Cory:
Unlock Your AI Assistant Now!
Abstract
Background: In patients with diabetes mellitus (DM) and high bleeding risk (HBR) undergoing percutaneous coronary intervention (PCI), the optimal duration of dual antiplatelet therapy (DAPT) remains uncertain.
Aims: We sought to compare early DAPT discontinuation in DM and non-DM patients enrolled in the prospective XIENCE Short DAPT programme.
Methods: The effects of 1- versus 3-month DAPT on ischaemic and bleeding outcomes were compared using propensity score stratification. The primary endpoint was a composite of all-cause death or myocardial infarction (MI) at 1 year. The incidence of Bleeding Academic Research Consortium (BARC) Type 2 to 5 bleeding was the key secondary endpoint.
Results: Out of 3,352 included patients, 1,299 (38.8%) had DM; diabetic patients had a higher 1-year incidence of death or MI (DM vs non-DM: 10.1% vs 6.6%) and similar BARC 2-5 bleeding (DM vs non-DM: 9.5% vs 9.2%). With 1- versus 3-month DAPT, the incidence of death or MI did not statistically differ in DM patients (adjusted hazard ratio [adjHR] 0.70, 95% confidence interval [CI]: 0.47-1.05) and non-DM patients (adjHR 1.26, 95% CI: 0.87-1.81), although heterogeneity by DM status was evident (p for interaction=0.015). BARC 2-5 bleeding was numerically lower with 1-month DAPT in both groups (DM: adjHR 0.67, 95% CI: 0.45-1.01; non-DM: adjHR 0.78, 95% CI: 0.56-1.07; p for interaction=0.973).
Conclusions: Among HBR patients with DM undergoing PCI, 1-month DAPT, as compared to 3-month DAPT, was not associated with an excess of fatal or non-fatal MI and even reduced the occurrence of bleeding. These findings should be interpreted in the context of a predominantly stable patient population with low procedural complexity and may not be generalisable to higher-risk cases.
Bleeding and ischaemic events after percutaneous coronary intervention (PCI) are associated with substantial morbidity and mortality12. A course of 6-12 months of dual antiplatelet therapy (DAPT) with aspirin and a P2Y12 inhibitor is the default strategy to prevent thrombotic complications after coronary stenting3. However, DAPT is encumbered by a considerable risk of bleeding, particularly in patients with clinical conditions predisposing to high bleeding risk (HBR)4. Increasing awareness of the negative prognostic impact of bleeding and technology refinements with new-generation drug-eluting stents (DES) have allowed a progressive shortening of DAPT duration, without compromising safety567.
Diabetes mellitus (DM) is an established cardiovascular risk factor that affects more than one-third of patients undergoing PCI8. Patients with DM are at higher risk for ischaemic events and are often considered for longer and more potent antithrombotic therapy after PCI9101112. However, the optimal strategy in patients with both DM and HBR status remains unclear13.
In the XIENCE Short DAPT programme, a 1-month DAPT regimen followed by aspirin resulted in fewer bleeding complications than a 3-month regimen, without increasing ischaemic risk in HBR patients undergoing PCI6. Therefore, we aimed to evaluate the efficacy and safety of 1- versus 3-month DAPT in DM and non-DM patients with HBR undergoing PCI.
Methods
Study design
The rationale, design and principal results of the XIENCE Short DAPT programme have been previously reported1415. In brief, the programme consisted of three international, open-label, prospective, single-arm studies: the XIENCE 28 USA Study (ClinicalTrials.gov: NCT03815175) and XIENCE 28 Global Study (NCT03355742) testing 1-month DAPT, and the XIENCE 90 study (NCT03218787) testing 3-month DAPT. The two short DAPT regimens were tested in high bleeding risk patients undergoing PCI with the cobalt-chromium everolimus-eluting XIENCE stent (Abbott). Patients presenting with chronic coronary syndrome (CCS) or non-ST-segment elevation acute coronary syndrome (NSTE-ACS) were eligible for inclusion if they had up to 3 target lesions, with a maximum of 2 target lesions per epicardial vessel, excluding lesions located in the left main artery, grafts, or restenosis sites. The clinical programme was funded by Abbott, which designed the protocol together with the principal investigators and executive and steering committee members. The study protocol was approved by institutional review boards or ethics committees at each site. Enrolled patients signed written informed consent, and an independent data safety monitoring board ensured safety.
Study population and treatment
We designed this analysis to investigate the effect of 1- versus 3-month DAPT in diabetic and non-diabetic patients15. Key inclusion and exclusion criteria are reported in Supplementary Table 1 and are the same as in the original studies. As per the XIENCE Short DAPT protocol, patients met HBR criteria if at least one of the following criteria was present: age ≥75 years, chronic therapy (>6 months) with anticoagulants, prior (within 12 months) history of major bleeding, prior stroke (ischaemic or haemorrhagic), anaemia (haemoglobin <11 g/dL), renal insufficiency (creatinine ≥2.0 mg/dL or dialysis), or systemic disease associated with higher bleeding risk (e.g., thrombocytopaenia or coagulation disorders). After the index PCI, patients received DAPT consisting of aspirin and a P2Y12 inhibitor. Patients were assessed for DAPT discontinuation at 1- and at 3-month follow-up in the XIENCE 28 studies and the XIENCE 90 study, respectively. P2Y12 inhibitor was discontinued in patients who were adherent to DAPT and did not experience myocardial infarction (MI), repeat revascularisation, stroke, or stent thrombosis. Follow-up occurred up to 12 months. For this analysis, the 1-month eligibility of patients from the XIENCE 90 study was retrospectively evaluated in order to match the design of the XIENCE 28 studies.
Clinical endpoints
The primary endpoint was the composite of all-cause death or MI between 1 and 12 months after the index PCI. Bleeding Academic Research Consortium (BARC) Type 2 to 5 bleeding was the key secondary endpoint. Other endpoints included net adverse clinical events (NACE), defined as the composite of death, MI, BARC Type 3 to 5 bleeding, and stroke; target lesion failure (TLF), defined as the composite of cardiovascular death, target vessel MI, or clinically indicated target lesion revascularisation (TLR); target vessel revascularisation (TVR); definite or probable stent thrombosis; and the individual components of the composite outcomes. Outcomes were adjudicated by an independent clinical events committee. MI and stent thrombosis were defined according to the Academic Research Consortium 2 definitions16. The full endpoint definitions are provided in Supplementary Table 2.
Statistical analysis
Continuous variables are presented as means with standard deviations, while categorical variables are presented as frequencies and percentages. Comparisons between groups for continuous variables were performed using the Student’s t-test, and comparisons between groups for categorical variables were performed using the chi-square test. Survival analysis was conducted using the Kaplan-Meier method, with comparisons between groups made using the log-rank test to assess the time to the first event. Cox proportional hazard models were applied to compare the unadjusted risks for the primary and secondary outcomes. Adjusted risks were derived using propensity score (PS) stratification into quintiles, consistent with the design of the main study14. For PS building, in case of missing data, multiple imputation with the Markov Chain Monte Carlo method and Rubin’s combination rule was used. The stratification weight was determined by the proportion of the sample size of each stratum and the overall sample size for both groups. All analyses were conducted using R software, version 3.6.2 (R Foundation for Statistical Computing) or SAS software, version 9.4 (SAS Institute).
Results
Population characteristics
Between July 2017 and February 2020, a total of 3,652 HBR patients were enrolled in the XIENCE Short DAPT programme. For the present analysis, the final cohort included in the study consisted of 3,352 patients, of whom 1,299 (38.8%) had DM (Figure 1). The baseline and procedural characteristics of patients, stratified according to diabetic status, are reported in Supplementary Table 3 and Supplementary Table 4. Diabetic patients were younger than non-diabetic patients but with more comorbidities; there were no differences between the groups in terms of clinical presentation, with most of the patients presenting with CCS (64.5% vs 65.9%; p=0.426) and up to one-third with NSTE-ACS (35.5% vs 34.1%; p=0.426). Among HBR criteria, anaemia and renal insufficiency were more frequently observed in the diabetic group. PCI complexity was generally low, with less than 6% receiving long stenting or more than 3-lesion, 3-stent, or 3-vessel PCI, with no difference between the groups.
When stratified according to DAPT duration, a total of 1,382 patients (41.2%) receiving 1-month DAPT and 1,970 patients (58.8%) receiving 3-month DAPT were included; of these, 512 and 787 were in the diabetic group, and 870 and 1,183 were in the non-diabetic group, respectively. The baseline clinical and procedural characteristics of patients with and without DM stratified according to the DAPT regimen are reported in Table 1 and Table 2, respectively. Of note, patients receiving 1-month DAPT were more likely to present with non-ST-segment elevation myocardial infarction in both the DM (17.4% vs 7.5%; p<0.001) and non-DM (17.98% vs 6.69%; p<0.001) strata. Regarding the antithrombotic regimen, clopidogrel was the most common P2Y12 inhibitor prescribed at discharge (>80%) across all groups (Table 2). DAPT was continued after the protocol-mandated time in 3.1% and 14.5% of patients in the 1- and 3-month DAPT groups, respectively, without differences according to diabetes status. In the latter group, 70.0% of patients completed 3-month DAPT, 14.5% were still on DAPT at 6 months, and 12.9% were on DAPT at 12-month follow-up. Within 90 days of follow-up, DAPT discontinuation occurred more frequently in non-diabetic patients on 3-month DAPT (Figure 2).
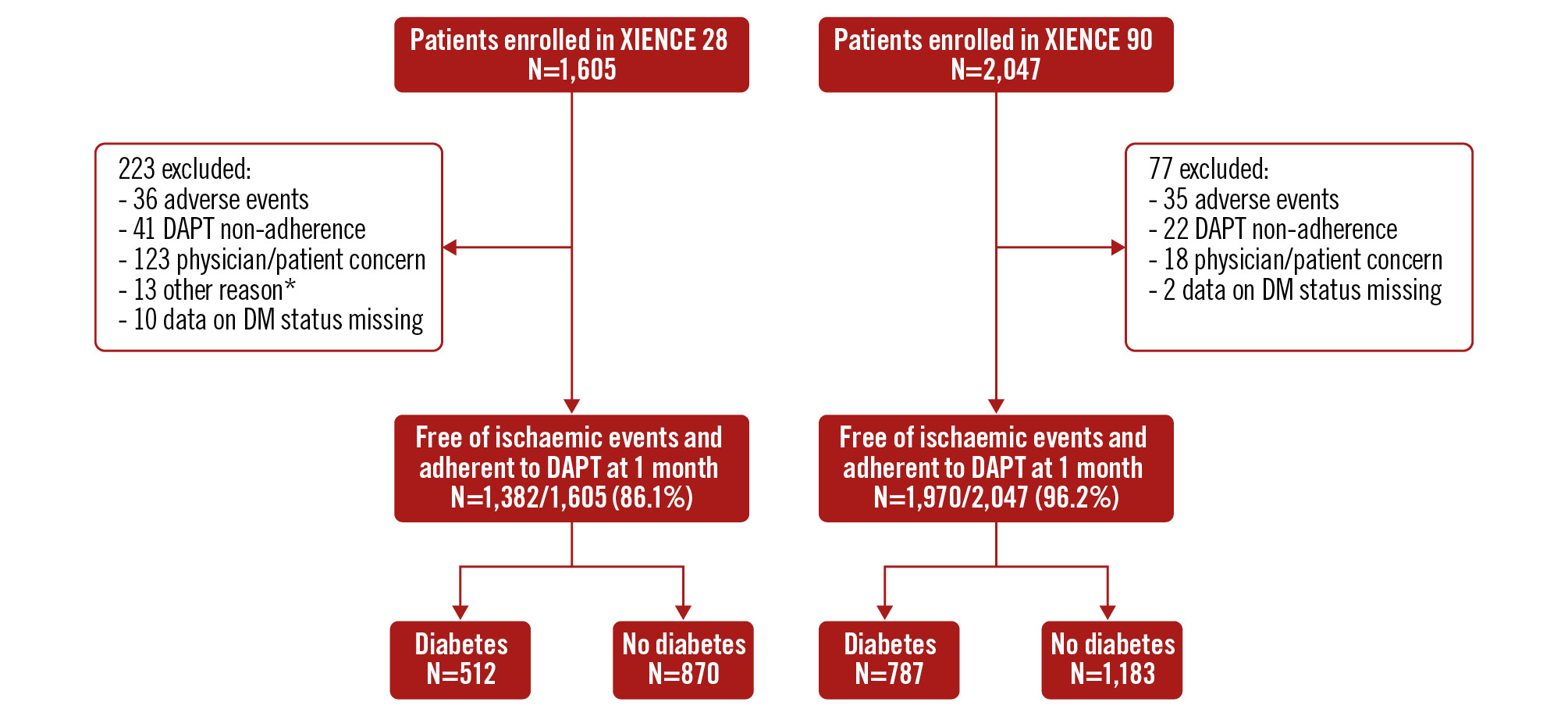
Figure 1. Patient flow diagram. *1 duplicate subject enrolment, 12 missed the 1-month visit. DAPT: dual antiplatelet therapy; DM: diabetes mellitus
Table 1. Baseline clinical characteristics in patients with and without diabetes, stratified according to DAPT regimen.
| Patients with DM (n=1,299) | Patients without DM (n=2,053) | |||||
|---|---|---|---|---|---|---|
| XIENCE 281-month DAPT (n=512) | XIENCE 903-month DAPT (n=787) | p-value | XIENCE 281-month DAPT (n=870) | XIENCE 903-month DAPT (n=1,183) | p-value | |
| Clinical characteristics | ||||||
| Age, years | 74.2±9.0 | 72.8±9.8 | 0.011 | 77.0±7.8 | 76.6±8.8 | 0.236 |
| Female sex | 171 (33.4) | 264 (33.5) | 0.956 | 279 (32.1) | 436 (36.9) | 0.024 |
| Race | ||||||
| White | 280 (76.3) | 651 (82.7) | 0.01 | 522 (87.6) | 1,086 (91.8) | 0.004 |
| Asian | 68 (18.5) | 29 (3.7) | <0.001 | 55 (9.2) | 16 (1.4) | <0.001 |
| Hispanic or Latino ethnicity | 49 (10.1) | 29 (3.7) | <0.001 | 88 (10.7) | 27 (2.3) | <0.001 |
| Black or African American | 17 (4.6) | 71 (9.0) | 0.009 | 19 (3.2) | 46 (3.9) | 0.457 |
| Hypertension | 460 (89.8) | 745 (94.7) | 0.001 | 712 (81.8) | 1,025 (86.6) | 0.003 |
| Dyslipidaemia | 376 (73.4) | 688 (87.4) | <0.001 | 558 (64.1) | 932 (78.8) | <0.001 |
| Chronic kidney disease | 238 (48.0) | 311 (39.8) | 0.004 | 389 (47.2) | 489 (41.7) | 0.016 |
| Prior PCI | 163 (31.8) | 257 (32.7) | 0.758 | 225 (25.9) | 350 (29.6) | 0.063 |
| Prior CABG | 51 (10.0) | 115 (14.6) | 0.014 | 61 (7.0) | 131 (11.1) | 0.002 |
| Chronic coronary syndrome | 333 (65.0) | 505 (64.2) | 0.748 | 576 (66.2) | 776 (65.6) | 0.773 |
| Acute coronary syndrome | 179 (35.0) | 282 (35.8) | 0.748 | 294 (33.8) | 407 (34.4) | 0.773 |
| NSTEMI | 89 (17.4) | 59 (7.5) | <0.001 | 155 (17.8) | 82 (6.9) | <0.001 |
| Unstable angina | 90 (17.6) | 223 (28.3) | <0.001 | 139 (16.0) | 325 (27.5) | <0.001 |
| PARIS bleeding score | 6.3±2.2 | 6.0±2.4 | 0.038 | 6.0±2.3 | 6.0±2.3 | 0.814 |
| PRECISE-DAPT score | 27.8±12.3 | 26.3±12.4 | 0.038 | 27.6±10.6 | 26.1±11.1 | 0.004 |
| High bleeding risk criteria | ||||||
| Age ≥75 years | 307 (60.0) | 436 (55.4) | 0.105 | 635 (73.0) | 854 (72.2) | 0.689 |
| Chronic anticoagulant therapy | 230 (44.9) | 319 (40.5) | 0.118 | 384 (44.2) | 486 (41.1) | 0.159 |
| Anaemia | 99 (19.3) | 172 (21.9) | 0.275 | 99 (11.4) | 141 (11.9) | 0.714 |
| History of stroke | 62 (12.1) | 108 (13.7) | 0.399 | 82 (9.4) | 115 (9.7) | 0.829 |
| Renal insufficiency | 73 (14.3) | 118 (15.0) | 0.714 | 42 (4.8) | 39 (3.3) | 0.077 |
| Thrombocytopaenia | 11 (2.2) | 23 (3.0) | 0.404 | 20 (2.4) | 14 (1.2) | 0.043 |
| History of major bleeding | 17 (3.3) | 20 (2.5) | 0.409 | 29 (3.3) | 37 (3.1) | 0.79 |
| Number of HBR criteria | 1.6±0.8 | 1.5±0.7 | 0.505 | 1.5±0.7 | 1.4±0.6 | 0.062 |
| ESC thrombotic risk enhancers40 | ||||||
| Diabetes | 512 (100) | 787 (100) | N/A | - | - | - |
| Insulin-dependent diabetes | 160 (31.2) | 264 (33.5) | 0.389 | - | - | - |
| Prior MI | 98 (19.3) | 143 (18.5) | 0.715 | 128 (14.8) | 174 (14.9) | 0.946 |
| Multivessel CAD | 253 (49.4) | 399 (50.7) | 0.651 | 317 (36.4) | 519 (43.9) | <0.001 |
| eGFR 15-59 ml/min/1.73 m² | 203 (39.6) | 269 (34.2) | 0.045 | 358 (41.1) | 447 (37.8) | 0.123 |
| Premature CAD (age <45 years) | 2 (0.4) | 4 (0.5) | 0.76 | 2 (0.2) | 3 (0.3) | 0.914 |
| Number of risk enhancers | 2.1±0.9 | 2.0±0.9 | 0.277 | 0.9±0.8 | 1.0±0.8 | 0.312 |
| Moderate or high thrombotic risk | 512 (100) | 787 (100) | N/A | 583 (67.0) | 809 (68.4) | 0.51 |
| Continuous variables are reported as mean±SD. Categorical variables are reported as n (%). CABG: coronary artery bypass graft; CAD: coronary artery disease; DAPT: dual antiplatelet therapy; DM: diabetes mellitus; eGFR: estimated glomerular filtration rate; ESC: European Society of Cardiology; HBR: high bleeding risk; MI: myocardial infarction; N/A: not applicable; NSTEMI: non-ST-segment elevation myocardial infarction; PARIS: Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients; PCI: percutaneous coronary intervention; PRECISE-DAPT: Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy; SD: standard deviation | ||||||
Table 2. Procedural features and therapy at discharge in patients with and without diabetes, stratified according to DAPT regimen.
| Patients with DM (n=1,299) | Patients without DM (n=2,053) | |||||
|---|---|---|---|---|---|---|
| XIENCE 281-month DAPT (n=512) | XIENCE 903-month DAPT (n=787) | p-value | XIENCE 281-month DAPT (n=870) | XIENCE 903-month DAPT (n=1,183) | p-value | |
| Procedural characteristics | ||||||
| Radial access | 349 (68.2) | 398 (50.6) | <0.001 | 629 (72.3) | 629 (53.2) | <0.001 |
| Number of lesions treated | 1.0 [1.0-1.0] | 1.0 [1.0-1.0] | 0.577 | 1.0 [1.0-1.0] | 1.0 [1.0-1.0] | 0.704 |
| Type B2/C lesion | 178 (34.8) | 299 (38.0) | 0.238 | 318 (36.6) | 387 (32.7) | 0.07 |
| Bifurcation lesion | 46 (9.0) | 61 (7.8) | 0.429 | 115 (13.2) | 92 (7.8) | <0.001 |
| Number of stents implanted | 1.0 [1.0-1.0] | 1.0 [1.0-1.0] | 0.76 | 1.0 [1.0-1.0] | 1.0 [1.0-1.0] | 0.593 |
| Total stent length, mm | 26.9±14.4 | 26.1±13.5 | 0.305 | 27.4±14.5 | 25.2±14.0 | <0.001 |
| Preprocedure RVD, mm | 3.0±0.5 | 3.0±0.5 | 0.377 | 3.0±0.5 | 3.0±0.5 | 0.625 |
| Preprocedure %DS | 83.2±9.7 | 83.8±9.8 | 0.278 | 82.1±10.7 | 84.0±9.4 | <0.001 |
| Complex PCI, any of the following | 30 (5.9) | 45 (5.7) | 0.915 | 44 (5.1) | 62 (5.2) | 0.853 |
| ≥3 stents implanted | 18 (3.5) | 31 (3.9) | 0.696 | 29 (3.3) | 40 (3.4) | 0.953 |
| ≥3 lesions treated | 11 (2.1) | 19 (2.4) | 0.755 | 23 (2.6) | 33 (2.8) | 0.841 |
| ≥3 vessel treated | 1 (0.2) | 0 (0) | 0.215 | 1 (0.1) | 5 (0.4) | 0.202 |
| Total stent length >60 mm | 17 (3.3) | 28 (3.6) | 0.819 | 35 (4.0) | 46 (3.9) | 0.877 |
| Antiplatelet therapy at discharge | ||||||
| Aspirin | 417 (81.4) | 728 (92.5) | <0.001 | 705 (81.0) | 1,071 (90.5) | <0.001 |
| Clopidogrel | 436 (85.2) | 634 (80.6) | 0.034 | 759 (87.2) | 977 (82.6) | 0.004 |
| Prasugrel | 10 (2.0) | 19 (2.4) | 0.583 | 4 (0.5) | 27 (2.3) | <0.001 |
| Ticagrelor | 66 (12.9) | 135 (17.2) | 0.038 | 107 (12.3) | 181 (15.3) | 0.053 |
| Continuous variables are reported as mean±SD, or median [IQR]. Categorical variables are reported as n (%). DAPT: dual antiplatelet therapy; DM: diabetes mellitus; DS: diameter stenosis; IQR: interquartile range; PCI: percutaneous coronary intervention; RVD: reference vessel diameter; SD: standard deviation | ||||||
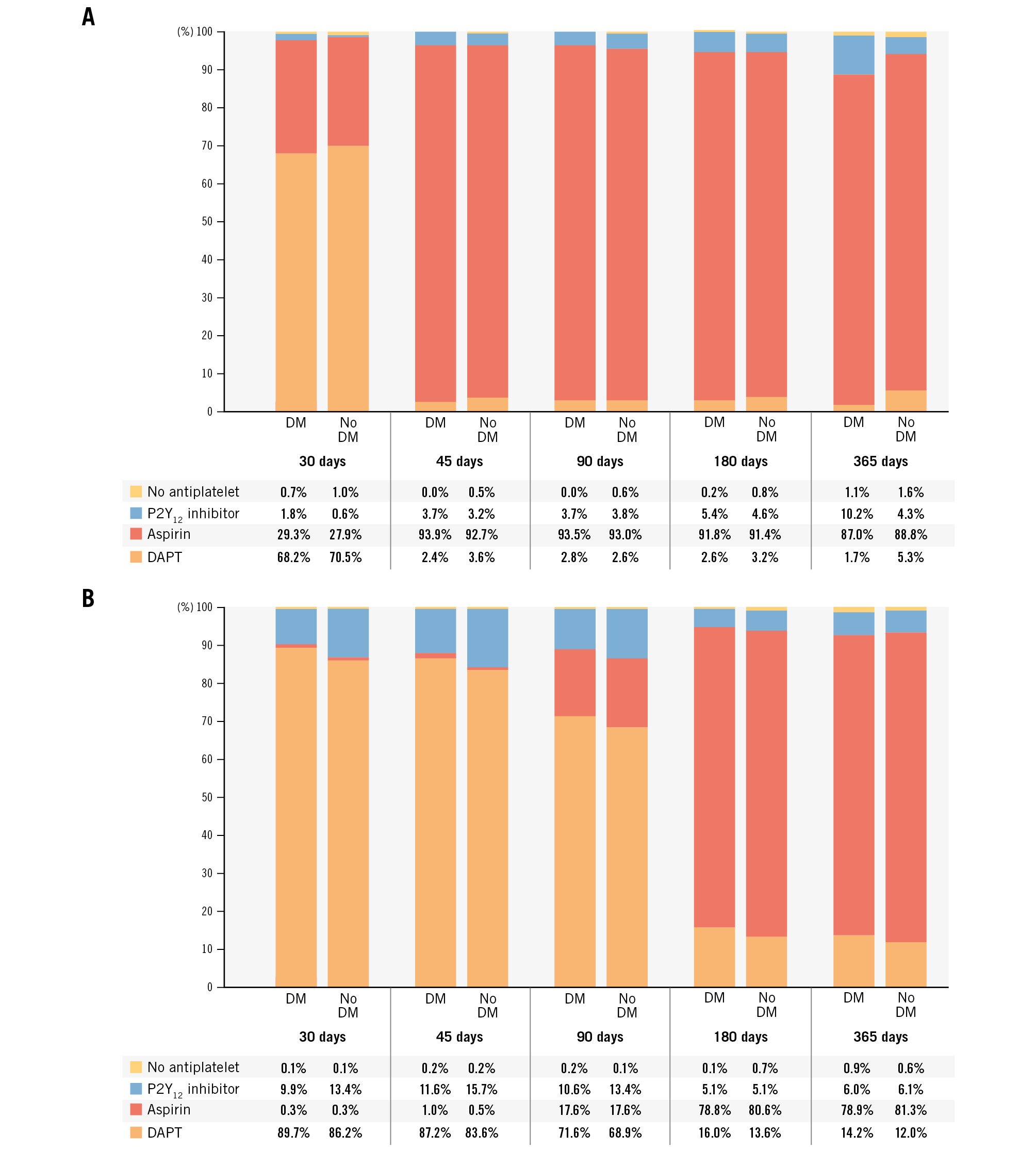
Figure 2. Antiplatelet regimens during the study. A) Antiplatelet regimens in patients enrolled in XIENCE 28, stratified by diabetes status. B) Antiplatelet regimens in patients enrolled in XIENCE 90, stratified by diabetes status. DAPT discontinuation was mandated at 1 month after percutaneous coronary intervention (PCI) in XIENCE 28 and at 3 months after PCI in XIENCE 90. DAPT: dual antiplatelet therapy; DM: diabetes mellitus
Outcomes according to diabetes status
At 1-year follow-up, patients with DM had a higher incidence of the primary endpoint (10.1% vs 6.6%, hazard ratio [HR] 1.51, 95% confidence interval [CI]: 1.18-1.94; p=0.001) (Figure 3A). Diabetic patients also experienced significantly higher risks of MI, TLF, TVR, and NACE (Supplementary Table 5). With respect to bleeding, there were no significant differences between diabetic and non-diabetic patients for either BARC Type 2-5 (9.5% vs 9.2%, HR 1.02, 95% CI: 0.80-1.29; p=0.902) (Figure 3B) or BARC Type 3-5 bleeding (4.6% vs 4.2%, HR 1.09, 95% CI: 0.78-1.54; p=0.610).
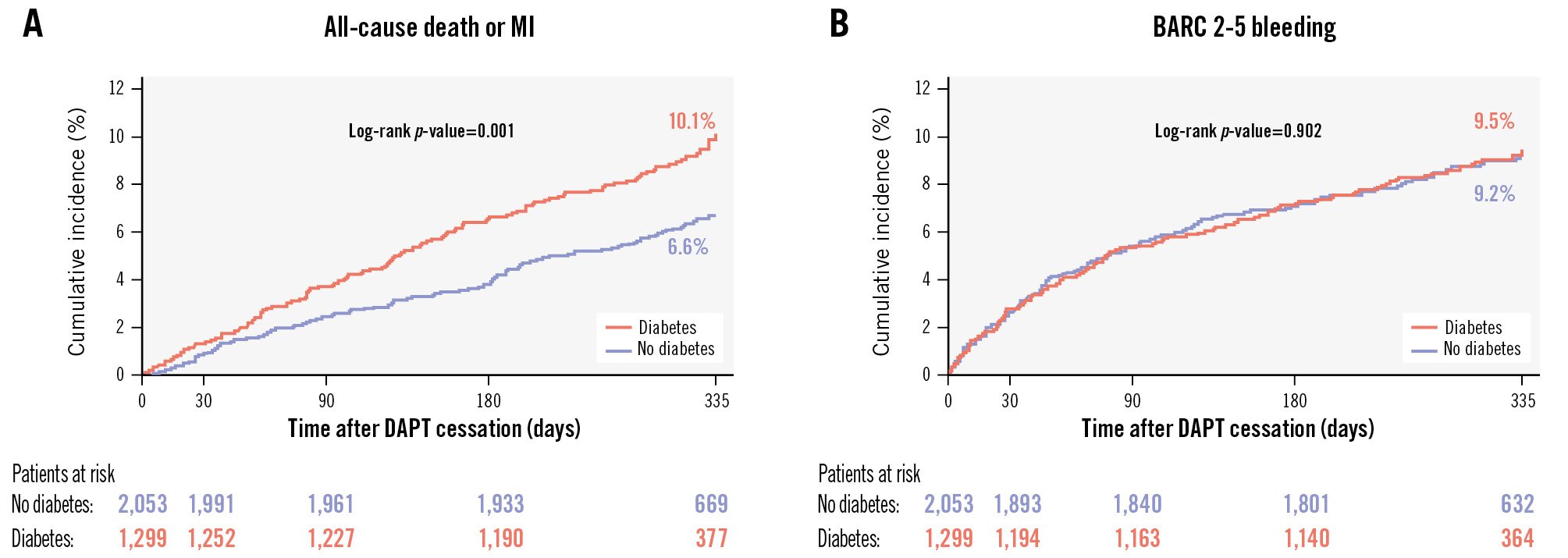
Figure 3. Cumulative incidence of the composite of all-cause death or MI and of BARC Type 2 to 5 bleeding in patients with and without diabetes mellitus. A) Cumulative incidence of all-cause death or MI. B) Cumulative incidence of BARC 2-5 bleeding. BARC: Bleeding Academic Research Consortium; DAPT: dual antiplatelet therapy; MI: myocardial infarction
Outcomes according to DAPT duration
The incidence and adjusted HRs (adjHRs) for clinical outcomes, stratified by DAPT duration and diabetic status, are reported in Table 3 and the Central illustration. Between 1 and 12 months after PCI, in patients with DM, all-cause death or MI occurred in 39 (8.4%) patients receiving 1-month DAPT and in 81 (11.3%) patients on 3-month DAPT; in the non-DM group, the primary endpoint occurred in 64 (7.8%) patients on 1-month DAPT and in 64 (5.7%) patients on 3-month DAPT. After PS stratification, there was a signal of treatment effect modification of 1- versus 3-month DAPT by diabetic status for the risk of the primary endpoint (DM: adjHR 0.70, 95% CI: 0.47-1.05; p=0.083; non-DM: adjHR 1.26, 95% CI: 0.87-1.81; p=0.224; p for interaction=0.015). The risk of MI associated with 1- versus 3-month DAPT was significantly lower in patients with DM (3.3% vs 6.8%, adjHR 0.46, 95% CI: 0.26-0.84; p=0.011), whereas it did not differ in patients without DM (3.1% vs 2.1%, adjHR 1.52, 95% CI: 0.84-2.75; p=0.171; p for interaction=0.004), mainly driven by a difference in target vessel MI. Similarly, the risk of target lesion failure associated with 1- versus 3-month DAPT was reduced in DM patients (4.8% vs 8.6%, adjHR 0.54, 95% CI: 0.33-0.89; p=0.016) but not in non-DM patients (5.8% vs 3.6%, adjHR 1.57, 95% CI: 1.01-2.45; p=0.045; p for interaction<0.001). Rates of stroke and stent thrombosis were generally low and were not statistically different between 1- and 3-month DAPT regimens in both patients with and without DM; the incidence of ischaemic stroke was significantly lower in DM patients receiving 1-month DAPT (0.8% vs 2.2%, adjHR 0.31, 95% CI: 0.10-0.98; p=0.045), compared to those receiving 3-month DAPT.
Between 1 and 12 months, the risk of BARC Type 2-5 bleeding tended to be lower with 1- versus 3-month DAPT in both DM (8.1% vs 10.4%; adjHR 0.67, 95% CI: 0.45-1.01; p=0.057) and non-DM patients (8.1% vs 10.1%; adjHR 0.78, 95% CI: 0.56-1.07; p=0.125; p for interaction=0.973). There was no difference in the risk of BARC 3-5 bleeding between 1- and 3-month DAPT in patients with (4.5% vs 4.7%, adjHR 0.77, 95% CI: 0.44-1.37; p=0.381) or without DM (3.5% vs 4.7%, adjHR 0.72, 95% CI: 0.44-1.16; p=0.178; p for interaction=0.538). Finally, 1-month DAPT significantly reduced the incidence of NACE in DM patients (11.0% vs 15.4%, adjHR 0.64, 95% CI: 0.46-0.90; p=0.010) but not in non-DM patients (11.2% vs 10.1%, adjHR 1.02, 95% CI: 0.76-1.37; p=0.889; p for interaction=0.047).
Table 3. One-year clinical outcomes of HBR patients with and without diabetes receiving 1- versus 3-month DAPT.
| Outcomes | Diabetes (N=1,299) | No diabetes (N=2,053) | Interactionp-value‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| XIENCE 28 1-month DAPT (N=512) | XIENCE 90 3-month DAPT (N=787) | Adjustedhazard ratio† (95% CI) | p-value | XIENCE 28 1-month DAPT (N=870) | XIENCE 90 3-month DAPT (N=1,183) | Adjustedhazard ratio† (95% CI) | p-value | ||
| All-cause death or MI | 39 (8.4) | 81 (11.3) | 0.70 (0.47-1.05) | 0.083 | 64 (7.8) | 64 (5.7) | 1.26 (0.87-1.81) | 0.224 | 0.015 |
| All-cause death | 24 (5.2) | 40 (5.9) | 0.86 (0.51-1.46) | 0.583 | 40 (4.8) | 48 (4.2) | 0.98 (0.63-1.53) | 0.928 | 0.521 |
| Cardiovascular death | 10 (2.3) | 26 (3.9) | 0.57 (0.27-1.22) | 0.147 | 22 (2.6) | 23 (2.0) | 1.15 (0.62-2.14) | 0.655 | 0.094 |
| MI | 15 (3.3) | 50 (6.8) | 0.46 (0.26-0.84) | 0.011 | 25 (3.1) | 23 (2.1) | 1.52 (0.84-2.75) | 0.171 | 0.004 |
| Definite or probable ST | 1 (0.2) | 4 (0.6) | 0.48 (0.05-4.42) | 0.516 | 3 (0.4) | 2 (0.2) | 2.09 (0.32-13.60) | 0.442 | 0.245 |
| Stroke | 5 (1.0) | 18 (2.5) | 0.38 (0.14-1.07) | 0.067 | 6 (0.9) | 15 (1.4) | 0.45 (0.17-1.22) | 0.118 | 0.708 |
| Ischaemic stroke | 4 (0.8) | 16 (2.2) | 0.31 (0.10-0.98) | 0.045 | 5 (0.8) | 14 (1.3) | 0.39 (0.13-1.15) | 0.088 | 0.74 |
| Target lesion failure | 22 (4.8) | 61 (8.6) | 0.54 (0.33-0.89) | 0.016 | 47 (5.8) | 40 (3.6) | 1.57 (1.01-2.45) | 0.045 | <0.001 |
| Target lesion revascularisation | 9 (2.0) | 13 (1.8) | 1.00 (0.41-2.43) | 0.996 | 9 (1.1) | 13 (1.2) | 1.08 (0.44-2.63) | 0.862 | 0.852 |
| Target vessel revascularisation | 13 (3.1) | 26 (3.5) | 0.73 (0.37-1.46) | 0.371 | 16 (2.2) | 20 (1.9) | 1.28 (0.64-2.55) | 0.479 | 0.446 |
| Target vessel MI | 12 (2.7) | 40 (5.4) | 0.45 (0.23-0.88) | 0.02 | 20 (2.6) | 17 (1.5) | 1.68 (0.85-3.31) | 0.134 | 0.007 |
| BARC 2-5 bleeding | 39 (8.1) | 73 (10.4) | 0.67 (0.45-1.01) | 0.057 | 64 (8.1) | 111 (10.1) | 0.78 (0.56-1.07) | 0.125 | 0.973 |
| BARC 3-5 bleeding | 21 (4.5) | 34 (4.7) | 0.77 (0.44-1.37) | 0.381 | 28 (3.5) | 52 (4.7) | 0.72 (0.44-1.16) | 0.178 | 0.538 |
| NACE | 53 (11.0) | 112 (15.4) | 0.64 (0.46-0.90) | 0.01 | 91 (11.2) | 114 (10.1) | 1.02 (0.76-1.37) | 0.889 | 0.047 |
| The percentages mentioned above represent Kaplan-Meier rates at 12 months after the index procedure. †Propensity-stratified outcomes according to sex, baseline serum creatinine, anticoagulation therapy, stroke, history of major bleeding, baseline platelet, baseline haemoglobin, body mass index, hypertension, hypercholesterolaemia, prior PCI, prior CABG, prior MI, multivessel disease, diabetes, type B2/C lesion, total lesion length, mean preprocedure RVD, mean preprocedure DS, bifurcation lesion, number of lesions treated, number of vessels treated, number of stents, total stent length, P2Y12 on discharge, PARIS risk score for major bleeding, PRECISE-DAPT risk score for bleeding. ‡p-value is obtained from the interaction test between the anticoagulant at discharge and DAPT after applying multiple imputation and propensity score stratification. BARC: Bleeding Academic Research Consortium; CABG: coronary artery bypass graft; CI: confidence interval; DAPT: dual antiplatelet therapy; DS: diameter stenosis; HBR: high bleeding risk; MI: myocardial infarction; NACE: net adverse clinical events; PARIS: Patterns of Non-Adherence to Anti-Platelet Regimens in Stented Patients; PCI: percutaneous coronary intervention; PRECISE-DAPT: Predicting Bleeding Complications in Patients Undergoing Stent Implantation and Subsequent Dual Antiplatelet Therapy; RVD: reference vessel diameter; ST: stent thrombosis | |||||||||
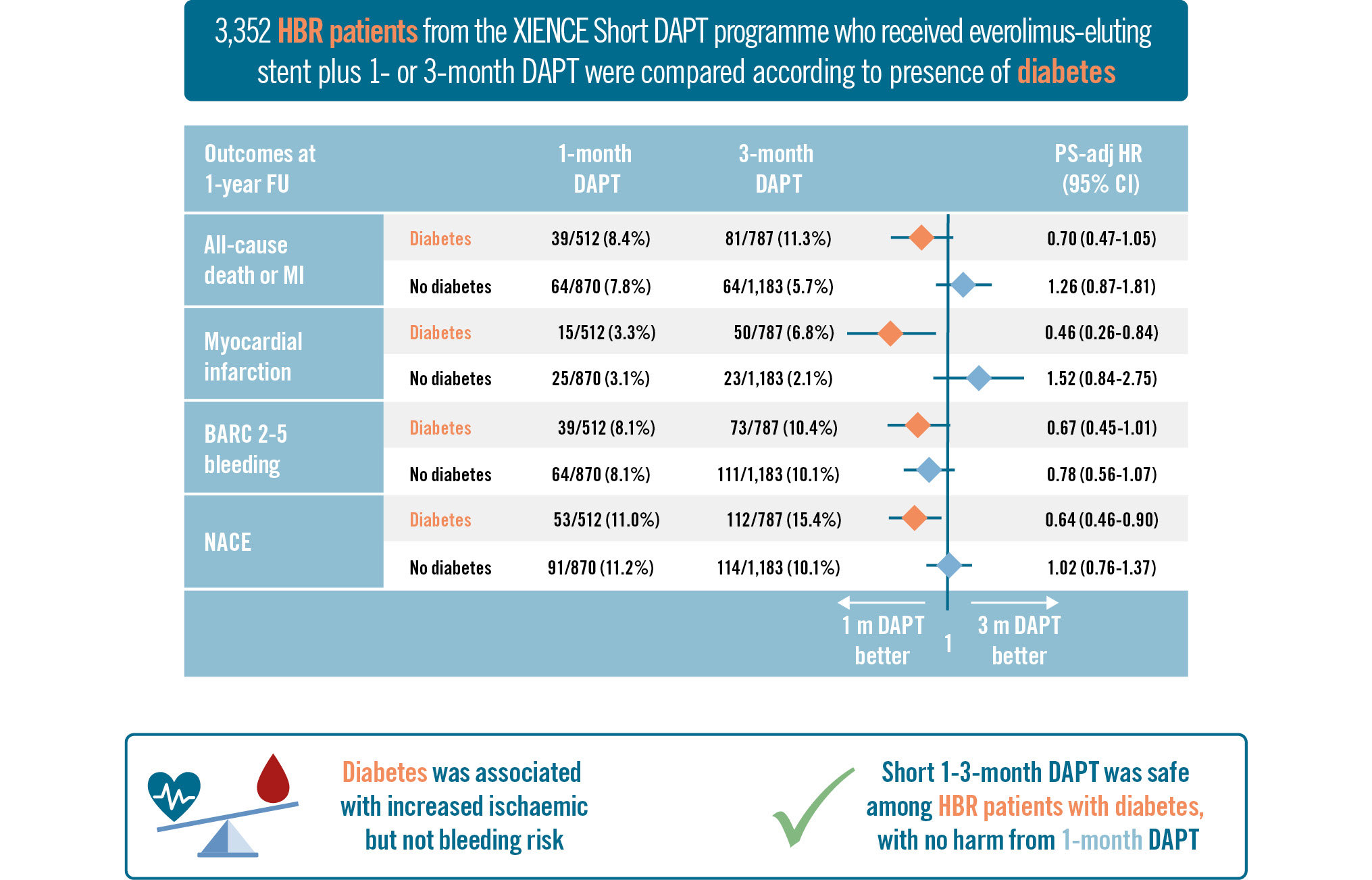
Central illustration. One- versus three-month DAPT in diabetic patients at high bleeding risk. adj: adjusted; BARC: Bleeding Academic Research Consortium; CI: confidence interval; DAPT: dual antiplatelet therapy; FU: follow-up; HBR: high bleeding risk; HR: hazard ratio; MI: myocardial infarction; NACE: net adverse clinical events; PS: propensity score
Secondary analysis
The results of an exploratory analysis stratifying patients by DM status and insulin treatment are reported in Supplementary Table 6 and were generally consistent with the primary analysis.
Discussion
In this study, we compared the safety and efficacy of 1- versus 3-month DAPT in DM and non-DM patients with HBR undergoing PCI with an everolimus-eluting stent within the XIENCE Short DAPT programme. The main findings are as follows: (1) HBR patients with diabetes incurred a significantly higher risk of ischaemic but not bleeding events; (2) HBR patients with DM did not derive ischaemic harm from 1-month DAPT compared with the 3-month regimen, in the presence of a significant interaction across comparisons with the non-DM group; (3) clinically relevant BARC Type 2-5 bleeding tended to be numerically lower in both DM and non-DM patients on 1-month DAPT, without heterogeneity between groups.
DM affects up to 40% of patients undergoing PCI. It is associated with a prothrombotic and proinflammatory state and increases the risk of ischaemic complications after myocardial revascularisation17. In the FREEDOM trial, DM patients with multivessel disease undergoing PCI had significantly higher rates of death or MI at 5-year follow-up, compared with those receiving coronary artery bypass graft18. The worse outcomes of DM patients were partly driven by higher rates of both spontaneous as well as stent-related adverse events19. The introduction of new-generation DES has substantially reduced the need for repeat revascularisation after PCI in the general population; yet, this benefit appears to be less pronounced among patients with DM202122. Moreover, owing to the established association of DM with thrombotic complications, prolonged DAPT is often considered after PCI in these patients13. Such an approach has shown some benefit in patients at very high ischaemic risk (e.g., those with acute coronary syndrome [ACS]) despite resulting in an increased incidence of bleeding events. In the PEGASUS-TIMI 54 trial, an extended course of aspirin and ticagrelor reduced the risk of the composite of cardiovascular death, MI, or stroke while increasing the risk of major bleeding in patients with a prior MI and additional risk factors including diabetes23. However, when a similar strategy was tested in diabetic patients with stable coronary artery disease in the THEMIS trial, the observed benefit in the overall population was modest, though larger in those with a history of PCI1024. Interestingly, in the DAPT Study, extended treatment with either clopidogrel or prasugrel plus aspirin beyond 12 months after stent implantation in the DM subgroup was associated with an attenuation of major adverse cardiovascular events reduction originally observed in the overall study population25.
Haemorrhagic complications may mitigate the benefit provided by a more intense or prolonged antithrombotic regimen, resulting in a null effect on overall survival1. Thus, when a thrombotic risk enhancer like diabetes coexists with high bleeding risk status, the scenario is even more complex. Among HBR patients undergoing PCI, our study confirmed a high prevalence of DM, which was associated with a worse ischaemic risk profile. Diabetic patients exhibited more comorbidities, a greater extent of coronary artery disease, and had twice the risk of suffering an MI at 1-year follow-up. Of note, despite patients with DM having higher rates of revascularisation, the incidence of stent thrombosis was reassuringly low – below 0.5% at 1 year – without any significant interaction for diabetes status and DAPT regimen. This is noteworthy as most of the advancements in stent technologies have allowed a reduction in the duration of DAPT while maintaining efficacy in preventing thrombotic events, especially in HBR patients26272829.
When patients were stratified according to DAPT regimen, we found a significant heterogeneity for the effects of 1- versus 3-month DAPT for the incidence of the primary endpoint, MI, and target lesion failure, which were overall lower in diabetic patients receiving 1-month DAPT. Moreover, 1-month DAPT was also associated with a numerically greater bleeding risk reduction in DM patients, and it is well recognised that bleeding itself can trigger ischaemic complications by precipitating type 2 MI or leading to DAPT disruption3031. A recent large meta-analysis showed that a longer DAPT regimen is effective in reducing major adverse cardiovascular events after complex PCI only when HBR features are not present, with an incremental effect in ACS patients32. Similarly, in our cohort of DM patients, we might speculate that the coexistence of HBR status shifted the balance of clinical benefit in favour of the shorter 1-month DAPT. Of note, DM patients presented with a slightly higher number of HBR criteria, mainly due to a higher prevalence of anaemia and renal insufficiency. Moreover, a higher number (14%) of diabetic patients assigned to the 3-month regimen were still on DAPT at 1 year, thus being exposed to a relatively more prolonged risk of bleeding. However, we interpret the reduced ischaemic risk observed with 1-month DAPT in diabetic patients as a signal of safety rather than a definitive sign of superiority. The interplay between competing risks may partly explain the observed MI patterns, though this remains speculative. Indeed, the recent MASTER DAPT trial, which randomised HBR patients undergoing PCI with a biodegradable-polymer sirolimus-eluting stent implantation to abbreviated or standard (≥3 months) DAPT, did not show any significant impact of diabetic status on the abbreviated treatment effects33. Our study was not powered to evaluate single ischaemic endpoints such as MI, and this finding may be attributed to chance. While propensity score matching was applied to balance baseline characteristics, the possibility of residual confounders cannot be entirely excluded, including differences in geographical representation. The 1-month DAPT arm included patients from two studies (XIENCE 28 USA and XIENCE 28 Global) conducted across the USA, Europe, and Asia, whereas the 3-month DAPT arm comprised exclusively US patients (XIENCE 90). For instance, Asian patients – who were predominantly represented in the 1-month DAPT arm and exhibited significant imbalances between diabetic and non-diabetic groups – are known to have a higher bleeding rather than ischaemic risk, and their characteristics may have contributed to the observed results. Additionally, most patients in this cohort received only a single stent, and the overall complexity of the PCI procedures was low; consequently, the applicability of these findings to the broader population of diabetic patients undergoing PCI – many of whom undergo more complex interventions, often in the setting of ACS – may be limited. The difference in target vessel MI, which was higher in diabetic patients – in the absence of any difference in stent thrombosis – may be attributed to the higher risk of restenosis and disease progression; however, the same limitations discussed above should be considered for the comparison between 1- and 3-month DAPT.
Finally, in XIENCE Short DAPT, most of the included patients received aspirin monotherapy after DAPT discontinuation. However, a strategy of short DAPT followed by the use of P2Y12 inhibitor monotherapy after early aspirin withdrawal has recently emerged as an alternative antithrombotic strategy for secondary prevention3435. In DM patients receiving everolimus-eluting stents, both clopidogrel and ticagrelor monotherapy after 1 to 3 months of DAPT have been proven effective in reducing bleeding without increasing ischaemic events, as compared to standard DAPT363738. Thus, P2Y12 inhibitor monotherapy can play a key role, especially in patients with HBR undergoing PCI339.
Limitations
This analysis should be interpreted in light of some limitations. First, as previously mentioned, due to the non-randomised design, we cannot exclude the presence of residual confounders, and the study was not powered to detect differences in individual ischaemic endpoints. Second, the observed findings should be interpreted in the context of the inclusion criteria and procedural characteristics of the patients enrolled in the XIENCE Short DAPT programme, which may limit generalisability to a larger population of DM patients undergoing PCI. Third, for patients enrolled in XIENCE 90, eligibility was retrospectively evaluated given the lack of 1-month follow-up. Finally, it is important to note that most of the enrolled patients were treated with clopidogrel (>80%) and mainly in the setting of chronic coronary syndrome; additionally, the findings may not be applicable to individuals who received different DAPT regimens and/or a stent different from the study stent.
Conclusions
In HBR patients undergoing PCI with the cobalt-chromium everolimus-eluting XIENCE stent, those with DM, although at a higher risk, did not experience ischaemic harm from 1-month DAPT compared with 3-month DAPT, and a numerical reduction of bleeding was observed at the 1-year follow-up. These findings should be interpreted in the context of a study population consisting predominantly of patients with chronic coronary syndrome and low procedural complexity and may not be generalisable to higher-risk cases.
Impact on daily practice
Diabetes mellitus is associated with a high ischaemic risk in patients with coronary artery disease undergoing percutaneous coronary intervention, often necessitating more intense and/or prolonged dual antiplatelet therapy (DAPT). In high bleeding risk (HBR) patients, shorter DAPT regimens have been proposed to mitigate bleeding events, though evidence supporting their safety in diabetic patients has been limited. Our analysis from the XIENCE Short DAPT programme demonstrates that a 1-month DAPT regimen is safe and effective, reducing bleeding complications without increasing ischaemic events in diabetic patients. These findings provide reassuring evidence for clinicians to consider shorter DAPT durations for HBR diabetic patients, particularly in stable presentations and low procedural complexity, as reflected in the majority of patients enrolled in the study. This approach helps balance the trade-off between bleeding and ischaemic risks in routine clinical practice.
Funding
This study was sponsored by Abbott (Chicago, IL, USA).
Conflict of interest statement
D.J. Angiolillo declares that he has received consulting fees or honoraria from Abbott, Amgen, Anthos, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi Sankyo, Eli Lilly, Faraday, Haemonetics, Janssen, Merck, Novartis, PhaseBio, PLx Pharma, Pfizer, and Sanofi; and institution research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Faraday, Gilead, Idorsia, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, and the Scott R. MacKenzie Foundation. M. Valgimigli reports grants and personal fees from Terumo, AstraZeneca, Alvimedica/CID, Abbott, Daiichi Sankyo, Bayer, CoreFlow, Idorsia Pharmaceuticals Ltd, Universität Basel - Departement Klinische Forschung, Vifor, Bristol-Myers Squibb, Biotronik, Boston Scientific, Medtronic, Vesalio, Novartis, Chiesi, and PhaseBio. D. Cao reports consulting fees from Terumo. D.L. Bhatt discloses the following relationships - advisory board: Angiowave, Bayer, Boehringer Ingelheim, CellProthera, Cereno Scientific, Elsevier Practice Update Cardiology, High Enroll, Janssen, Level Ex, McKinsey, Medscape Cardiology, Merck, MyoKardia, NirvaMed, Novo Nordisk, PhaseBio, PLx Pharma, and Stasys; board of directors: American Heart Association New York City; Angiowave (stock options), Bristol-Myers Squibb (stock), DRS.LINQ (stock options), and High Enroll (stock); consultant: Broadview Ventures, GlaxoSmithKline, Hims, SFJ, and Youngene; data monitoring committees: Acesion Pharma, Assistance Publique-Hôpitaux de Paris, Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Boston Scientific (Chair, PEITHO trial), Cleveland Clinic, Contego Medical (Chair, PERFORMANCE 2), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo; for the ABILITY-DM trial, funded by Concept Medical; for ALLAY-HF, funded by Alleviant Medical), Novartis, Population Health Research Institute, and Rutgers University (for the NIH-funded MINT Trial); honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org; Chair, ACC Accreditation Oversight Committee), Arnold and Porter law firm (work related to Sanofi/Bristol-Myers Squibb clopidogrel litigation), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor-in-Chief, Harvard Heart Letter), Canadian Medical and Surgical Knowledge Translation Research Group (clinical trial steering committees), CSL Behring (AHA lecture), Cowen and Company, Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor-in-Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), K2P (Co-Chair, interdisciplinary curriculum), Level Ex, Medtelligence/ReachMD (CME steering committees), MJH Life Sciences, Oakstone CME (Course Director, Comprehensive Review of Interventional Cardiology), Piper Sandler, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), WebMD (CME steering committees), and Wiley (steering committee); other: Clinical Cardiology (Deputy Editor); patent: sotagliflozin (named on a patent for sotagliflozin assigned to Brigham and Women’s Hospital who assigned to Lexicon; neither he nor Brigham and Women’s Hospital receive any income from this patent); research funding: Abbott, Acesion Pharma, Afimmune, Aker BioMarine, Alnylam, Amarin, Amgen, AstraZeneca, Bayer, Beren, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Cardax, CellProthera, Cereno Scientific, Chiesi, CinCor, Cleerly, CSL Behring, Eisai, Ethicon, Faraday Pharmaceuticals, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Garmin, HLS Therapeutics, Idorsia, Ironwood, Ischemix, Janssen, Javelin, Lexicon, Eli Lilly, Medtronic, Merck, Moderna, MyoKardia, NirvaMed, Novartis, Novo Nordisk, Otsuka, Owkin, Pfizer, PhaseBio, PLx Pharma, Recardio, Regeneron, Reid Hoffman Foundation, Roche, Sanofi, Stasys, Synaptic, The Medicines Company, Youngene, and 89Bio; royalties: Elsevier (Editor, Braunwald’s Heart Disease); site co-investigator: Abbott, Biotronik, Boston Scientific, CSI, Endotronix, St. Jude Medical (now Abbott), Philips, SpectraWAVE, Svelte, and Vascular Solutions; trustee: American College of Cardiology; and unfunded research: FlowCo. J. Hermiller has been part of the Abbott advisory board for the last two years. F.-J. Neumann received consultancy honoraria from Novartis and Meril Life Sciences; speaker honoraria from Boston Scientific, Amgen, Daiichi Sankyo, and Meril Life Sciences; reports speaker honoraria paid to his institution from BMS/Pfizer, Daiichi Sankyo, Boston Scientific, Siemens Healthineers, and Amgen; as well as research grants paid to his institution from Boston Scientific and Abbott. R. Mehran reports institutional research payments from Abbott, Abiomed, Affluent Medical, Alleviant Medical, Amgen, AM-Pharma, Arena, AstraZeneca, AtriCure Inc., Biosensors, Biotronik, Boston Scientific, Bristol-Myers Squibb, Cardiawave, CeloNova, CERC, Chiesi, Concept Medical, Cytosorbents, Daiichi Sankyo, Duke, Element Science, Essential Medical, Faraday, Idorsia Pharmaceuticals, Janssen, MedAlliance, Mediasphere, Medtelligence, Medtronic, MJH Healthcare, Novartis, OrbusNeich, Penumbra, PhaseBio, Philips, Pi-Cardia, PLx Pharma, Population Health Research Institute, Protembis, Recor Medical, RenalPro, RM Global, Sanofi, Shockwave Medical, Vivasure, and Zoll; personal fees from Affluent Medical, Cardiovascular Research Foundation (CRF), Cordis, Daiichi Sankyo Brasil, E.R. Squibb & Sons, Esperion Science/Innovative Biopharma, Europa Group/Boston Scientific, Gaffney Events, Educational Trust, Henry Ford Health Cardiology, Ionis Pharmaceuticals, Medcon International, Novartis, Novo Nordisk, PeerView Institute for Medical Education, Terumo Europe N.V., Vectura, Vox Media, WebMD, IQVIA, Radcliffe, and Tarsus Cardiology; no fees from AMA (Scientific Advisory Board), SCAI (Women in Innovations Committee Member); faculty: CRF; honorarium: JAMA Cardiology (Associate Editor), ACC (BOT Member, SC Member CTR Program); and equity <1% in Applied Therapeutics, Elixir Medical, Stel, and ControlRad (spouse). The other authors have no relevant conflicts of interest to declare regarding the present manuscript.
Supplementary data
To read the full content of this article, please download the PDF.

