Cory:
Unlock Your AI Assistant Now!
Abstract
Relevant calcified coronary artery disease (CCAD) may be present in around 20% of patients undergoing percutaneous coronary interventions, and it is known to add procedural challenges and risks. Careful patient selection and specific expertise in multimodality imaging and plaque modification techniques are required to plan and adopt the most appropriate therapeutic strategy. This review aims to present the contemporary clinical approach and procedural planning for CCAD patients, describing the available tools and strategies in view of the most recent scientific evidence.
Calcification of atherosclerotic plaques represents the predominant pathological process underlying coronary lesions that are resistant to or unsuitable for conventional percutaneous dilatation techniques. Poor procedural planning and inappropriate selection of interventional tools can not only lead to a failure in the treatment of heavily calcified coronary artery disease (CCAD) but may also result in complications and worse outcomes.
This review aims to provide a clear picture of the current knowledge and best practices in CCAD assessment and treatment, based on the most relevant scientific evidence and recently published expert consensus1. Furthermore, key practical considerations, as well as visual algorithms, are presented, where appropriate, for each main topic. Epidemiology and pathological aspects are detailed in Supplementary Appendix 1.
Interventional planning
In calcified coronary stenosis (CCS), calcium modification by ablation or fracture leads to better stent expansion and improved clinical outcomes. Over the last decade, an improved understanding of coronary calcium detection, characterisation, and quantification through non-invasive and invasive imaging modalities has aided procedural planning and appropriate device selection for percutaneous coronary interventions (PCI).
Computed tomography coronary angiography (non-invasive)
Computed tomography coronary angiography (CTCA) has proved to be a reliable non-invasive diagnostic modality for the evaluation of coronary artery disease. Coronary calcium may be assessed using the widely adopted Agatston score, which reflects a global within-slice quantification. In the MESA study2, an Agatston score>0 was shown to add prognostic significance over recognised clinical scores, defining a significant risk increment even in patients otherwise deemed at low risk. Regional CCAD evaluation and per-vessel analysis have also been implemented and may be of help for ischaemic event prediction3. Dedicated CTCA imaging software allows three-dimensional qualitative and quantitative analyses of plaque components like fat, fibrosis and calcium based on attenuation values. Furthermore, computed tomography-derived fractional flow reserve (FFR-CT) can provide an estimate of the functional significance of lesions. In the presence of a high calcium burden, CTCA can determine the distribution and volume of calcified plaques, including the detection of concentric calcium rings. In a cohort of 66 calcified plaques (32 vessels) in 31 patients, Monizzi et al compared calcium volume derived by CTCA versus optical coherence tomography (OCT). No differences were noted in calcium length, calcium arc or minimal lumen area between the two modalities (Pearson correlation coefficient 0.92)4. However, CTCA overestimated calcium volume by approximately 60% when compared to OCT (18.23 mm3 vs 10.03 mm3). This was more often seen in densely calcified plaques with higher Hounsfield units and attenuation, which created blooming artefacts and blurring of adjacent tissues. The FFR-CT has a high sensitivity (87%) but low specificity (54%) when compared to invasive FFR in CCAD, thus resulting in more false positives5. The latest-generation photon-counting CT significantly improves the evaluation of plaque components, calcium volume, thickness and arc, thereby facilitating the planning of PCI procedures6.
These imaging and functional analyses are helpful in the selection of calcium modification devices, optimisation of cath lab resources, and better time allocation prior to invasive procedures. Sekimoto et al demonstrated that a per-lesion CTCA Agatston calcium score >450 could predict the need for rotational atherectomy (RA) with 93% sensitivity and 88% specificity7. Furthermore, CTCA is valuable for the assessment of chronic total occlusions (CTO) by defining the anatomy of the vessel and the presence of severe calcifications within the proximal cap and the occluded segment itself. Thus, alternative procedural techniques, like retrograde approaches, can be pre-empted to overcome these challenges.
The advancements and advantages of CTCA in procedural planning have encouraged the development of dedicated artificial intelligence (AI)-enabled software to integrate CTCA into the cath lab workflow during PCI (QAngio CT [Medis Medical Imaging Systems])8. The P4 (Precise Procedural and PCI Plan; ClinicalTrials.gov: NCT05253677) study aims to compare CT-guided precision PCI versus intravascular ultrasound (IVUS)-guided PCI: for the CT guidance arm, CTCA will be utilised for preprocedural planning and for online guidance in the cath lab. Resource utilisation, immediate procedural outcomes, and major adverse cardiac events (MACE) during follow-up will be compared between both modalities at 12 months9.
Invasive coronary angiography
Identification of CCAD at invasive coronary angiography (ICA) is the first step in the management pathway for PCI of CCAD. CCAD is defined as moderate when radiopacities are observed only during cardiac motion before injection of the contrast and as severe when radiopacities are observed without cardiac motion and are visible on both sides of the arterial lumen, resembling tram tracks. However, ICA is unable to define the arc extension or depth of calcium.
Compared to IVUS and OCT, ICA has poor sensitivity (<50%) for calcium detection but a high specificity (>95%)1011. However, the sensitivity of ICA increases with increasing calcium burden at the lesion site, suggesting that when calcium is identified on ICA, it is usually thicker, longer, and extends to more than two quadrants. These features are associated with a higher chance of stent underexpansion.
Despite its limitations, ICA is an extremely useful and universally adopted tool for procedural planning in PCI of CCAD, and severe lesion calcification is included in the calculation of the SYNTAX score.
Prior to PCI, ICA gives guidance on vessel size and taper as well as the quantitative distribution of calcium along the vessel length. It defines calcified bends, tortuosity, and angles which could either hamper device/stent delivery, thereby pre-empting the need for extra support wires or guide extension catheters, or help to anticipate complications, including coronary perforation. One may then consider alternative balloon-based calcium modification tools like intravascular lithotripsy (IVL) or cautious atherectomy techniques that would be performed by experienced operators. The presence of luminal filling defects in severely calcified arteries often represents nodular calcification that may require a combination of calcium modification devices for optimal treatment and stent expansion (Figure 1).
During PCI, ICA identifies potentially serious complications, such as dissections and perforations that occur with greater frequency during the treatment of CCAD. Prior to stent implantation, the adequacy of lesion preparation can be assessed at ICA by inflating a non-compliant (NC) balloon (sized 1:1 to the vessel) to high pressures and checking for uniform expansion in two orthogonal projections (Figure 2). The use of stent-enhancement software may help in visualising stent expansion and optimisation (Figure 3). Finally, post-PCI angiography is key to identifying relevant complications.
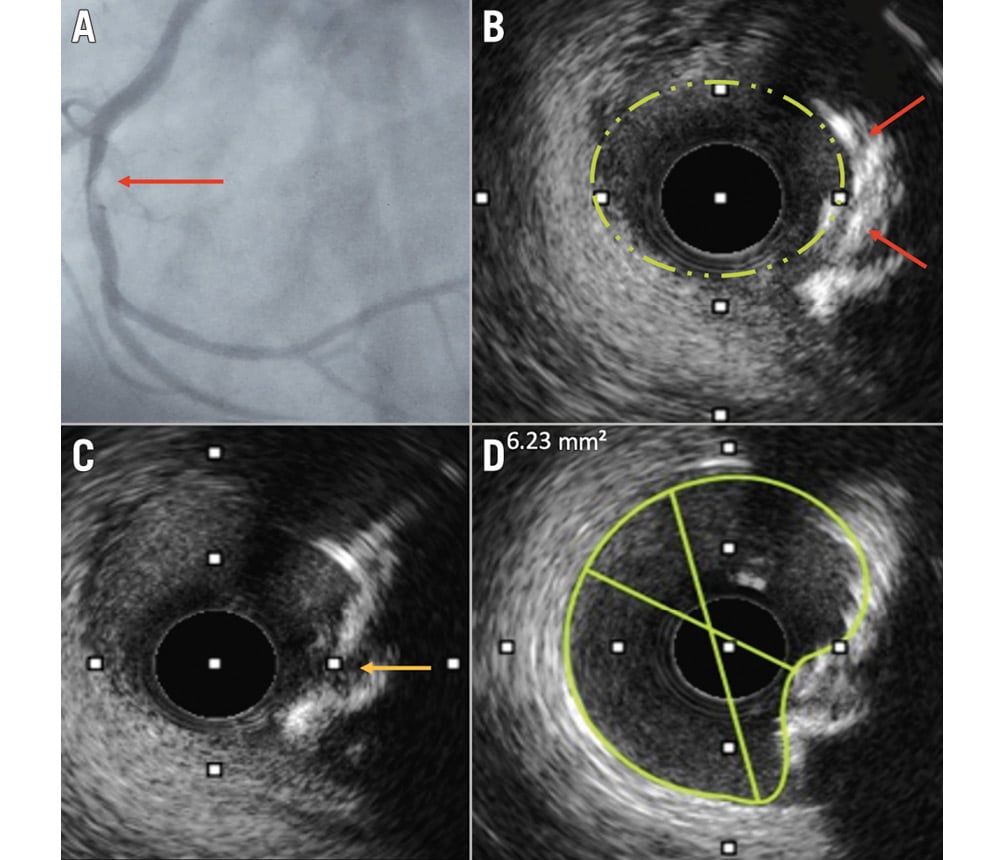
Figure 1. A patient with acute coronary syndrome and a luminal filling defect at angiography, assumed to be a thrombus. A) Filling defect in the RCA (red arrow). B) An intravascular ultrasound revealed an eruptive calcium nodule (red arrows). C) Fractures in the calcium plate were seen after intravascular lithotripsy (yellow arrow). D) Following stent implantation, despite the elliptical lumen, optimal stent expansion was achieved. RCA: right coronary artery
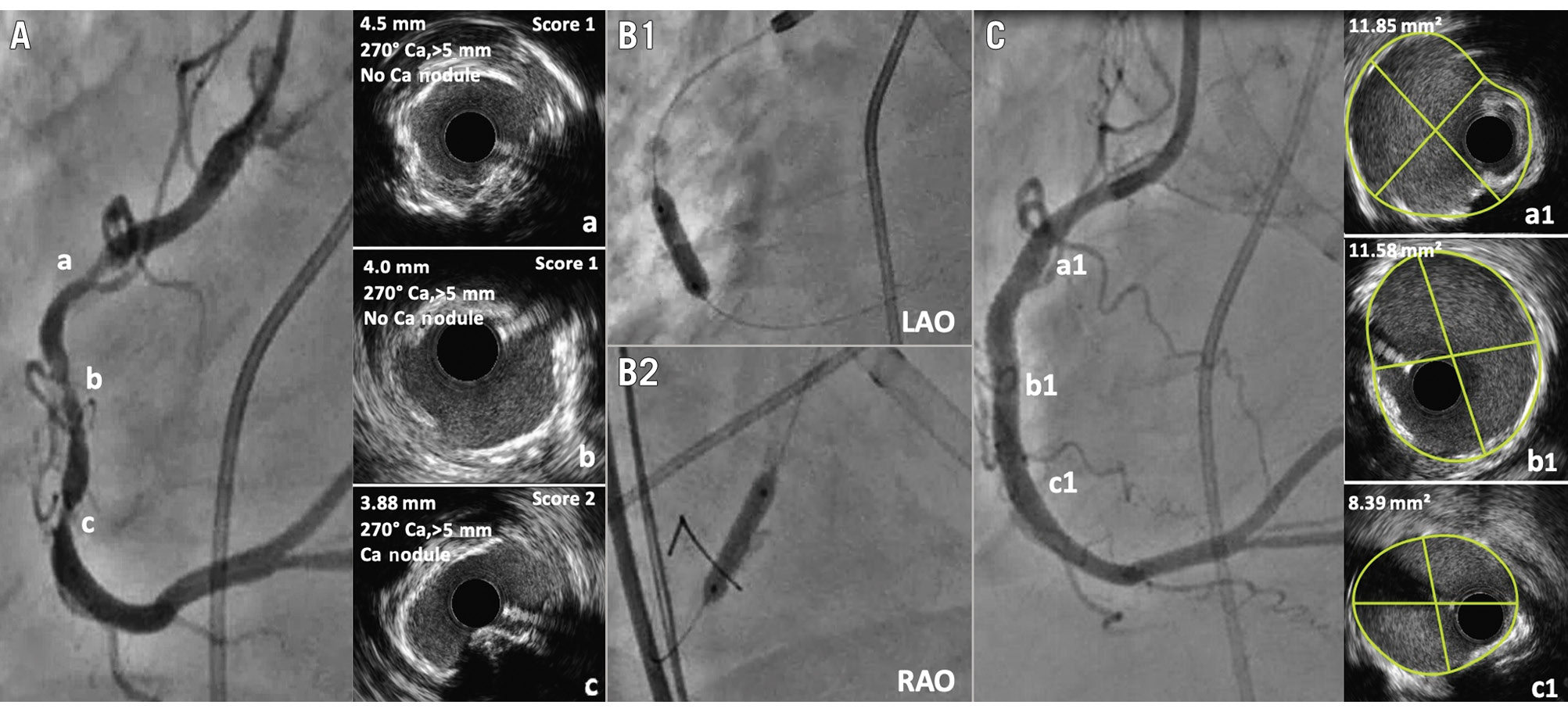
Figure 2. In severely calcified stenosis, intravascular imaging also guides when non-compliant (NC) balloon dilatation may be adequate. A) A tandem severely calcified stenosis in the right coronary artery (RCA). IVUS demonstrated a calcium score of 1 for proximal (a) and mid lesions (b) and a score of 2 for distal lesions (c). B) Based on the score, a 3.5 mm NC balloon was selected and showed full expansion at 18 atm in two orthogonal projections (B1 & B2). C) Following stent implantation, greater than 90% stent expansion and optimal minimal stent area were achieved (a1=11.85 mm²; b1=11.58 mm²; c1=8.39 mm²). Ca: calcium; IVUS: intravascular ultrasound; LAO: left anterior oblique; RAO: right anterior oblique
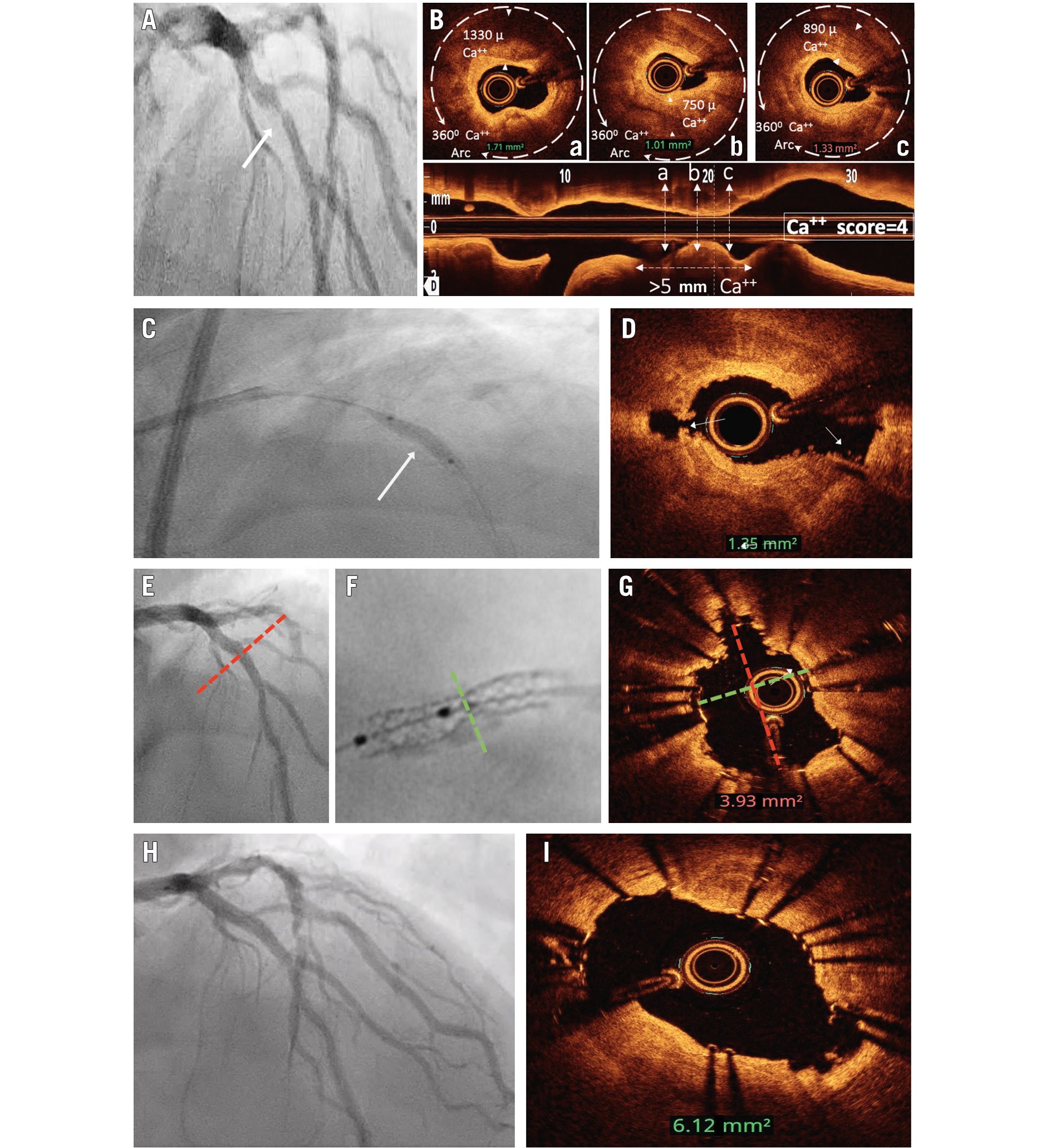
Figure 3. Focal, type A stenosis with moderate angiographic calcification. In a PCI procedure, there is value in visualising full balloon expansion in two orthogonal projections prior to stent implantation and the use of intravascular imaging for optimisation. A) Focal, moderately calcified proximal left anterior descending artery stenosis. B) Optical coherence tomography (OCT) demonstrated a calcium score of 4. C) Following 40 pulses of intravascular lithotripsy (IVL), the lesion showed full expansion in the AP cranial projection. D) Post-IVL, OCT demonstrated calcium fracture (white arrows). E) Following stent implantation, angiography showed full stent expansion in the AP cranial projection. F) Significant stent underexpansion was seen in the RAO caudal projection on “stent enhancement”. G) OCT demonstrated suboptimal MSA (3.9 mm2) and elliptical stent expansion. H) Following intrastent delivery of residual 40 pulses of IVL and ultrahigh-pressure balloon dilatation, OCT demonstrated the optimal MSA had been achieved (6.12 mm2) despite elliptical stent expansion (I). AP: anterior-posterior; Ca: calcium; MSA: minimal stent area; PCI: percutaneous coronary intervention; RAO: right anterior oblique
Intravascular imaging
Accurate and detailed evaluation of CCAD is only feasible through intravascular imaging (IVI) with IVUS and OCT. In those with moderate to severe calcium at ICA, IVI provides invaluable information for procedural planning and device selection to safely achieve an optimal result.
Intravascular ultrasound
IVUS, the most sensitive and specific imaging modality for CCAD identification11, displays calcium as a bright echogenic leading arc with shadowing behind it. IVUS can accurately characterise calcium as superficial when the bright leading edge is located within the shallowest half of the plaque and media thickness or as deep when the bright leading edge is within the deepest half of plaque and media thickness. Calcium extension is accurately quantified as degrees of a circumferential arc, while calcified plaque length can be estimated using automated pullback techniques. Nodular calcium is usually seen as a protruding convex-shaped eccentric mass of calcium with an underlying severely calcified plate (Figure 1B). On occasion, dense fibrous tissue can be mistaken for calcium as it is also echogenic and causes shadows behind it. However, “reverberations” (multiple reflections creating concentric arcs beyond the calcium) are highly specific for calcium. The limitation of IVUS is that it cannot define the thickness of calcium due to the complete reflection of ultrasound waves, thus causing acoustic shadowing behind the calcified leading edge. As a surrogate, the smooth surface of the calcium plate with reverberation behind it represents a thinner calcium plate, and the irregular surface without reverberation behind it represents thicker calcium12 (Figure 4). IVUS also has procedurally relevant advantages in patients with chronic renal failure, calcified CTO, left main interventions, and aorto-ostial lesions, given its capability of substituting repeated angiographic views.
Prior to PCI, an IVUS-based scoring system proposed by Zhang et al13 helps predict stent underexpansion and, therefore, the need for effective calcium modification prior to stent implantation. Each of the following IVUS-identified CCAD characteristics is assigned a score of 1: (a) calcium arc >270; ≥5 mm length; (b) calcium arc of 360; (c) calcified nodule (CN); (d) vessel diameter <3.5 mm. A total score of ≥2 is predictive of stent underexpansion, and therefore, calcium modification with appropriate devices should be strongly considered.
During the PCI procedure, the adequacy of lesion preparation is ascertained by visualising cracks and fractures in the calcium plate following IVL/ultrahigh-pressure balloon dilatation (Figure 1) or by identification of reverberations beyond the calcium arc (signifying thinning and volumetric reduction of calcium) when ablative atherectomy is used (Figure 4Cb). Volume reduction of nodular calcium can also be visualised, causing vessel lumen enlargement. Landing zones and stent length can be identified through an automated pullback. Following stent implantation, IVUS imaging is performed to optimise stent expansion based on predefined validated criteria14 (Figure 4F). Finally, vessel and stent analysis that is assisted by machine learning/AI-based software (e.g., the AVVIGO+ IVUS system [Boston Scientific]) may help operators in image interpretation and procedural optimisation.
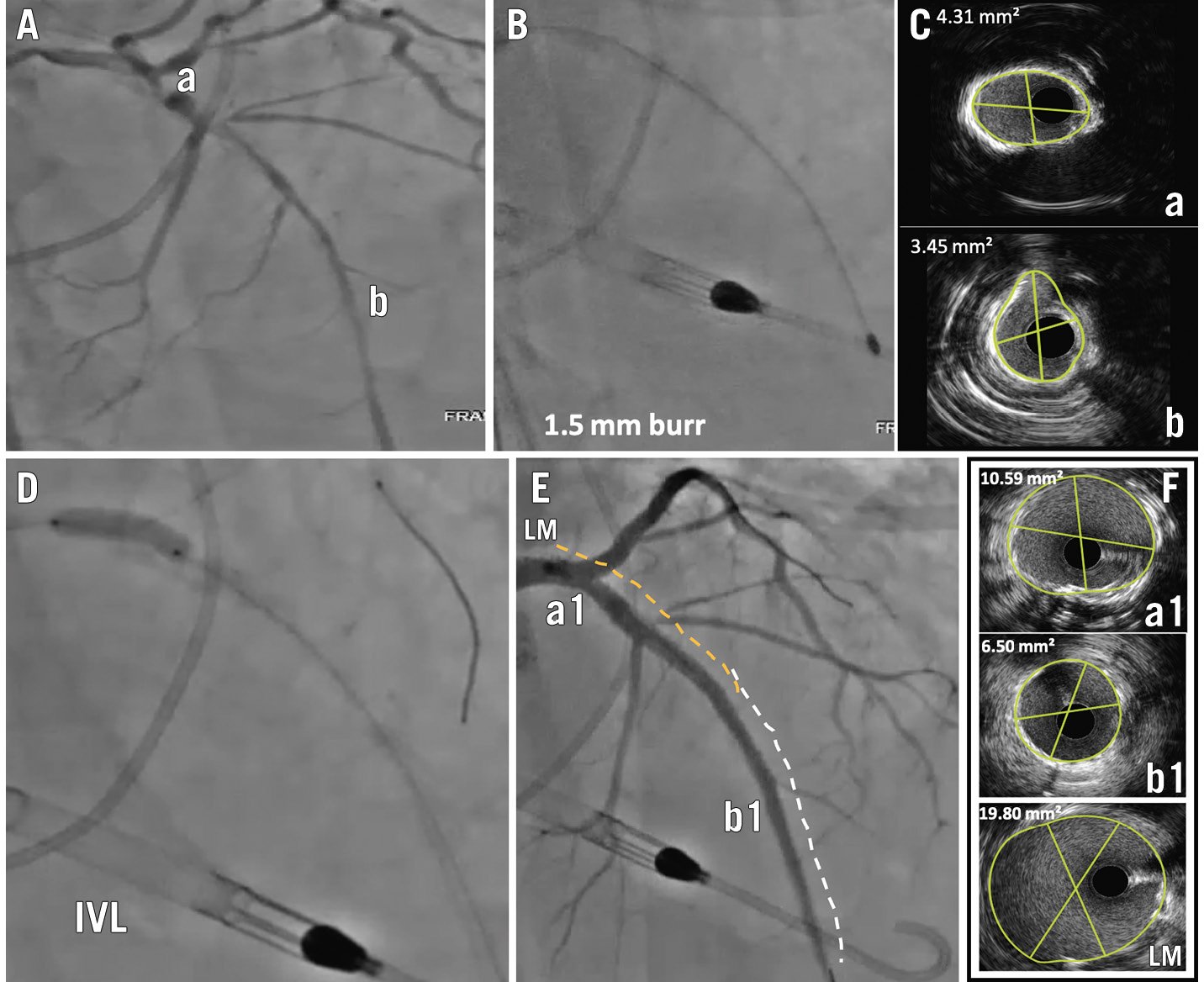
Figure 4. Long, severely calcified coronary stenosis. Intravascular imaging was able to guide different calcium modification devices for different segments of the vessel to achieve optimal results. A) Long segment stenosis of the left main (LM), ostial-proximal and mid-segment left anterior descending artery (LAD). B) The lesion was balloon uncrossable; therefore, rotational atherectomy (RA) with a 1.5 mm burr was performed. C) Post-RA, intravascular ultrasound (IVUS) showed a 360° calcium arc in the ostial-proximal LAD (image Ca), suggesting a need for additional plaque modification, and reverberations around the calcium in the mid-LAD (image Cb), suggesting adequate ablation and volume reduction. D) The mid-LAD lesion was dilated with a non-compliant optimal-sized balloon which achieved full expansion; intravascular lithotripsy (IVL) with a 4.0 mm catheter was used to treat the ostial-proximal LAD and LM, resulting in full expansion after 60 pulses (E). Two long overlapping stents were implanted with a good angiographic result (F). IVUS revealed an optimal MSA (mid-LAD 6.5 mm2, ostial-proximal LAD 10.59 mm2). MSA: minimal stent area
Optical coherence tomography
OCT is less sensitive than IVUS for the identification of calcium: in fact, CCAD cannot be precisely identified by OCT when superficial light-attenuating lipid, necrotic or heterogeneous (mixed) plaques overlie deeper calcifications. However, when borders are identifiable, OCT can define the thickness of calcium in addition to its arc extension and length (Figure 3). Nodular calcium is usually identified more accurately by OCT than by IVUS (Figure 5). Furthermore, OCT can define the specific morphology of the CN, eruptive or not, thus allowing the operator to seek the best procedural result and account for the prognostic impact of each type of lesion, as described earlier. Preprocedure, a validated OCT-based scoring system, proposed by Fujino et al15, helps to predict stent underexpansion, taking into account maximum calcium arc (>180°=2 points), maximum calcium thickness (>0.5 mm=1 point), and calcium length (>5 mm=1 point). A lesion calcium score of 4 predicts stent underexpansion (Figure 3B).
During the procedure, following plaque modification, OCT can identify plaque grooving with calcium volume reduction as it is clearly visible as arc fractures of calcium plates or at the base of a CN (Figure 5). The post-stent implantation minimal stent area (MSA) and stent expansion can also be assessed accurately and optimised as per well-defined criteria14. The new Ultreon Software (Abbott) utilises AI to automatically quantify calcification for length, arc and thickness to guide calcium modification strategies as per the scoring system.
As a final word, several studies have highlighted that the higher special resolution of OCT usually permits more precise definition of abluminal calcium thickness and morphology, together with a more precise evaluation of procedural results, leading to a larger percentage of stent expansion. However, it is not clear whether this translates into prognostic benefits, as the literature reports mixed results, and recent insights from a comparison of OCT versus IVUS guidance in complex PCI failed to identify a clear winner even in specific subgroups such as CCAD patients16.
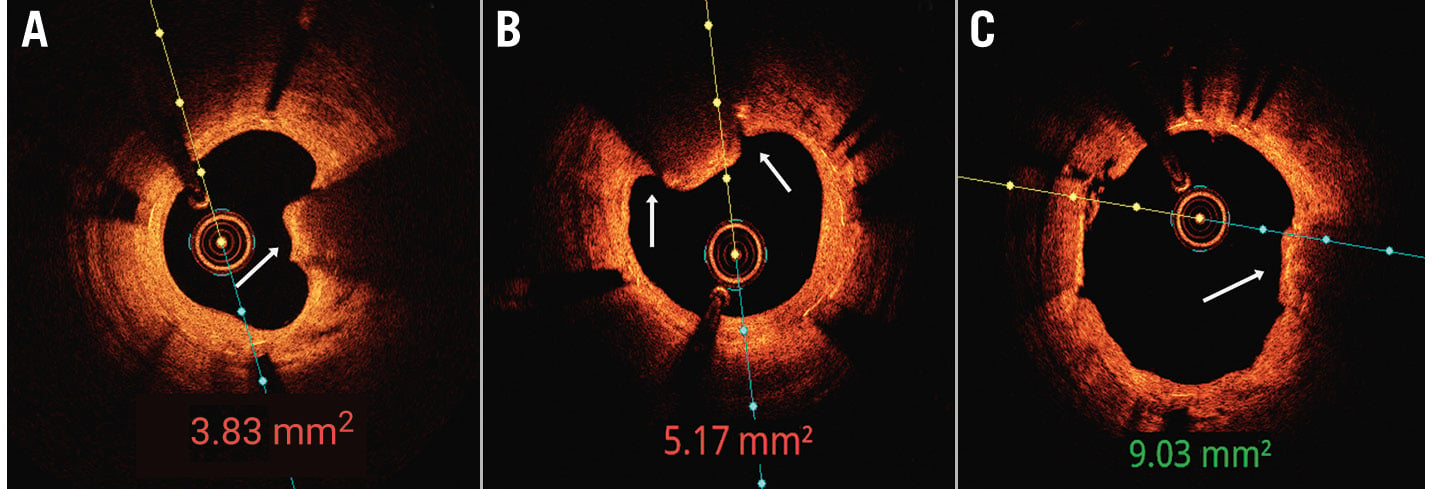
Figure 5. In-stent restenosis 5 months after stent implantation in a severely calcified artery. A) Optimal coherence tomography (OCT) showed a calcified nodule (CN) protruding through the stent struts as well as stent underexpansion. B) Following intravascular lithotripsy, fractures (white arrows) were seen at the base of the nodule. C) Following non-compliant balloon dilatation and use of a drug-coated balloon, OCT revealed extrusion of the CN and a large minimal stent area (9.03 mm2).
Additional observations at IVI which influence PCI planning
IVI can demonstrate guidewire bias towards or away from the calcified plaque/nodule, defining the possible impact of RA and helping in the choice of burr size. Calcifications with a large circumferential arc (>227°) but a thin plate (<0.67 mm) are more likely to fracture with NC balloon dilatation alone.
Eccentric/nodular CCAD may result in eccentric stent expansion despite adequate calcium modification leading to a residual stenosis seen with ICA and with stent enhancement. Chasing angiographic residual stenosis for complete stent expansion with larger NC balloons or higher pressures may increase the risk of vessel perforation (Figure 3). The MSA by IVI, rather than an angiographic estimation of residual stenosis, is the key procedural endpoint to achieve favourable long-term outcomes.
Dedicated devices for PCI of calcified coronary artery disease
High-pressure and super-high-pressure non-compliant balloons
NC balloons are used as a standalone or adjunctive therapy for modifying calcified coronary plaques. NC balloons allow uniform balloon expansion, reducing the risk of overexpansion of compliant segments that would otherwise cause complications such as dissection and perforation1718. Heavily calcified plaques can be resistant to high-pressure inflation of the NC balloons. Balloon angioplasty may fail to provide sufficient stent expansion when the thickness of the calcified plaques is more than 0.24 mm19. One super-high-pressure NC balloon (OPN NC [SIS Medical]) has a twin-layer design allowing inflation at a higher pressure, with a rated burst pressure of 35 atm. It can be effective in highly resistant, undilatable lesions17, and the inflation pressure may be increased slowly up to 45-50 atm18. In the randomised trial ISAR-CALC20, the use of super-high-pressure balloons in patients in whom predilatation with conventional non-compliant devices had been unsuccessful was associated with better OCT-derived minimum lumen diameter and diameter stenosis compared to scoring balloons, without particular differences in terms of stent expansion index or angiographic procedural success.
Cutting/scoring balloons
The cutting balloon (e.g., WOLVERINE [Boston Scientific]) is an NC balloon with 3 or 4 atherotomes attached longitudinally to its surface. In non-calcific atherosclerotic plaque, these atherotomes create microincisions in the vessel wall and score the vessel longitudinally, allowing controlled dilatation of the vessel rather than causing an uncontrolled disruption of the plaque. A recent randomised trial demonstrated that predilatation with the cutting balloon resulted in a higher final minimal stent area than that with NC balloons21. In calcified lesions, the blades act as a fulcrum for the application of force, leading to calcium fracture. When combined with rotational artherectomy, RA followed by the cutting balloon was associated with more calcium fracture and better stent expansion compared with RA followed by conventional balloons22. Scoring balloons are either semicompliant (e.g., AngioSculpt [Philips], NSE Alpha [B. Braun]) or NC (e.g., Scoreflex [OrbusNeich]) balloons surrounded by helical scoring elements on the surface. This design reduces the risk of balloon slippage and allows controlled and uniform balloon inflation with a relatively better trackability than the cutting balloon.
The advantage of the balloon-based strategy is the ease of use without additional training or special equipment. However, the limitations of the balloon-based strategy are (1) deliverability within heavily CCS; (2) eccentric expansion of balloons in the presence of eccentric plaque or a CN; (3) limited impact on thick, nodular, and eccentric calcified plaques; and (4) possibility of barotrauma-related complications.
Rotational atherectomy
RA (e.g., ROTAPRO [Boston Scientific]) is one of the atheroablative techniques which has been used for more than 35 years to physically ablate and remove the calcified plaques using a diamond-encrusted elliptical metallic burr (1.25-2.5 mm) rotated at high speeds. RA works based on two fundamental principles. First, a differential cutting mechanism allows preferential ablation of inelastic calcified plaque that is unable to stretch away from the burr while sparing healthy elastic tissue23. Second, most of the microdebris produced by RA are small enough (~5 μm) to traverse the coronary microcirculation and be cleared by the reticuloendothelial system24.
Two randomised trials from the drug-eluting stent (DES) era suggested the feasibility and effectiveness of an RA-based plaque modification strategy2526. Although these trials did not show relevant differences in clinical outcomes between RA- and balloon-based strategies, the RA-based strategy was associated with significantly higher procedural success2526. In addition, a pooled analysis of these trials showed that there was a 14% crossover rate to the RA-based strategy due to the inability to cross with any balloon (48.9%), undilatable lesions (31.9%), and inability to deliver a stent (17.0%)27. Predictors of the need for bailout RA were tortuosity >45â°, angiographic severe calcification, lesion length >20 mm, and bifurcation lesion27. These results suggest that the RA-based strategy has a role in selected cases where the balloon-based strategy is highly likely to fail (upfront) or has already failed (bailout).
Optimal RA technique is important to minimise procedural complications such as dissection, perforation, slow-flow, or no-reflow and burr entrapment. Based on the studies which demonstrated more procedural complications and no clinical benefits with the maximum debulking strategy with a large burr28, the use of a small burr (burr/artery ratio <0.7) is recommended to modify calcified plaques but not aggressively debulk them29. A recommended rotational speed is between 135,000 and 180,000 rpm30, and some experts prefer a speed between 140,000 and 150,000 rpm, with higher speeds reserved for selected cases to reduce the risk of complications29. It is important to gradually advance the burr with a pecking motion, avoid deceleration during rotablation >5,000 rpm, and limit the duration of ablation to <30 seconds per individual run30. Since the dedicated 0.009” wire may be difficult to steer and advance, especially in the case of tortuous anatomy, the use of microcatheters for wire-exchange techniques may be extremely useful to achieve distal positioning. Finally, the latest iteration of this wire (ROTAWIRE Drive [Boston Scientific]) is designed for easier manoeuvring and may hopefully facilitate the adoption of this therapy.
Orbital atherectomy
The Diamondback 360 (Abbott) orbital atherectomy (OA) system (OAS) is an atheroablative technology to treat severely calcified coronary lesions. The OAS has one size of diamond-coated crown (1.25 mm) which is eccentrically mounted to a drive shaft and advanced over a dedicated 0.012” ViperWire Advance (Abbott) coronary wire with a 0.014” tip31. There are distinct features related to the mechanism of action of OA. In contrast to RA, which uses the rotational motion of the burr, the crown of the OAS orbits around the vessel lumen at high speeds and ablates calcified plaques using a differential sanding mechanism32. OA works at low (80,000 rpm) and high (120,000 rpm) speeds. The orbital diameter of the crown expands radially with a higher speed via centrifugal force3132. This mechanism of action allows circumferential ablation of eccentric calcified plaques with less wire bias than RA and the use of a single 1.25 mm crown for a vessel size ranging from 2.5 mm to 4 mm. The orbital movement of the small crown allows maintenance of blood flow to the distal vessel while it is sanding the calcified plaques into microdebris with an average size of 2 μm. This contributes to the less occlusive nature of OA with a lower risk of slow-flow or no-reflow than RA3132. Another important difference between RA and OA is that the crown of the OAS allows bidirectional ablation of the calcified plaques, reducing the risk of device entrapment.
The ORBIT (Evaluate the Safety and Efficacy of OAS in Treating Severely Calcified Coronary Lesions) II trial was a prospective single-arm study that demonstrated the safety and efficacy of OA in the treatment of severely calcified de novo lesions (30-day MACE: 10.4%, procedural success rate: 88.9%)33. Complications after OA were relatively low: slow-flow or no-reflow was seen in 0.2%, severe dissection in 2.3%, abrupt closure in 0.9%, and perforation in 0.9%33. In a real-world registry, OA also showed favourable 1-year clinical outcomes compared to historical controls34. Data are lacking regarding the direct comparison of OA with other plaque modification strategies. To this point, RA has had 40 years of clinical application, accumulated scientific evidence, and extensive clinical use in daily practice worldwide (the manufacturer declared that more than 1,000,000 cases have been performed after 30 years of commercialisation); this device has therefore revealed practically all its potential, and limitations, while OA still has open questions awaiting answers derived from more extensive use. Despite the lack of adequate evidence-based studies, the latest consensus of European experts dedicated to the treatment of severely calcified lesions still recognises a unique role for RA when facing uncrossable and undilatable lesions, one of the most challenging scenarios of PCI that, so far, remains the domain of RA1. Finally, a head-to-head comparison of the clinical outcomes of the two atherectomy devices has never been conducted and the potential advantages of OA over RA remain only hypotheses. On the contrary, a recently published randomised head-to-head study revealed that RA seems to yield significantly better tissue modification, a larger lumen area and better stent expansion compared to OA35, while the recent ECLIPSE trial (Kirtane, AJ. ECLIPSE: A Large-scale, Randomized Trial of Orbital Atherectomy vs. Conventional Balloon Angioplasty in Severely Calcified Coronary Arteries Prior to DES Implantation. TCT 2024. 29 October 2024, Washington, D.C., USA) failed demonstrate better results with OA compared to balloon angioplasty in severely calcified lesions.
Intravascular lithotripsy
IVL is a novel therapeutic option for calcified coronary lesions. The Shockwave IVL catheter (Shockwave Medical) contains multiple lithotripsy emitters along the shaft of an integrated semicompliant balloon36. Electric energy discharged from the lithotripsy emitters creates rapidly expanding and collapsing vapour bubbles within the integrated balloon, producing acoustic pressure waves (or shockwaves) that propagate circumferentially and transmurally37. The shockwaves travel through soft tissue with a minimal effect, but if they encounter a tissue with differing acoustic impedance, such as calcium, compressive stress is created, which fractures the calcium36.
Clinical studies have shown that circumferential and longitudinal calcium fracture was the major mechanism of action of IVL that facilitated acute luminal gain and stent expansion3637. In a pooled analysis of the Disrupt CAD I-IV studies, procedural success was achieved in 92.4% of cases with a 30-day MACE of 7.3%, which was largely driven by in-hospital non-Q wave myocardial infarction38. The rates of any angiographic complication and perforation were 0.3% and 0.2%, respectively, and no IVL-associated perforations, abrupt closure, or no-reflow were observed39.
IVL has several advantages over other plaque modification strategies. IVL uses the acoustic energy delivered by a semicompliant balloon inflated at 4 atm. The risk of barotrauma is lower than other strategies requiring high-pressure balloon inflation. IVL has a unique advantage in the treatment of both superficial and deep as well as concentric and eccentric calcium due to circumferentially and transmurally propagating acoustic energy without being affected by wire bias. One of the caveats of using IVL in the treatment of heavily calcified coronary lesions is the deliverability of the IVL catheter to the target lesions. In such cases, the use of guide extension, predilatation with balloon angioplasty, or a combined strategy with other ablative therapies can be considered. The new Shockwave C2+ platform is designed for improved deliverability and guarantees 120 pulses instead of 80, which may be useful for treating longer segments, modifying heavier calcifications or spare IVL balloon runs/pulses to treat different regions.
Excimer laser coronary atherectomy
Excimer laser coronary atherectomy (ELCA) debulks and modifies tissue with the photochemical, photothermal, and photokinetic properties of monochromatic coherent ultraviolet light without causing significant vessel injury. While thought to be more effective on fibrotic plaques and thrombi, it proved to be a useful tool also for calcified uncrossable or undilatable lesions, and it may also be effective to facilitate modification of the proximal cap and allow its penetration into a CTO40. Although off-label, laser atherectomy with slow contrast rather than saline injection can be used by experienced operators to increase its effect, especially within underexpanded stents41. The ELCA catheter comes in four sizes (0.9 mm, 1.4 mm, 1.7 mm and 2.0 mm), and it can be advanced over a regular workhorse wire. Finally, although in the past its preparation has been cumbersome, the new device carries significant advances that allow for an easier setup.
Practical considerations and algorithmic approach
The Central illustration presents an algorithmic approach for the treatment of calcified coronary lesions. The most important step in the treatment algorithm is to diagnose, characterise, and quantify the calcified plaques with intravascular imaging, which not only helps determine the optimal treatment strategies but, as previously stated, can also improve clinical outcomes after PCI42.
Once IVUS or OCT images are obtained, evaluation of the characteristics of the calcified plaques should be performed. In the presence of superficial (luminal) calcified plaques, calcium scores are calculated to determine the need for adjunctive plaque modification therapies131415.
Calcified plaques which are located deep in the vessel wall covered by superficial fibrosis can be treated with conventional NC or speciality balloons43. IVL would also be effective because of its transmural propagation of acoustic energy. Among atheroablative therapies, ELCA may be considered given its ability to generate sonic waves affecting compliance and ablating superficial fibrous tissue. RA has a limited role in the treatment of deep calcium.
Treatment of CN remains challenging. Because of their distinct morphological and prognostic features, it would be helpful to differentiate between eruptive and non-eruptive CN by intravascular imaging. IVL may be effective in the treatment of CN, especially eruptive44, and can be considered as an upfront or adjunctive therapy. Among the atheroablative strategies, OA may have benefits over RA given its circumferential shaving mechanism, although this has not been proved. Some of the CN are highly non-deformable and may require a combined approach. A combination of other ablative therapies and IVL may have a synergistic effect on the treatment of CN by debulking and fracturing the CN.
After initial plaque modification, it is advised to repeat IVI, and if no calcium fractures are seen, additional therapies should be considered prior to stent implantation. A combination of RA or OA with IVL appears to be promising in certain cases. In fact, deep calcium may not be adequately addressed by RA, and IVL may not be possible as the upfront strategy due to difficulties in device advancement. Combination therapy is in line with contemporary goals, from maximum plaque debulking as plaque modification45, and case series of the “RotaTripsy” strategy support feasibility and efficacy. A combination of atherectomy and modified-balloon techniques, such as RA and cutting balloon, has also been proposed and may improve acute gain and stent expansion in selected patients46. After plaque modification is achieved, a DES is implanted, followed by IVI, which provides improved outcomes by addressing edge dissection, malapposition, and underexpansion. For underexpanded stents, despite postdilatation with high-pressure NC balloons, super-high-pressure NC balloon inflation or IVL can be considered as a bailout. Note that such an indication is not considered in the instructions for use of IVL although it is extensively used within that scope in real-world practice4748.
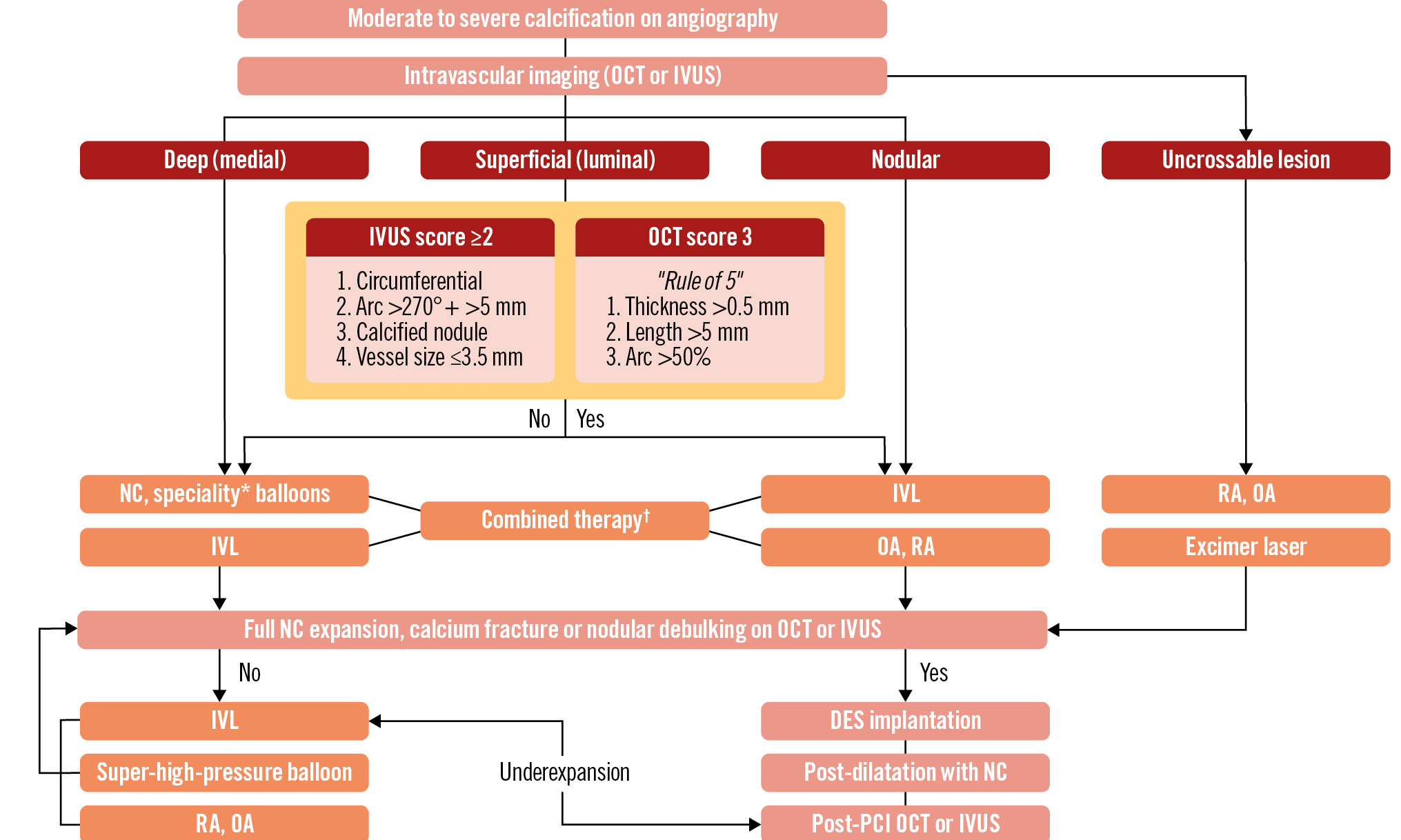
Central illustration. Algorithmic approach for the optimal management of calcified coronary lesions. Intravascular imaging with OCT or IVUS is the first step in the management algorithm. For uncrossable lesions, upfront atheroablative therapies can be used. Deep calcified plaques can usually be treated with NC or speciality balloons, and IVL can be considered. Calcified nodules are sometimes non-deformable, and IVL, atheroablative therapy (especially OA), or a combination of both can be considered. For superficial luminal calcified plaques, an IVUS- or OCT-based scoring system can be used to determine the need for adjuvant plaque modification therapies. If those scores are high, adjunctive plaque modification therapies should be considered. Prior to stent implantation, effectiveness of the plaque modification should be assessed. If there is no calcium fracture, additional plaque modification therapies are needed prior to the stent implantation. If the stent is implanted but underexpanded, further treatment with a super-high-pressure balloon or IVL can be considered to achieve optimal PCI results. *Speciality balloons include cutting or scoring balloons. †Combination of IVL and an atheroablative therapy. DES: drug-eluting stent; ELCA: excimer laser coronary atherectomy; IVL: intravascular lithotripsy; IVUS: intravascular ultrasound; NC: non-compliant; OA: orbital atherectomy; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; RA: rotational atherectomy
Management of complications in PCI of calcified vessels
Although plaque modification devices may cause common PCI complications, this chapter will describe those specific to each individual tool only.
Specific complications related to RA are burr stall, burr entrapment, burr detachment, and RotaWire tip separation. Similarly, OA carries specific complications of ViperWire fracture, crown entrapment, and crown detachment. A recent evaluation of the MAUDE (Manufacturer and User Facility Device Experience) database revealed that the most common OA complications are related to equipment failure49. Indeed, separations were the most common complications of OA (wire separation was most commonly followed by drive shaft detachment and crown detachment). The next most common complication was crown entrapment.
Burr and catheter size
Selecting smaller burr sizes (burr:artery ratio <0.7) reduces angiographic complications without compromising procedural success50. Smaller burrs permit smaller guide catheter and arterial sheath use and also allow for radial rather than femoral access, with fewer resultant vascular complications. Cautious burr selection is required in situations of uncrossable lesions, tortuosity, eccentric lesions, and wire bias.
Burr or crown entrapment
The incidence of burr entrapment is 0.4% to 1%51. A burr stall might occur in cases of aggressive advancement of the burr through very eccentric and extremely calcified lesions. If the burr passes distal to an incompletely ablated lesion, proximal retrieval of the burr is restricted by the absence of diamond chips on the back surface of the burr. This prevents retrograde ablation. Again, this complication can be avoided by meticulous RA technique and smaller burr sizes. The burr should be rotating throughout its passage through the lesions, preventing decelerations of more than 5,000 rpm. The burr should not be stopped from spinning within the lesion. When platforming the burr, all tension should be removed from the drive shaft before starting the burr in order to prevent the burr from lurching forward into the stenosis. Warning signs of impending burr entrapment include lack of smooth advancement under fluoroscopy, change in auditory pitch with variations in resistance encountered by the burr, or the tactile feel of resistance in the advancer knob with or without excessive drive shaft vibration.
A burr stall is often associated with entrapment and difficult burr retrieval. When a burr is entrapped, both advancement and removal are prevented. Attempts to restart the burr should be avoided.
During OA, heavily calcified tortuous vessels carry the highest risk of entrapment. It is recommended to avoid high speed and to maintain the same steady rate of advancement and retraction to reduce the risk of crown detachment while maximising plaque ablation.
Methods for retrieval of a stuck burr (methods for a stuck OAS crown are the same):
1. Administer high-dose intracoronary vasodilators.
2. Most times, steady, controlled traction on the drive shaft will release the burr. A disconcertingly aggressive pull on the drive shaft may be needed. Care must be taken not to apply too much force. Be aware of the risk of proximal dissection from deep guide catheter intubation. This can be attempted in both Dynaglide-on and Dynaglide-off modes.
3. Traction on the guidewire may help to release the burr. The spring tip of the RotaWire is 0.014 inches and acts as a stopper at the burr tip. Thus, pulling the wire will pull on the burr. This may, however, lead to wire tip separation (see below). The ViperWire also has a stopper to assist traction on the drive shaft.
4. Entrapped burrs (or crowns) may be retrieved by inflation of small balloons. Careful advancement of a second guidewire (usually requiring a second guiding catheter) beyond the entrapped burr, with inflation of a small buddy balloon (1.0-1.25 mm) next to the burr to release it. It may be necessary to navigate a wire into the subintimal space adjacent to the burr and to inflate a small balloon next to the burr. With such subintimal dilatation, every attempt should be made to maintain the RotaWire in the distal vessel to avoid losing access to the distal true lumen.
5. Advance a guide extension catheter over the drive shaft. Use the biggest guide extension that the guide catheter can accommodate. Cut the drive shaft outside the body and remove the sheath that covers the drive shaft. Push the guide extension over the drive shaft and up to the burr. This allows a fulcrum for steady traction on the burr or crown52.
6. Subintimal tracking beyond the burr with distal vessel re-entry, as with antegrade dissection re-entry CTO techniques, is a final option. The burr is then stented into the vessel wall. This is only possible if the drive shaft has come loose from the burr and only the burr or crown remains53.
7. If catheter-based strategies fail, surgical removal and coronary artery bypass grafting (CABG) may be required.
Crown detachment
Complete detachment of the crown is more common than crown entrapment. The risk of this complication increases with prolonged ablation and with multiple passes, particularly at high speed49. Using the same speed in each direction of plaque clearance with a slow and steady advancement is recommended to lower the risk of crown detachment while maximising plaque clearance54. According to the manufacturer, the risk is greatest after a total treatment time of 8 minutes.
Use of a gooseneck snare over a parallel guidewire (described in more detail for a separated wire below) or a guide extension over the ViperWire, such as described above for a stuck burr, may retrieve the crown.
Wire fracture or entrapment
Wire fracture, with or without distal entrapment of the wire, can occur during RA. The transition between the distal radio-opaque portion and the stiffer shaft of the RotaWire guidewire is susceptible to fracture. The burr will not pass beyond this transition from the 0.009” wire to the 0.014” tip, and this zone should be avoided during burring in order to prevent tip separation. Operators should take caution not to bend or kink any portion of the wire (especially portions of the wire upon which RA is to be performed).
The ViperWire may also detach. A wire with bends or kinks and prolonged ablation, lasting more than 5 minutes, predisposes to wire fracture.
If the wire is trapped in a small branch, a microcatheter can be advanced up to the point of entrapment, and an attempt can be made to free the wire by pulling. Not infrequently, this will result in wire tip separation. If the wire tip is in a small branch, it may be left there, usually without major consequences. If the wire is in a main vessel, it can be retrieved or pushed into a small, less important branch.
The following methods may be considered5455:
1. A parallel wire and a partially inflated balloon can be used to push the wire into a distal small branch.
2. Wire wrap: two or more additional wires can be advanced past the broken wire tip, and these are rotated multiple times in opposite directions to wrap around the broken wire and each other. All the wires are then pulled back into the guide catheter together.
3. Microsnare: A parallel wire is advanced adjacent to the broken wire. A microsnare is advanced over the second wire, and an attempt is made to snare the broken wire. Both wires are then removed.
4. Alternatively, the wire may be stented into the vessel wall.
Calcified-vessel PCI in specific settings
Bifurcation and left main lesions
Bifurcation lesions (BL) may be encountered in up to 20% of PCI cases, and the presence of calcium is an established risk factor for side branch (SB) damage56. Registry data confirm higher rates of target lesion failure (TLF) after PCI in the presence of severely calcified BL57. However, evidence regarding the best strategies for BL treatment is limited. The PREPARE-CALC trial compared scoring/cutting balloon versus RA for CCAD treatment and found higher rates of SB compromise in the modified-balloon arm26. Furthermore, its bifurcation subanalysis in 104 patients confirmed this finding together with significantly higher rates of myocardial injury58. A recent retrospective study further supports the safety of main-branch RA even when compared with conventional bifurcation techniques59: in 302 calcified bifurcation lesions, Mizuno et al found significantly less SB compromise (in terms of reduced Thrombolysis in Myocardial Infarction [TIMI] flow) in RA-treated patients. Finally, a pooled analysis of the PREPARE-CALC and ROTAXUS trials identified BL in CCAD as a predictor of the bailout performance of RA28, together with lesion length and vessel tortuosity. Regarding the treatment with OA, data from a large retrospective registry report relatively low 30-day MACE rates for both bifurcation (10.1%) and non-bifurcation (6.2%) lesions60, mainly driven by myocardial infarctions at 30 days (8.4% and 4.9%). Therefore, despite the lack of SB wiring, treatment with atherectomy devices has proved to be among the safest techniques for calcified bifurcation lesions. Side branch wire protection with a microcatheter has been performed and proposed for very high-risk left main (LM) atherectomy procedures, but there is probably little need to adopt this technique that has never been proved to improve outcomes. Furthermore, since both in vivo and in vitro experiences report frequent damage to the microcatheter and the wire, this technique cannot be recommended. IVL has the potential advantage of allowing SB wiring during the plaque modification procedure, although no relevant evidence is available on the performance of this strategy compared to atherectomy. Provisional single stenting is the most supported strategy in BL treatment, and CCAD is no exception as some data, like the COBIS II registry, confirm higher rates of repeat PCI with a 2-stent strategy57. Upfront use of the latter may be considered in selected patients, especially when the calcified SB serves a large territory, is >2.5 mm in diameter, has a plaque length >5 mm, and >70% stenosis. Available evidence on calcified LM treatment is limited. However, a recent European registry reported 1-year MACE rates of RA in this group as comparable with those of non-LM RA PCI. Nonetheless, the marked clinical and procedural complexity of this subgroup was confirmed by numerically higher 30-day MACE and 1-year target vessel revascularisation (TVR)61. Finally, an initial experience with IVL for the treatment of calcified unprotected LM suggests its safety and feasibility, also as a second-line therapy following ablative techniques, but stronger evidence is required to confirm this finding62.
Thrombotic lesions and acute coronary syndromes
Lesion preparation in the presence of an evident thrombus or acute coronary syndrome (ACS) is often feared as an adjunctive risk of distal embolisation or slow/no-reflow. However, in a challenging anatomical and clinical context like CCAD-ACS, adequate plaque modification is crucial to reduce the risk of procedural failure, potentially life-threatening complications such as dissection or perforations, and stent undersizing/underexpansion. As reported by an OCT analysis in ACS patients, up to 13% of subjects may have a calcified culprit plaque, mainly classified as superficial calcific sheets (67%) followed by eruptive CN (26%) and calcified protrusions (7%)63. Superficial calcific sheets seem to be more represented in left anterior descending artery lesions and linked to more extensive myocardial damage, while calcified CN may be responsible for 2-7% of sudden cardiac deaths64. PCI of CCAD in ACS was an independent predictor of stent thrombosis and unplanned ischaemia-driven target lesion revascularisation (TLR) in a pooled analysis of 6,855 patients from the ACUITY and HORIZONS-AMI trials65, where severe and moderate target lesion calcifications were reported in 6% and 26% of patients, respectively. A recent comparison of CCAD RA treatment in patients with chronic coronary syndromes versus unstable angina (UA) or non-ST-segment elevation myocardial infarction (NSTEMI) reported comparable procedural success and 1-year clinical outcomes (hazard ratio [HR] 1.39, 95% confidence interval [CI]: 0.91-2.12), despite a slightly higher rate of acute slow-flow in UA patients, suggesting the safety and feasibility of RA in this complex subgroup66. Similarly, patients with acute myocardial infarction (81% NSTEMI) treated with RA showed 99% feasibility and comparable acute results with a stable RA control group, also confirming higher MACE rates from 30-day to 1-year follow-up in the ACS group67. Few data are available for IVL in the specific ACS setting, even if a recent comparison of a high-risk real-world subgroup (47% ACS) with Disrupt CAD III trial patients suggests high procedural success and low complication rates68.
In summary, as proposed by the most recent European consensus, appropriate calcified plaque modification techniques should also be used in the ACS context. The opportunity to perform ad hoc procedures is largely dependent on the operator’s and other team members’ experience. In fact, if the cath lab has not completed training with available plaque modification devices, TIMI-3 flow should be re-established without stenting during the index procedure whenever possible, planning staged interventions with proficient operators or, if not achievable, considering the transfer of the patient to a high-expertise centre1.
Chronic total occlusion
The severity of calcifications in CTO seems to worsen with lesion age69 and the presence of bypass grafting, probably due to reduced shear stress secondary to competitive flow. A large retrospective analysis on >13,000 CTO-PCI between 2012 and 2023 reported moderate to severe calcifications in 47% of patients. These patients were older, more likely to have had previous bypass surgery, and had higher Japanese CTO (J-CTO) scores. The presence of moderate/severe calcifications was also significantly associated with MACE and perforations, and it was an independent predictor of lower technical success rates70. Furthermore, contrast medium volume, fluoroscopic time, and total radiation dose were significantly associated with severe calcifications. A recent expert review of the European CTO Club71 strongly endorsed preprocedural calcium assessment with CTCA in order to plan the treatment on the basis of a complete non-invasive anatomical evaluation, including the occluded segments. IVUS is usually preferred over OCT for intravascular imaging because the need for contrast fillings may worsen coronary dissections and haematomas as well as renal function. If intraplaque tracking can be achieved, microcatheter-assisted distal positioning of a Rota- or ViperWire should be possible, and an atherectomy can be performed, if appropriate. A series of CTO patients treated with RA showed comparable long-term outcomes with non-RA subjects (MACE 15% vs 13%; p=0.7), despite their relevantly higher complexity72. Extra-plaque crossing may be obtained with knuckle techniques using polymer-jacketed wires. While atherectomy is generally not advised in case of complex extra-plaque tracking, cautious use of small-burr RA may still be evaluated by expert operators to modify the intra-extra plaque transition of the proximal cap. Initial experience with IVL suggests safety and efficacy also in this context, and cautious extra-plaque use has been described, bearing in mind the higher risk of perforations73. Aggressive balloon dilatation with pressures higher than 14-16 atm within extra-plaque segments is generally not advisable because of the risk of extensive coronary perforations. ELCA may be potentially useful for uncrossable lesions, but the supportive evidence is scarce74.
Concomitant aortic stenosis and TAVI patients
The calcification process of atherosclerotic plaques and of the aortic valve apparatus share similar pathophysiological mechanisms, involving inflammation, oxidative stress, fibrosis, angiogenesis and osteogenesis differentiation and, consequently, aortic stenosis (AS) and CAD coexist in 50% to 75% of patients75.
Indeed, the presence of severe aortic valve calcifications is a strong predictor of extensive CAD76, although the clinical impact of CAD in patients with AS is unclear. The ACTIVATION (PercutAneous Coronary inTervention prIor to transcatheter aortic VAlve implantaTION) trial enrolled AS patients with stable CAD (Canadian Cardiovascular Society class ≤2 angina) that were randomised to PCI versus medical treatment of the associated CAD. The study demonstrated no benefits of PCI but a higher incidence of bleeding in the PCI group77. However, when PCI of CCAD in AS patients is deemed necessary, plaque/calcium modification techniques should be adopted, if appropriate. A recent large US database search of AS patients treated with PCI reported the use of RA in 9.1% and OA or excimer laser atherectomy in 2.1% of cases, with increasing absolute numbers over the years and a trend towards fewer MACE with IVUS guidance78. A retrospective study of 32 AS patients treated with OA suggested the feasibility and safety of OA in this context79. Reported experience with CCAD-IVL in AS is currently limited to case series.
Although there is little evidence available, PCI treatment of CCAD in patients undergoing transcatheter aortic valve implantation (TAVI) is a topic of interest. Several experiences report that a pre-TAVI CT scan may be useful to anticipate the presence and distribution of CCAD80. In a monocentric study, RA that was performed before valve replacement was safe and effective in TAVI patients81. A case series of 18 OA patients also reported acceptable 30-day and 1-year outcomes, but OA was performed exclusively before the TAVI procedure82.
Need for mechanical circulatory support
Very limited evidence is available on the role of mechanical support devices during CCAD-PCI, but their main goal is to maintain acceptable blood pressure and perfusion in patients with severely impaired left ventricular function undergoing complex PCI on vessels that supply extensive areas of viable myocardium83. In fact, a case series of high-risk RA reported relevant rates of prolonged hypotension and bailout need for assist device placement84. Given the multiple procedural steps for lesion preparation and stent optimisation together with the higher complication risk, usage of upfront or bailout active supportive devices such as Impella (Johnson & Johnson) pumps or extracorporeal membrane oxygenators (ECMO) during CCAD-PCI may be of help. Special care in the management of femoral access, including echo-guided puncture, is strongly suggested as bleeding and vascular complications may limit the net clinical benefit of these devices.
Calcified in-stent restenosis
Calcified neoatherosclerosis (CNA) has been found in around 15% of patients with very late in-stent restenosis (ISR) and presents on OCT as a calcified sheet (well-delineated and signal-poor regions with sharp borders) and may be related to suboptimal treatment results. In 5% of ISR, nodular calcium may be present and possibly linked to worse outcomes85. Even if substantial evidence is lacking on the best treatment strategy for this challenging subgroup, current data suggest the feasibility and safety of both RA and IVL for CNA modification86.
Clinical management and medical therapy optimisation
Since PCI and CABG in heavily calcified coronary lesions carry an increased risk of periprocedural complications, a clear indication for intervention needs to be established before embarking on a PCI or CABG strategy87. Acute coronary syndrome as the initial presentation will likely result in the recommendation of an invasive strategy. As discussed above, calcium modifying tools can be used in ACS3688, and the latest experts’ consensus suggests that the use of RA and OA in ACS including ST-segment elevation myocardial infarction (STEMI) is feasible1. In chronic coronary syndromes, the benefit of PCI or CABG in lesions other than left main stenosis is in symptom relief and quality-of-life improvement rather than prognosis89. Thus, the risk-benefit ratio of intervention needs to be carefully evaluated, and medical therapy is the recommended first step, especially when the procedural risk is high. SYNTAX scores I and II and the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II should be used to guide decision-making on a case-by-case basis. The decision between PCI versus CABG should be made by a Heart Team, taking into account the extent of disease, anatomical suitability for PCI and CABG, comorbidities, and local expertise in complex PCI and CABG, whilst considering patient preference. Extensively calcified proximal disease with good surgical targets should generally favour CABG in patients who are otherwise good surgical candidates, whereas diffuse distal disease or chronic total occlusions may be better treated by medical therapy.
Once a clear indication for intervention has been established, careful PCI planning using all the available information obtained by the already discussed tools (CT, fluoroscopy, IVI) is of paramount importance.
Most lesions can be treated by the modality with which the operator has the most experience, but there are factors that may lead to choosing one modality over another. IVL is best suited to large proximal vessels with larger residual lumen diameters, thick calcium ≥0.5 mm and in the presence of deep calcium. Extreme angulation and tortuosity increase the risk of RA and OA and may favour IVL. However, IVL delivery may also be challenging. Atherectomy is preferred in smaller vessels, long lesions with diffuse disease, and balloon-uncrossable lesions. Eccentric calcification with favourable wire bias may be best treated by RA.
There is evidence of delayed stent healing in calcified arteries90, and the ideal duration of dual antiplatelet therapy (DAPT) post-PCI in calcified vessels has not been formally evaluated. Several studies indicate that patients with complex PCI features and the need for RA are at high risk for both ischaemic complications and bleeding91. Thus, the duration of DAPT following PCI for calcified lesions requires a complex balance between these 2 risks, and a tailored individualised approach is needed. In low bleeding risk cases, erring on longer-duration DAPT up to 2 years may be considered, whilst shortening DAPT to 1-3 months can be needed in very high bleeding risk cases92. A visual algorithmic approach for clinical decision-making is provided in Figure 6.
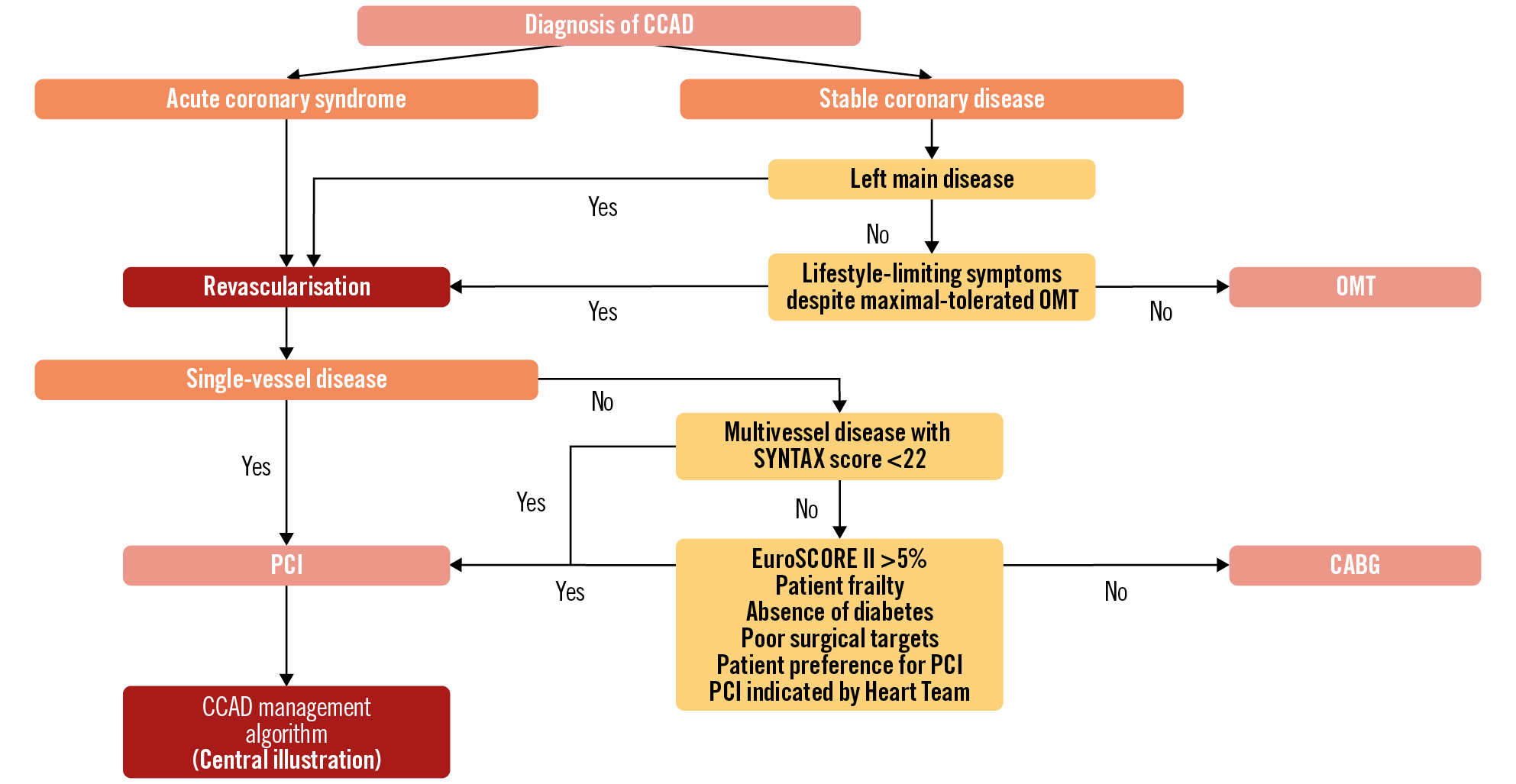
Figure 6. Algorithmic approach for clinical decision-making. In complex CCAD, the prognostic value of revascularisation in ACS justifies an early invasive strategy, while an extremely careful risk/benefit evaluation should be carried out in non-ACS. The choice between PCI and CABG must take into account both anatomical/angiographic and clinical/patient characteristics. Staged procedures and Heart Team discussions are advised in these complex cases. ACS: acute coronary syndrome; CABG: coronary artery bypass grafting; CCAD: chronic coronary artery disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; OMT: optimal medical therapy; PCI: percutaneous coronary intervention
Limitations
Basically, the major limitation of the literature related to the treatment of CCAD is the lack of high-level clinical evidence (Level of Evidence [LoE] A). Therefore, the divulgation of expert opinion and continuous education in the field are essential tools to improve the safety and efficacy of revascularisation techniques in this demanding setting.
Conclusions
A Heart Team approach to complex calcified CAD is key in defining the most appropriate therapy on an individualised basis. In selected patients, PCI can address challenging anatomies provided that the interventional team is proficient in procedural planning, integration of the available data, and device selection and utilisation. In stable patients, accurate risk-benefit analysis often portends in favour of medical therapy. Young patients with multivessel disease should be discussed with surgeons.
CCAD-ACS patients represent the most challenging subset for interventionalists, since interventions can impact positively on patient prognosis, provided that good results are obtained at the price of low complication rates.
Conflict of interest statement
F. Hellig received proctoring fees from Boston Scientific. A. Seth is an advisory board member for Abbott; and speakers bureau member for Boston Scientific and Shockwave Medical. F.L. Ribichini is a consultant for Boston Scientific, Shockwave Medical, and Abbott. R.A. Shlofmitz and G. Pesarini have no conflicts of interest to declare regarding this manuscript.
Supplementary data
To read the full content of this article, please download the PDF.

