Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Calcified nodules (CNs) are an increasingly important, high-risk lesion subset.
Aims: We sought to identify the emergence of new CNs and the relation between underlying plaque characteristics and new CN development.
Methods: Patients who had undergone two optical coherence tomography (OCT) studies that imaged the same untreated calcified lesion at baseline and follow-up were included. New CNs were an accumulation of small calcium fragments at follow-up that were not present at baseline. Cardiac death, myocardial infarction (MI), or clinically driven revascularisation related to OCT-imaged, but untreated, calcified lesions were then evaluated.
Results: Among 372 untreated calcified lesions, with a median of 1.5 (first and third quartiles: 0.7-2.9) years between baseline and follow-up OCTs, new CNs were observed in 7.0% (26/372) of lesions at follow-up. Attenuated calcium representing residual lipid (odds ratio [OR] 3.38, 95% confidence interval [CI]: 1.15-9.98; p=0.03); log10 calcium volume index (length×maximum arc×maximum thickness; OR 2.76, 95% CI: 1.10-6.95; p=0.03); angiographic Δangle between systole and diastole, per 10° (OR 2.30, 95% CI: 1.25-4.22; p=0.01); and time since baseline OCT, per year (OR 1.36, 95% CI: 1.05-1.75; p=0.02) were all associated with new CN development. Clinical events were revascularisation and/or MI and were more frequent in lesions with versus without a new CN (29.3% vs 15.3%; p=0.04).
Conclusions: New CNs developed in untreated, lipid-containing, severely calcified lesions with a larger angiographic hinge motion (between systole and diastole), compared with lesions without CNs, and were associated with worse clinical outcomes.
Calcified nodules (CNs) are an important lesion subset because they are one of the thrombotic causes of acute coronary syndrome (ACS) and are associated with poor clinical outcomes after percutaneous coronary intervention (PCI)1234. From a pathological perspective, CNs are characterised by the luminal protrusion of an accumulation of small calcium deposits formed by fragmentation of sheet calcification. CNs have been classified into two types: (1) CNs with disruption of an overlying fibrous cap with thrombus (eruptive CN) or (2) the presence of an intact fibrous cap without thrombus (non-eruptive nodular calcium)5.
Optical coherence tomography (OCT) has a high resolution of 10-20 μm and is the only imaging modality to visualise features of an eruptive CN or non-eruptive nodular calcium similar to histopathological findings678. Previous OCT studies have reported that the prevalence of a CN ranged from 3.1 to 8.0%1910111213141516 and that, compared with lesions without CNs, older patient age and chronic kidney disease (CKD)915, especially haemodialysis11, as well as a greater amount of calcium and a greater hinge motion between systole and diastole11 were associated with the presence of a CN. However, these clinical, pathological, and OCT observations were all based on cross-sectional studies.
To date, there are limited data on the natural history of a new CN, the time course of CN development, and the characteristics of lesions leading to a new CN. The principal study objective was to use serial OCT to document new CN development from calcified plaque and to establish the relation between high-risk characteristics of untreated calcified plaque and new CN development.
Methods
Study design and patient population
This was a single-centre, retrospective, observational study at St. Francis Hospital (Roslyn, NY, USA) from January 2012 to December 2022. Patients who had undergone two OCT studies that imaged the same untreated calcified lesion in a native coronary artery were included (Supplementary Figure 1). CNs observed at baseline were excluded. In the case of multiple eligible calcified lesions in a single vessel, only the most proximal calcified lesion was included (1 calcified lesion per vessel). The study complied with the Declaration of Helsinki, and the institutional review board at St. Francis Hospital approved the protocol. Informed consent for retrospective record review was waived because of minimal risk. For patients without outcome records in the electronic database, a waiver of documentation of consent was approved by the institutional review board; after obtaining informed consent, these subjects were contacted by telephone to confirm subsequent outcomes.
OCT imaging acquisition
Frequency domain OCT (C7-XR, ILUMIEN, or ILUMIEN OPTIS [all Abbott]) was used. OCT was performed at the operator’s discretion, primarily to guide PCI or assess a stenosis. The OCT technique has been previously described17. In brief, the OCT catheter (Dragonfly, Dragonfly Duo, Dragonfly OPTIS, or Dragonfly OpStar [all Abbott]) was advanced distal to the lesion, contrast media was flushed through the guiding catheter at a rate of 3 to 4 mL/s, and the OCT fibre was pulled back automatically at a rate of 18 to 36 mm/s.
Quantitative coronary angiography
Quantitative and qualitative angiographic analyses were performed by experienced analysts (Y.-W. Chen and T. Tsukui), who were blinded to clinical and OCT findings, using QAngio XA (Medis Medical Imaging) and standardised methodology18. Angiographic calcification was classified as moderate (radiopacities noted only with cardiac motion) or severe (radiopacities seen without cardiac motion)19. A radiolucent mass was defined as a radiolucent filling defect in the lumen during contrast injection. The change in the angiographic angle of the lesion (Δangle) was the difference in the angle between systole and diastole using the view showing the maximum Δangle1120. Distal left main bifurcation was defined as a left main lesion that extended into the bifurcation including 5 mm segments of either the left anterior descending artery (LAD) and/or left circumflex artery ostium.
OCT definitions and analysis
All OCT images were analysed offline using proprietary OCT software (Offline Review Workstation software, version E.4.1 [Abbott]) as described previously17, and baseline (first) and follow-up (second) OCT images were compared side by side. A consensus of two experienced OCT analysts (Y. Sugizaki and M. Matsumura) was required; a third analyst (A. Maehara) was consulted if the two primary analysts disagreed. Calcium was defined as a signal-poor or heterogeneous region with a sharply delineated border. Calcium with signal attenuation, presumably representing residual lipidic plaque adjacent to or within the calcium, was defined as calcium with a sharply delineated inner border and a partially obscured outer border due to strong signal attenuation7821 excluding gradual signal attenuation due to the limited OCT penetration depth. Untreated calcified lesions without a CN at baseline OCT were identified in a non-stented segment in a native coronary artery (Supplementary Figure 2). A CN was defined as an accumulation of small calcium fragments that protruded into the lumen (Figure 1); each CN was categorised as either an eruptive CN (disruption of the fibrous cap with irregular surface) or non-eruptive nodular calcium (smooth intact fibrous cap)6.
The maximum calcium arc, the maximum and minimum calcium thicknesses at the maximum calcium arc site, and contiguous calcium length were measured. The calcium volume index was calculated as calcium length×maximum calcium arc×maximum calcium thickness. Lipidic plaque was defined as a region with strong attenuation with an overlying fibrous cap, and a lipid arc >90° was defined as a lipid-rich plaque. Thin-cap fibroatheroma was defined as a lipid-rich plaque with a fibrous cap thickness <65 μm.
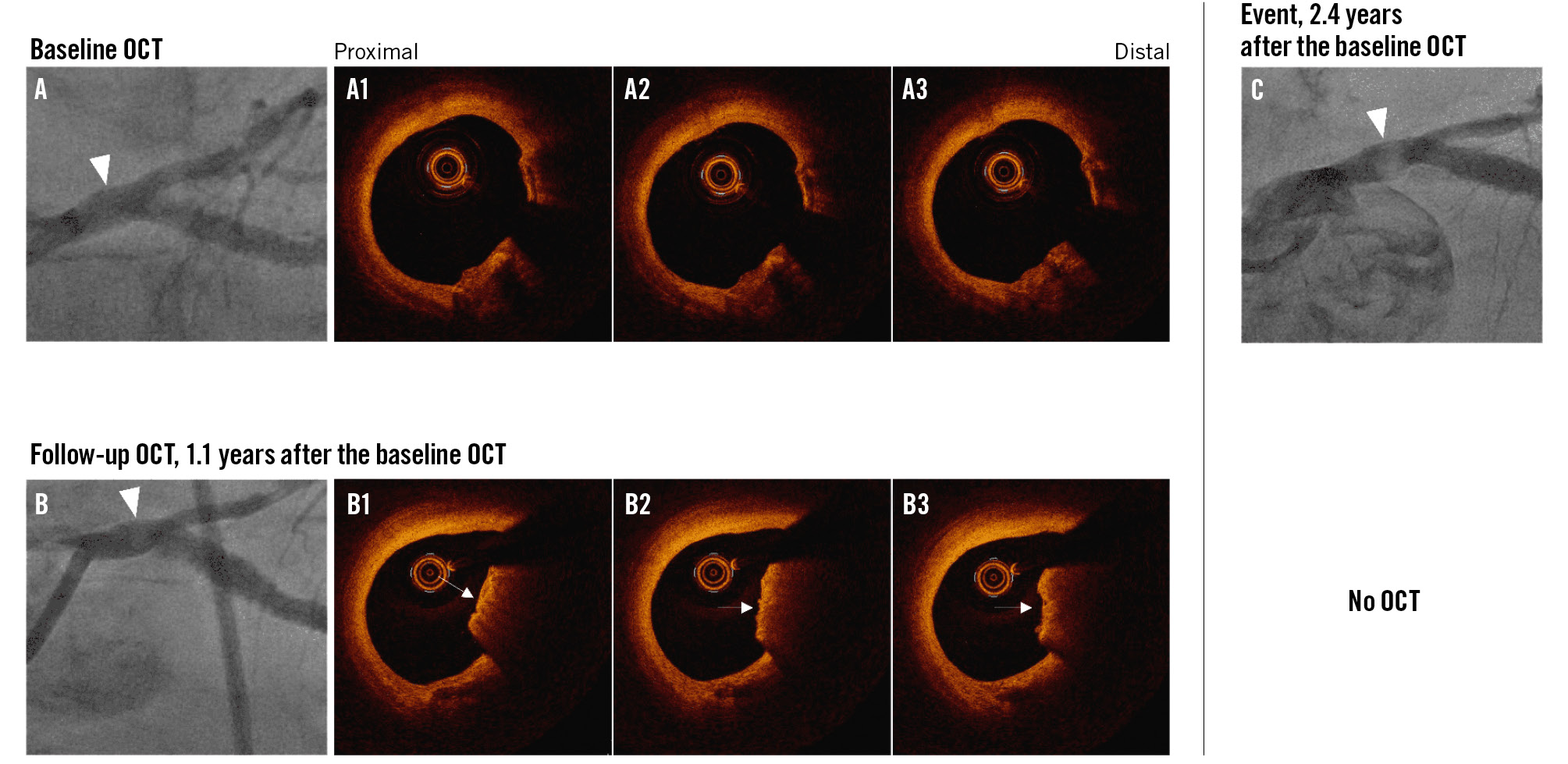
Figure 1. Representative images of new calcified nodule development. A, A1-3) An OCT-imaged untreated calcified lesion of the left main coronary artery at baseline. B, B1-3) A new non-eruptive calcified nodule in the same lesion at follow-up, 1.1 years later. C) About 2.4 years after baseline OCT, an angiographic radiolucent mass, representing a massive, calcified nodule was seen. White arrowheads indicate lesions of interest. White arrows indicate CNs. CN: calcified nodule; OCT: optical coherence tomography
Clinical endpoints
The primary clinical outcome was OCT-imaged, but untreated, calcified lesion-related major adverse cardiac events (MACE): a composite of cardiac death, myocardial infarction (MI), or clinically driven revascularisation since the baseline OCT22. Based on event angiography, events were classified as attributable to OCT-imaged untreated calcified lesions, to other lesions, or were indeterminate. Definite or probable stent thrombosis was also determined. Clinical events after the baseline OCT were confirmed by investigators based on their review of electronic medical records and/or telephone contacts.
Statistical analysis
Categorical variables are presented as frequencies and were compared using the chi-square or Fisher’s exact test. Continuous variables are presented as medians with first and third quartiles and were compared using the Mann-Whitney U test or Wilcoxon signed-rank test. Inter- and intraobserver variability for the diagnosis of calcium with attenuation were evaluated with kappa statistics using 40 randomly selected cases. Interobserver variability was assessed by two independent observers (Y. Sugizaki and M. Matsumura), and intraobserver variability was assessed with reanalysis by a single observer four weeks later. A multivariable logistic regression model was performed to evaluate the association between new CN development and clinical or morphological factors on baseline OCT. Covariates included calcium with attenuation, logarithm of calcium volume index, calcified lesion angiographic Δangle between systole and diastole, and the duration between baseline and follow-up OCT. Covariates were chosen based on their clinical and pathophysiological relevance or univariable relationship to each dependent variable. The cumulative event rates were estimated using a Kaplan-Meier curve and compared using a log-rank test. A multivariable Cox proportional hazard model was performed to evaluate the association between new CN development and the primary clinical outcome, adjusting for sex, age, diabetes mellitus, and haemodialysis. Covariates in the adjusted models were chosen based on their clinical relevance and prior reports61123. All statistical tests were 2-sided, and significance was defined as p<0.05. Statistical analyses were performed using the statistical package R, version 4.3.0 (R Foundation for Statistical Computing).
Results
Incidence of a calcified nodule
Among a total of 720 patients with baseline and follow-up OCT imaging of the same native vessel, 361 patients were excluded for the following reasons: 259 patients had no calcium in the untreated segment on baseline OCT, 36 patients were lacking OCT imaging for the same calcified lesion either at baseline or at follow-up, 34 patients had an untreated CN lesion at baseline, and 32 patients had insufficient image quality (Supplementary Figure 1). Thus, we identified 372 untreated calcified lesions in 372 vessels (1 calcified lesion per vessel) in 359 patients with baseline and follow-up OCT. The median duration between baseline and follow-up OCT was 1.5 (first and third quartiles [Q1-Q3]: 0.7-2.9) years. Among 259 patients who had no calcium in the untreated segment at baseline OCT, no new CN developed during a median interval of 1.7 (Q1-Q3: 0.9-1.8) years.
Among the 372 calcified lesions, we identified 26 calcified lesions in 26 patients with a new CN seen on the follow-up OCT, whereas there were 346 calcified lesions in 333 patients that did not develop a new CN. The incidence of a new CN was 7.0% (26/372) (Central illustration), and a new CN was most frequent in the distal left main bifurcation (Figure 2). CNs developed more often in lesions with a greater maximum calcium arc seen on baseline OCT: 14.0% (6/43) in >270°, 11.4% (5/44) in 181-270°, 9.3% (14/151) in 91-180°, and 0.7% (1/134) in ≤90° (Supplementary Figure 3A). The incidence of a new CN tended to increase after 2 years beyond baseline OCT, although there were 7 new CNs (5.0%) within the first year (Supplementary Figure 3B).
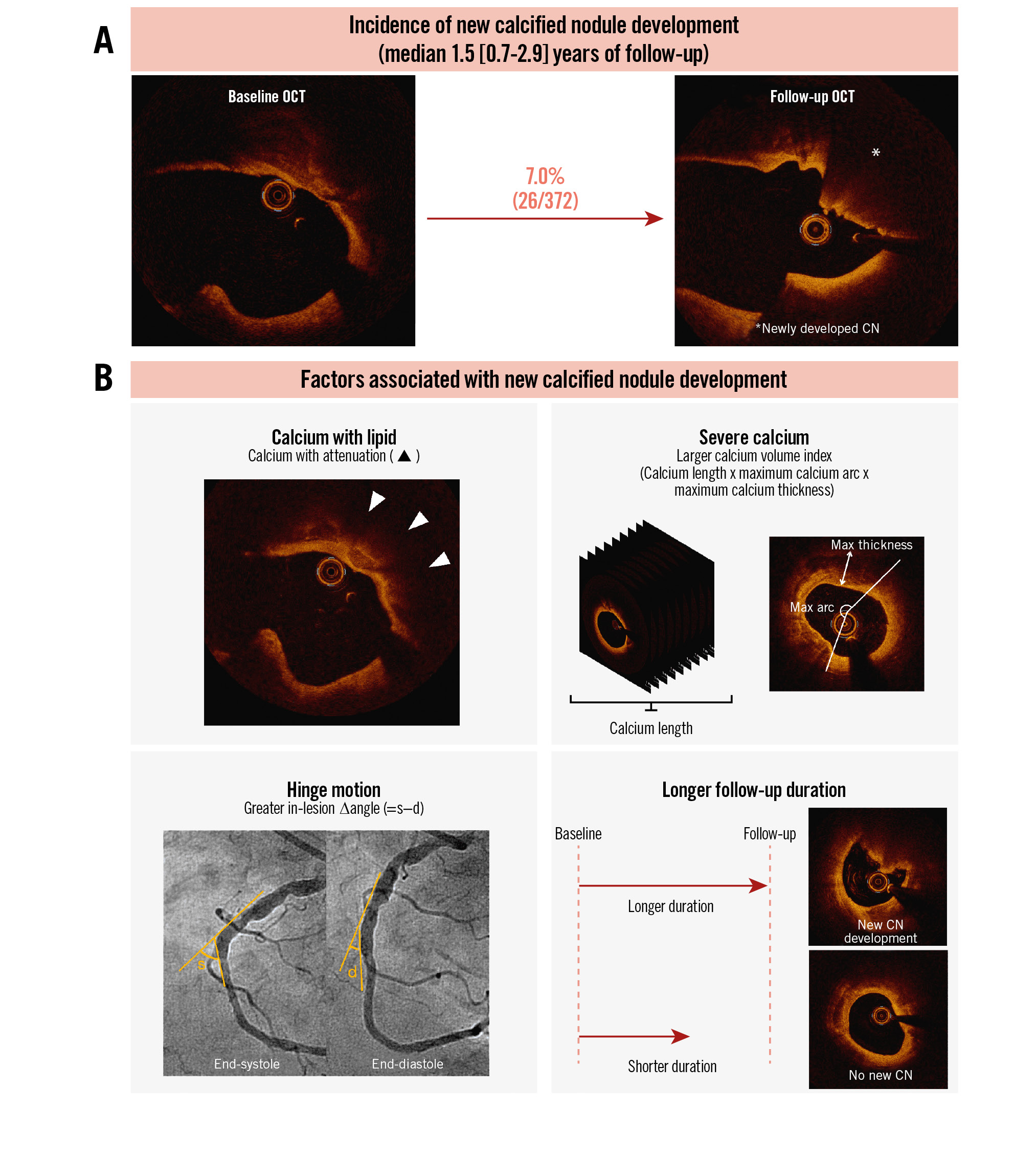
Central illustration. Natural history of new calcified nodule development and its predictors. A) The incidence of new CN development was 7.0% (26/372) per lesion with a median duration between the 2 OCTs of 1.5 (Q1-Q3: 0.7-2.9) years. B) In the multivariable logistic model, calcium with attenuation, log10 calcium volume index*, in-lesion ∆angle, and time since baseline OCT were associated with the presence of new CN development. *Calcium volume index=calcium length (mm)×maximum calcium arc (°)×maximum calcium thickness (mm). CN: calcified nodule; d: diastole; OCT: optical coherence tomography; Q: quartile; s: systole
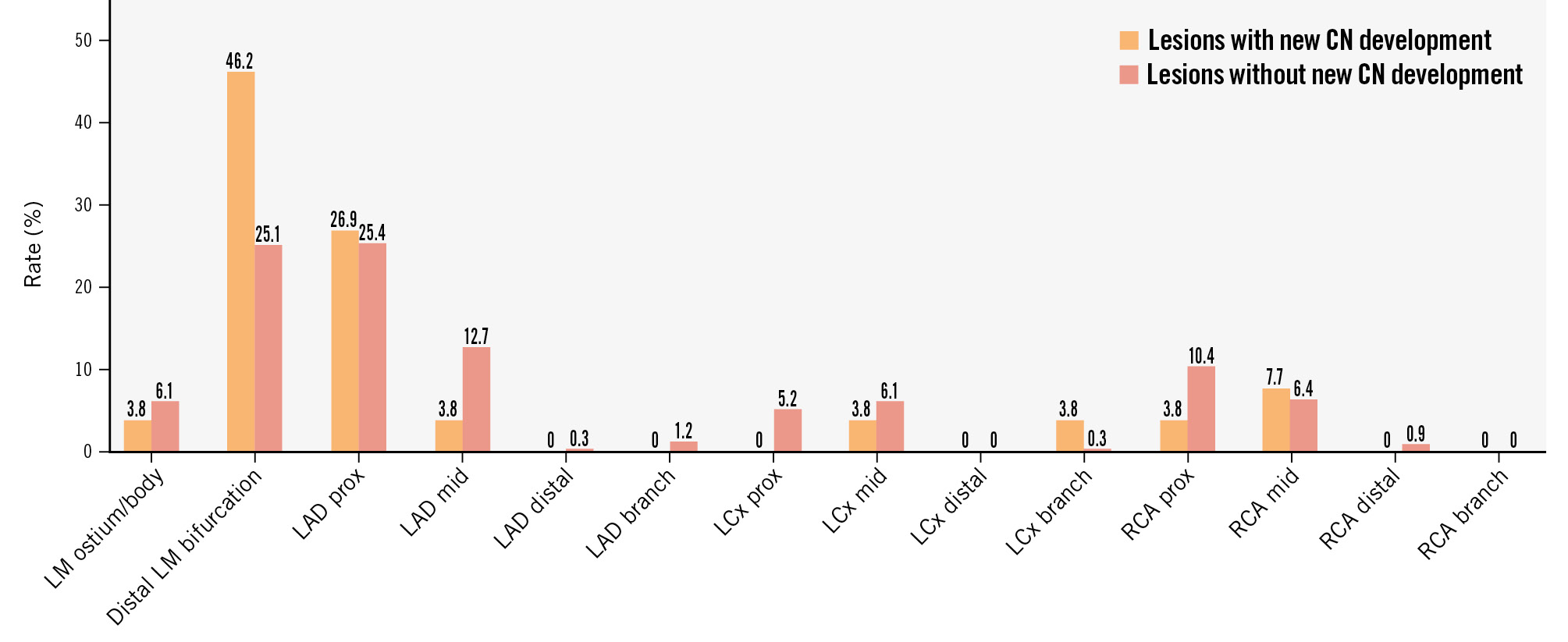
Figure 2. Distribution of calcified nodules. CN: calcified nodule; LAD: left anterior descending artery; LCx: left circumflex artery; LM: left main coronary artery; RCA: right coronary artery
Baseline clinical, angiographic, and OCT findings
Patients with new CN development were more frequently on haemodialysis than patients without new CN development (Table 1). Patients with new CN development tended to be older and to have a longer duration between baseline and follow-up OCTs than those without new CNs. Lesions that developed a new CN more often had baseline angiographic severe calcification and a greater Δangle between systole and diastole (10.1° vs 4.9°; p=0.01) than lesions that did not develop a new CN (Table 2, Supplementary Table 1, Supplementary Table 2).
Compared with lesions without new CN development, lesions that developed a new CN had greater baseline OCT calcium (longer calcium length [14.0 mm vs 6.9 mm; p<0.001] and a larger maximum calcium arc [157° vs 105°; p=0.001]), a higher prevalence of calcium with attenuation (46.2% vs 11.6%; p<0.001), and a greater increase in OCT calcium length, maximum calcium arc, and maximum calcium thickness from baseline to follow-up (Supplementary Figure 4).
Among 26 lesions with a new CN, there were 12 (46%) eruptive CNs, and 14 (54%) were classified as non-eruptive nodular calcium. Six eruptive CNs had thrombi, but no non-eruptive nodular calcium had thrombus (50.0% vs 0.0%; p=0.03). Lesions with new non-eruptive nodular calcium tended to have a larger maximum calcium arc on baseline OCT (Supplementary Table 3), compared with those that developed eruptive CNs.
Table 1. Baseline and follow-up patient characteristics.
| New CN at follow-up (26 patients) |
No new CN at follow-up (333 patients) |
p-value | |
|---|---|---|---|
| Age, years | 73 (59-79) | 68 (59-76) | 0.07 |
| Male | 76.9 (20) | 78.4 (261) | 1 |
| Diabetes mellitus | 42.3 (11) | 39.3 (131) | 0.97 |
| Hypertension | 92.3 (24) | 85.6 (285) | 0.51 |
| Dyslipidaemia | 96.2 (25) | 91.6 (305) | 0.65 |
| Current smoker | 7.7 (2) | 8.7 (29) | 1 |
| Chronic kidney disease* | 34.6 (9) | 23.4 (78) | 0.26 |
| Haemodialysis | 11.5 (3) | 2.1 (7) | 0.02 |
| Prior percutaneous coronary intervention | 69.2 (18) | 57.1 (190) | 0.32 |
| Prior coronary bypass grafting | 23.1 (6) | 17.7 (59) | 0.68 |
| Prior myocardial infarction | 34.6 (9) | 28.8 (96) | 0.69 |
| Medication at discharge from the baseline procedure | |||
| Statin | 80.8 (21) | 87.1 (290) | 0.54 |
| Aspirin | 92.3 (24) | 89.5 (298) | 0.9 |
| P2Y12 inhibitor | 100 (26) | 89.8 (299) | 0.17 |
| Anticoagulant | 15.4 (4) | 8.7 (30) | 0.43 |
| Medication at admission to the follow-up procedure | |||
| Statin | 84.6 (22) | 89.2 (297) | 0.7 |
| Aspirin | 88.5 (23) | 85.0 (283) | 0.68 |
| P2Y12 inhibitor | 42.3 (11) | 39.0 (130) | 0.75 |
| Anticoagulant | 23.1 (6) | 12.0 (40) | 0.19 |
| Clinical presentation at lesion level | 26 lesions | 346 lesions | |
| Duration between baseline and follow-up OCT, years | 2.6 (1.0-3.2) | 1.5 (0.7-2.8) | 0.09 |
| Clinical presentation at baseline procedure | 0.88 | ||
| STEMI | 0 (0) | 0.6 (2) | |
| NSTEMI | 3.8 (1) | 7.8 (27) | |
| Unstable angina | 38.5 (10) | 37.9 (131) | |
| Stable coronary artery disease | 57.7 (15) | 53.8 (186) | |
| Clinical presentation at follow-up procedure | 0.22 | ||
| STEMI | 3.8 (1) | 1.2 (4) | |
| NSTEMI | 15.4 (4) | 6.6 (23) | |
| Unstable angina | 30.8 (8) | 40.2 (139) | |
| Stable coronary artery disease | 50.0 (13) | 52.0 (180) | |
| Values are median (Q1-Q3) or % (n). *Chronic kidney disease was defined as glomerular filtration rate <60 mL/min/1.73 m2 using the Modification of Diet in Renal Disease equation. CN: calcified nodule; NSTEMI: non-ST-elevation myocardial infarction; OCT: optical coherence tomography; Q: quartile; STEMI: ST-elevation myocardial infarction | |||
Table 2. Angiographic and OCT findings at baseline OCT.
| New CN at follow-up (26 lesions) |
No new CN at follow-up (346 lesions) |
p-value | |
|---|---|---|---|
| Angiographic findings | |||
| Calcium | 73.1 (19) | 43.3 (150) | 0.001 |
| Moderate | 26.9 (7) | 25.7 (89) | |
| Severe | 46.2 (12) | 17.6 (61) | |
| Radiolucent mass | 0 (0) | 0 (0) | NA |
| ∆angle of lesion, ° | 10.1 (6.5-13.1) | 4.9 (2.0-10.0) | 0.01 |
| OCT findings | |||
| Minimum lumen area, mm2 | 5.10 (4.05-7.71) | 5.44 (4.04-7.30) | 0.92 |
| Calcium length, mm | 14.0 (8.5-20.6) | 6.9 (3.5-11.3) | <0.001 |
| Maximum calcium arc, ° | 157 (106-254) | 105 (73-166) | 0.001 |
| Maximum calcium thickness, mm | 0.92 (0.67-1.10) | 0.90 (0.69-1.09) | 0.93 |
| Minimum calcium thickness, mm | 0.37 (0.23-0.46) | 0.43 (0.27-0.59) | 0.29 |
| Calcium volume index, mm×mm×° | 2,127 (764-5,310) | 650 (218-1,846) | 0.03 |
| Calcium with attenuation | 46.2 (12) | 11.6 (40) | <0.001 |
| Lipidic plaque | 38.5 (10) | 27.7 (96) | 0.35 |
| Thin-cap fibroatheroma | 7.7 (2) | 5.2 (18) | 0.93 |
| Lipid-rich plaque (lipid arc >90°) | 11.5 (3) | 15.0 (52) | 0.84 |
| Maximum lipidic arc, ° | 81 (74-115) | 94 (81-129) | 0.46 |
| Values are median (Q1-Q3) or % (n). CN: calcified nodule; NA: not applicable; OCT: optical coherence tomography | |||
Relation between high-risk characteristics of untreated calcified plaque and new CN development
In the multivariable logistic regression model, calcium with attenuation (odds ratio [OR] 3.38, 95% confidence interval [CI]: 1.15-9.98; p=0.03); log10 calcium volume index (OR 2.76, 95% CI: 1.10-6.95; p=0.03); angiographic Δangle, per 10° (OR 2.30, 95% CI: 1.25-4.22; p=0.01); and time since baseline OCT, per year (OR 1.36, 95% CI: 1.05-1.75; p=0.02) were significantly associated with new CN development (Table 3, Supplementary Table 4). There was excellent interobserver and intraobserver agreement for the diagnosis of calcium with attenuation (κ=0.95 and 0.90, respectively).
Table 3. Factors associated with new calcified nodule development in the multivariable logistic regression model.
| Odds ratio (95% CI) | p-value | |
|---|---|---|
| Calcium with attenuation | 3.38 (1.15-9.98) | 0.03 |
| Log10 calcium volume index* | 2.76 (1.10-6.95) | 0.03 |
| ∆angle of lesion, per 10° | 2.30 (1.25-4.22) | 0.01 |
| Time since baseline OCT, years | 1.36 (1.05-1.75) | 0.02 |
| *Calcium volume index=calcium length (mm)×maximum calcium arc (°)×maximum calcium thickness (mm). Covariates were chosen based on clinical and pathophysiological relevance or univariable relationship to each dependent variable (Supplementary Table 4). CI: confidence interval; OCT: optical coherence tomography | ||
Clinical outcomes
Follow-up after baseline OCT was 5.0 (Q1-Q3: 3.3-6.8) years. The cumulative rate of OCT-imaged, but untreated, calcified lesion-related MACE was 16.4% (45 events) (Table 4). There were 45 clinically driven revascularisations, of which 6 were for an MI. There were no cardiac deaths that were clearly associated with an OCT-imaged, but untreated, calcified lesion. There were no episodes of definite or probable stent thrombosis.
Out of a total of 372 calcified lesions, the MACE rate for lesions that developed a new CN was higher than for the lesions that did not develop a new CN (29.3% vs 15.3%; p=0.04) (Figure 3). The multivariable Cox proportional hazard models demonstrated that new CN development was associated with untreated calcified lesion-related MACE (hazard ratio 2.03, 95% CI: 1.07-3.85; p=0.04) (Supplementary Table 5). At the follow-up procedure, 38 calcified lesions were revascularised. The MACE rate at the time of the follow-up OCT tended to be higher for lesions with new CN development compared with lesions without new CN development (22.1% vs 13.9%; p=0.18). Out of 334 untreated calcified lesions at follow-up, the subsequent MACE rate beyond the follow-up OCT was also higher for lesions with new CN development compared with lesions without new CN development (9.1% vs 1.7%; p=0.04) (Supplementary Figure 5).
Among 26 newly developed CNs in 26 patients, 5 CNs were revascularised at the time of the follow-up OCT; and an additional 2 new CNs were revascularised after the follow-up OCT (details are shown in Supplementary Table 6). Eruptive CNs had more events compared with non-eruptive nodular calcium (41.7% [5/12] vs 14.3% [2/14]), and CNs with thrombus had more events compared with CNs without thrombus (83.3% [5/6] vs 10.0% [2/20]), although this was statistically inconclusive.
Table 4. Clinical outcome after baseline OCT.
| Lesions with a new CN (26 lesions) |
Lesions without a new CN (346 lesions) |
p-value | |
|---|---|---|---|
| OCT-imaged untreated calcified lesion-related MACE | 29.3 (7) | 15.3 (38) | 0.04 |
| Clinically driven revascularisation | 29.3 (7) | 15.3 (38) | 0.04 |
| Myocardial infarction | 8.3 (2) | 1.5 (4) | 0.02 |
| Indeterminant MACE | 14.6 (3) | 3.4 (4) | 0.001 |
| Cardiac death | 14.6 (3) | 3.4 (4) | 0.001 |
| Data are presented as Kaplan-Meier estimates (number of events). CN: calcified nodule; MACE: major adverse cardiac events; OCT: optical coherence tomography | |||
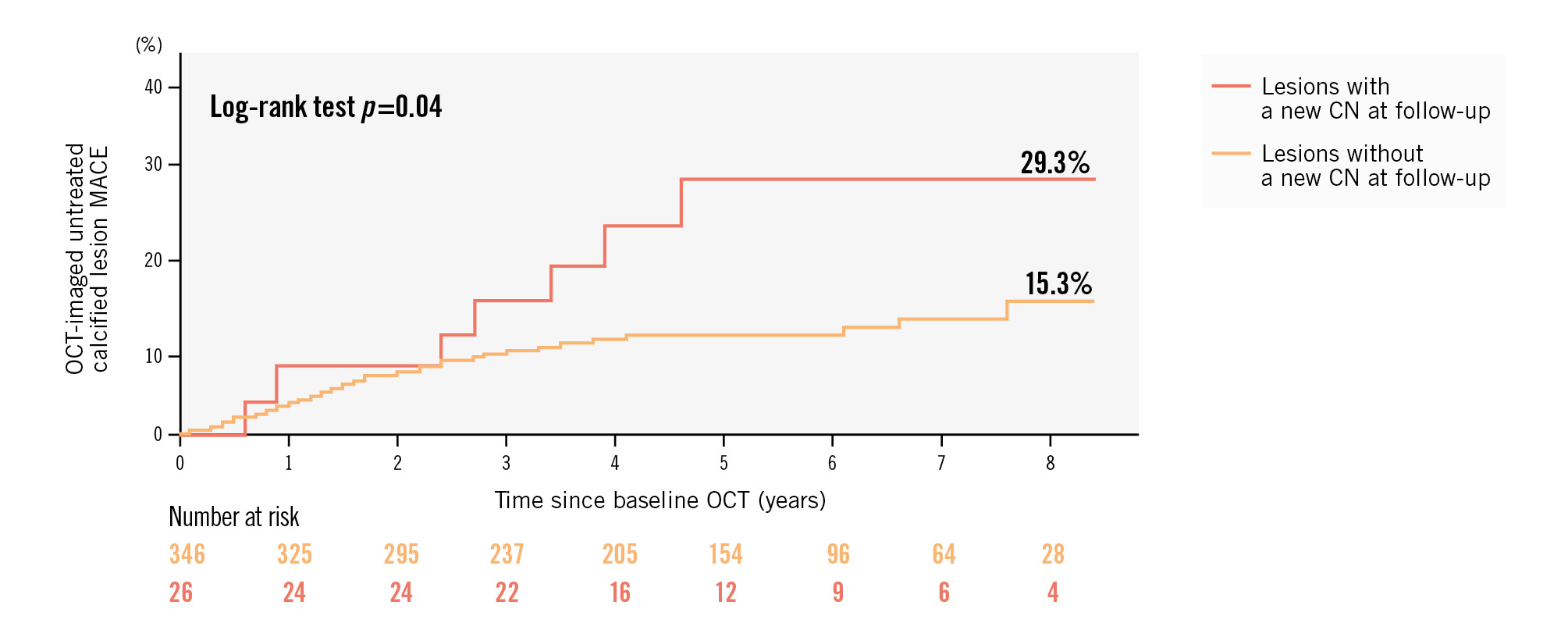
Figure 3. Kaplan-Meier curves for OCT-imaged untreated calcified lesion-related MACE after baseline OCT in patients with new CN development versus those without new CN development. CN: calcified nodule; MACE: major adverse cardiac events; OCT: optical coherence tomography
Discussion
This serial OCT investigation is the first to report the natural history of a new CN – from its development within an untreated calcified lesion to subsequent cardiac events. The major findings were as follows: (1) the incidence of a new CN was 7.0% within a previously untreated calcified lesion; (2) greater amounts of calcium (length, arc, and thickness), calcium with attenuation (indicating calcium with residual lipid), greater hinge motion in the calcified lesion at baseline, and longer follow-up duration were associated with a new CN; and (3) the development of a new CN was associated with worse clinical outcomes.
New calcified nodule development
A CN has been reported by pathologists in 2.5-7.0% of patients with sudden cardiac death2425. An OCT multicentre registry, including 702 ACS patients treated with OCT-guided PCI, demonstrated a similar prevalence of CNs (4.0%) as an underlying cause of thrombotic events15. Similarly, multiple OCT studies have shown a prevalence of CNs ranging from 3.1-8.0% in ACS patients19101112131415, and even higher (~30%) when only lesions with a large amount of calcium (>270°) were included11. In the current report, in which we observed a 7.0% incidence of a new CN, half of the patients presented with ACS. Thus, our findings indicated that there were high-risk calcified lesions that developed calcified nodules and that these calcified lesions were not stable.
High-risk untreated calcified plaque associated with new CN development
CNs have been found more frequently in older patients or those with CKD915, especially those receiving haemodialysis11. Additionally, Lee et al showed that angiographic hinge motion of the lesion and maximum calcium arc were associated with the presence of a culprit lesion CN11. Although the detailed mechanisms by which haemodialysis affects new CN development are still unclear, a larger amount of coronary calcium in renal dysfunction, especially haemodialysis26, may contribute to developing a new CN. Thus, our findings in the current serial OCT study were consistent with previous cross-sectional OCT studies.
In addition, we found that calcium with attenuation was associated with new CN development. Although the mechanism of CN development has been debated, one possibility suggested by pathologists is that small calcium deposits are formed by the fragmentation of necrotic core calcification (necrotic core adjacent to or within the calcium) within a severely calcified lesion coupled with external mechanical forces due to the cyclic hinge movement of the coronary artery5. Necrotic core calcification seems to be weak against external forces owing to the absence of underlying collagen, whereas flanking calcium sheets with a collagen matrix have greater strength. As a result, CNs tend to arise from necrotic core calcification rather than from collagen-rich calcification. Our findings seemed to support this hypothesis. A near-infrared spectroscopy/OCT study of de novo culprit lesions in patients with acute MI reported that the maximum lipid core burden index in 4 mm (maxLCBI4mm) was largest in OCT-defined plaque rupture (705 [interquartile range {IQR} 545 to 854]), followed by OCT-defined CN (355 [IQR 303 to 478]) and finally OCT-defined plaque erosion (300 [IQR 126 to 357]; p<0.001)27, also supporting the hypothesis that CN lesions have residual lipid components.
Previous ex vivo comparisons of histopathological and OCT findings reported that calcified plaque with strong attenuation had residual lipidic components that were adjacent to or within the calcium and that 45% of superficial dense calcium had an obscure outer border of calcification that contained a residual necrotic core78. A new CN develops more frequently in calcium with attenuation, presumably representing necrotic core calcification, rather than in calcium without attenuation. This is supported by a pathological report5.
Clinical implications
Lipid-rich plaques are strongly associated with future cardiac events28, whereas high-density matured calcified lesions are associated with a lower risk of future cardiac events29. In general, rupture-prone lipid-rich plaques evolve into stable calcified plaque30. However, CNs are also associated with future cardiac events in both stented1223 and untreated lesions16. The subanalysis in the CLIMA Study of untreated proximal LADs analysed by OCT reported that the presence of eruptive CNs was associated with future cardiac events16. In the current study, untreated calcified lesions with large amounts of calcium with strong signal attenuation at a hinge motion location evolved into a CN resulting in clinical events. Taken together, there is a subset of high-risk calcified lesions that can develop into a CN; it is important to recognise that these calcified lesions may not be stable.
Limitations
This was a single-centre retrospective study. The duration between index and follow-up OCTs was relatively short, and the occurrence of a CN was low (26 newly developed CNs). Our study did not perform 3-vessel OCT evaluation. The indication of OCT and its timing were per clinical discretion. Not all patients with calcified lesions at the index OCT underwent follow-up OCT. Not all CNs were linked to clinical events, resulting in a small number of events due to lesions with a new CN development. However, the clinical events rate associated with a newly developed CN were not trivial even when compared with known high-risk plaques. The lesion-level event rate from untreated high-risk plaque was 10.4% at 1 year (19 events from 183 OCT-detected thin-cap fibroatheroma [TCFA] of untreated proximal LAD lesions) in CLIMA31; 3.9% at 4 years (20 events from 515 OCT-detected TCFAs) in a single-centre study from China32; 4.4% at a median of 3.4 years (26 events from 596 virtual histology TCFAs) in PROSPECT33; and 3.5% at a median of 3.7 years (30 events from 851 near-infrared spectroscopy-defined lipid-rich plaques) in PROSPECT II28. Furthermore, the interpretation of calcified nodule composition using OCT is supported by limited pathology data.
Conclusions
Untreated lesions with a large amount of calcium and strong OCT signal attenuation with a larger hinge motion movement and longer follow-up were associated with subsequent development of a new CN, and the development of a new CN was associated with worse clinical outcomes.
Impact on daily practice
In the natural history of calcified nodules (CNs) in our study, a new CN developed in 7.0% of untreated calcified lesions. Compared with lesions without new CNs, a larger amount of calcium, larger systolic/diastolic in-lesion angiographic hinge motion, longer follow-up duration and calcified lesions with residual lipidic plaque were associated with the formation of new CNs, resulting in future cardiac events. Taken together, there is a subset of high-risk calcified lesions that can develop into a CN, and it is important to recognise that these calcified lesions may not be stable.
Conflict of interest statement
Y. Sugizaki - grant for research abroad from Daiichi Sankyo Foundation of Life Science; consultant for Boston Scientific. M. Matsumura - consultant for Terumo and Boston Scientific. E. Shlofmitz - consultant for Abbott, ACIST Medical, Amgen, Boston Scientific, Janssen Pharmaceuticals, Novo Nordisk, Philips, Shockwave Medical, and Terumo. J.W. Moses - equity in Orchestra Biomed and Xenter; and consultant for Medtronic. D.J. Cohen - research grant support from Boston Scientific, Abbott, Philips, and CathWorks; and consultant for Medtronic and Abbott. G.S. Mintz - honoraria from Boston Scientific, Abbott, SpectraWAVE, and Gentuity. A. Jeremias - consultant for Philips, Abbott, ACIST Medical, CathWorks, and Shockwave Medical. Z.A. Ali - institutional grant support for Abbott, Abiomed, ACIST Medical, Amgen, Boston Scientific, CathWorks, Canon, Conavi, HeartFlow, Inari, Medtronic, National Institute of Health, Nipro, Opsens Medical, Medis, Philips, Shockwave Medical, Siemens, SpectraWAVE, and Teleflex; consulting fees from Abiomed, AstraZeneca, Boston Scientific, CathWorks, Opsens, Philips, and Shockwave Medical; and equity in Elucid, Lifelink, SpectraWAVE, Shockwave Medical, and VitalConnect. A. Maehara - consultant for Boston Scientific, Philips, and SpectraWAVE; and speaker honoraria from Nipro. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

