural left ventricular contractile reserve. Patients who underwent surgical aortic valve replacement were not included in the study, preventing any inference on the interplay between the LFLG status and procedure type. Although the exams were performed by experienced certified operators, the absence of a core lab for standardised TTE acquisition and evaluation may have resulted in bias in image interpretation. We considered only all-cause death as the endpoint, not reporting data on cardiovascular mortality or other common postprocedural outcomes. Moreover, no specific quality-of-life questionnaire nor cognitive status assessment was routinely performed at follow-up.
Conclusions
Our findings suggest that the well-known higher mortality rates characterising patients with cLFLG-AS undergoing TAVI may be more related to their higher baseline cardiovascular risk than the low-flow status itself. Conversely, pLFLG patients represent a population similar to HG-AS subjects in terms of both baseline characteristics and long-term outcomes. Our study confirmed that LVEF recovery after TAVI is a common finding among patients affected by cLFLG-AS and correlates with postprocedural survival. While awaiting further studies and randomised data, performing TAVI in carefully selected patients with LFLG-AS seems to provide both safe and effective long-term results.
Impact on daily practice
While classical low-flow, low-gradient aortic stenosis (AS) patients exhibit lower survival rates compared to high-gradient AS patients at unadjusted analysis, this disparity diminishes when accounting for potential baseline confounders, suggesting that a patient’s risk profile may play a more significant role than low-flow status alone. Thus, accurate patient selection during preprocedural planning might improve transcatheter valve implantation outcomes and could be useful to avoid futile interventions.
Conflict of interest statement
G. Tarantini reports honoraria for lectures/consulting from Medtronic, Edwards Lifesciences, Boston Scientific, Abbott, GADA, MicroPort, and SMT. L. Nai Fovino reports honoraria for lectures from Edwards Lifesciences. G. Masiero reports honoraria for lectures/consulting from GE HealthCare. C. Fraccaro reports honoraria for lectures/consulting from Edwards Lifesciences. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

