Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The JenaValve Trilogy System (JVTS) is the only dedicated transcatheter heart valve system approved for treating patients with aortic regurgitation (AR). Recently, several studies have revealed high rates of permanent pacemaker implantation (PPI) exceeding 20% in patients with AR.
Aims: The aim of this study was to evaluate the incidence and risk factors for new PPI after transcatheter aortic valve implantation (TAVI) with the JVTS.
Methods: This retrospective multicentre registry included 141 patients without prior PPI who underwent transfemoral TAVI with the JVTS. Comparative analyses were performed regarding baseline and procedural parameters between patients with and without new PPI at discharge. Logistic regression models were fitted to identify predictors of PPI.
Results: The median age of patients was 81 (interquartile range [IQR] 76-85) years, 41% were female, and the median European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 3.6% (IQR 2.0-6.4). All patients presented with ≥moderate AR. At discharge, 34 patients (24.1%) required a new PPI. Pre-existing first-degree atrioventricular block and right bundle branch block were identified as independent predictors of new PPI. Anatomical characteristics, including annular and left ventricular outflow tract perimeters, were not predictive. Procedural factors such as implantation depth and valve oversizing were also not statistically different between patients with or without new PPI.
Conclusions: Overall, 24.1% of patients undergoing TAVI with the JVTS required a new PPI. While rates of new PPI were strongly associated with pre-existing first-degree atrioventricular block and right bundle branch block using the JVTS, no modifiable risk factors were identified.
Transcatheter aortic valve implantation (TAVI) is a treatment option for patients with aortic regurgitation (AR)12. The JenaValve Trilogy System (JVTS; JenaValve Technology) is a transcatheter heart valve (THV) system designed for the transfemoral treatment of patients with AR. While non-dedicated TAVI systems have a high rate of procedural failure, treatment with this dedicated THV system has shown a high technical success rate, with recent studies demonstrating excellent haemodynamic performance, with notably low rates of paravalvular regurgitation (PVR) and low rates of valve embolisation1234.
However, these studies also report that cardiac conduction disturbances resulting in permanent pacemaker implantation (PPI) occurred in 19.6% to 24% of patients treated with the JVTS12. Overall, these rates are comparable to those of new-generation non-dedicated THV systems when used off-label for AR treatment34. Among aortic stenosis (AS) patients, anatomical, electrocardiographic, and procedural factors – including modifiable variables like lower implantation depth and degree of THV oversizing – influence the risk for postprocedural PPI567. However, the pathomechanism and anatomy of AR differ significantly from AS. Larger average annular diameters and minimal or even an absence of leaflet calcification to restrict THV expansion might affect the conduction system differently.
Whether the same predictors apply to patients with non-calcified AR as for AS is still unknown. Therefore, the purpose of the present study was to investigate potentially modifiable and non-modifiable predictors in patients undergoing TAVI with a dedicated THV for AR.
Methods
Patient population & data acquisition
This registry included patients with AR and no prior history of PPI, treated at four experienced European centres (London, Bad Oeynhausen, Mainz, and Cologne). Patients undergoing transfemoral TAVI with the JVTS were retrospectively identified. Data were collected for cases performed between September 2021 and April 2024, plus one patient treated in April 2019. All patients referred for TAVI were discussed during multidisciplinary Heart Team meetings. AR was categorised as moderate, moderate-to-severe, and severe AR based on guideline recommendations8. For patients with mixed aetiology where AR was predominant, inclusion in the current analysis also required a mean aortic valve gradient of ≤20 mmHg. Principal exclusion criteria for the JVTS have been previously reported2. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines.
Device and procedure
The JVTS is a low-profile, self-expanding, supra-annular porcine pericardial bioprosthetic valve with a nitinol frame, available in three sizes to cover aortic annular perimeters ranging from 66 mm to 90 mm. A key feature of this valve is its three locators, specifically designed for active anchoring by clipping onto the aortic leaflets. This mechanism ensures a secure seal between the native valve and the prosthesis while minimising protrusion into the left ventricular outflow tract (LVOT). Further design aspects of the JVTS and details of the implantation procedure have been described previously9. After TAVI, all patients were continuously monitored to assess clinical events and underwent postprocedural electrocardiography, echocardiography, and laboratory investigations.
Data collection and definitions
Demographic data, clinical findings, and details of electrocardiographic and echocardiographic findings were collected as part of routine clinical care. Preprocedural multidetector computed tomography (CT) of the entire aorta and iliofemoral arteries was performed and analysed according to local standard methods by a blinded investigator (M. Stukenberg) using 3mensio Structural Heart software, version 10.3 (Pie Medical Imaging).
The standardised assessment included measuring the aortic annular dimensions, defined as the virtual basal plane encompassing the basal attachment of the three aortic cusps, with the area and perimeter primarily quantified in mid-systole. LVOT dimensions were assessed at 6 mm below the aortic annular plane. The “cover index” was calculated to assess the congruence between the aortic annulus and the device, defined as 100 × [(prosthesis diameter − CT-derived annulus diameter) / prosthesis diameter]. Valve oversizing at the level of the annulus and LVOT was calculated using multidetector CT: [(valve waist diameter/annulus perimeter) − 1] × 100 and [(device inflow diameter/LVOT perimeter) − 1] × 100. The length of the infra-annular portion of the membranous septum (MS) was measured in the coronal plane as the distance between the aortic annulus and the top of the muscular septum10.
Implantation depth, measured by aortography, was defined either as (1) the maximum distance (in millimetres) between the end of the intraventricular distal prosthesis and the aortic annulus at the level of the non-coronary and left coronary cusps, or (2) the deepest point of the distal prosthesis, regardless of the anatomical orientation. A pigtail catheter was used for calibration. The fluoroscopic projection for aortography was individualised to obtain a coplanar view for the patient’s anatomy and the implanted THV.
Statistical analysis
Continuous variables are expressed as median and interquartile range (IQR; 25th-75th percentile) and were compared using the Wilcoxon rank-sum test. Categorical baseline characteristics were compared using the chi-square test or Fisher’s exact test as appropriate. Univariable logistic regression was conducted to identify predictors for new PPI, with odds ratios (ORs) and 95% confidence intervals (CIs) reported. Factors with a p-value<0.10 and considered clinically relevant were included in the multivariable logistic regression model. P-values<0.05 were considered statistically significant. All statistical analyses were performed using R, version 4.4.2 (R Foundation for Statistical Computing).
Results
Baseline characteristics
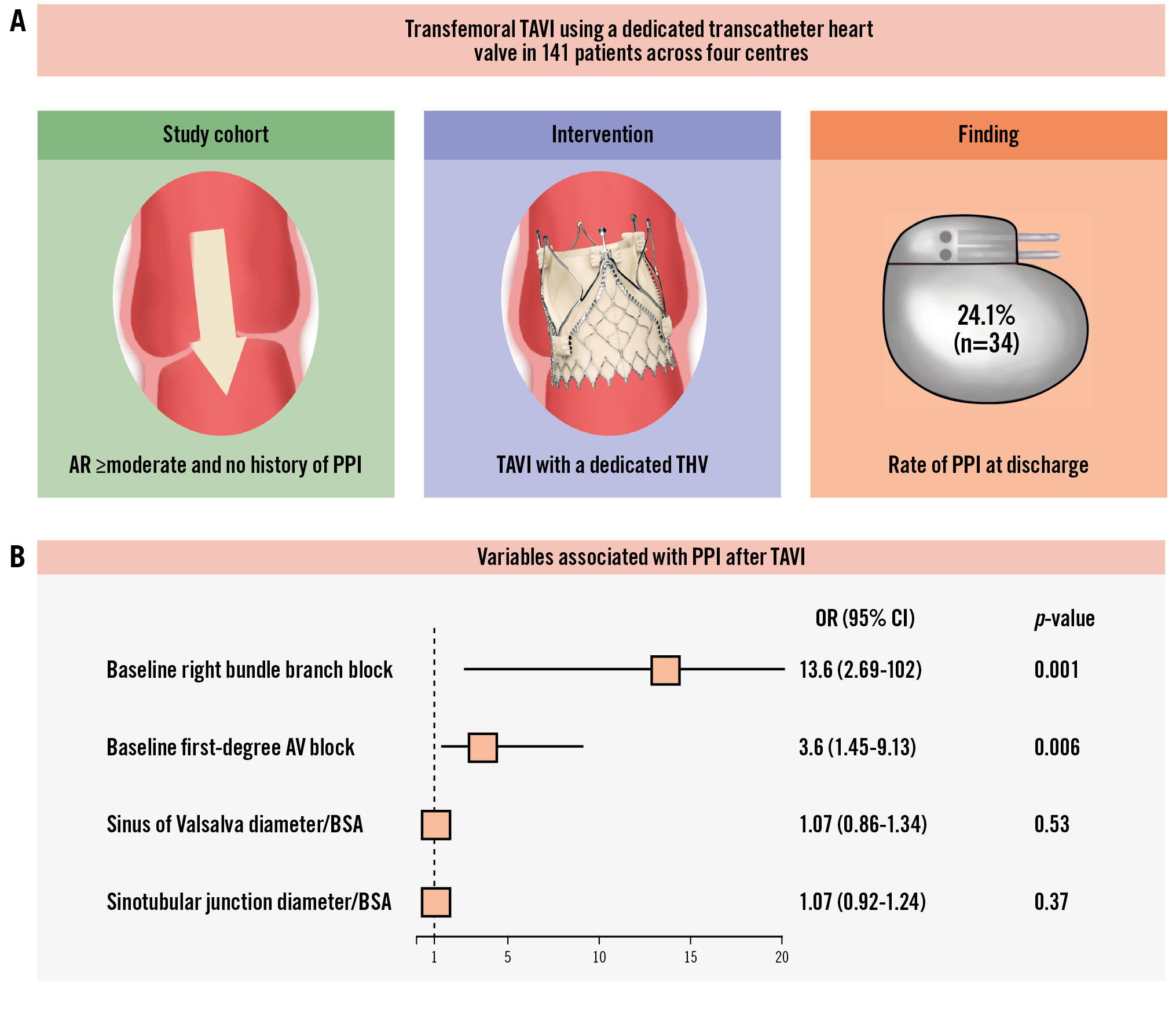
Baseline characteristics are summarised in Table 1. The median age of the cohort was 81.0 (IQR 76.0-84.8) years, with 41% being female. At discharge, 34 patients (24.1%) required a new PPI (Central illustration). The primary indication for PPI was complete heart block, which accounted for 74.0% (n=25) of all patients.
Baseline characteristics were generally similar between groups, except for a trend towards reduced renal function in patients requiring PPI (estimated glomerular filtration rate: 48.5 [IQR 36.0-71.0] ml/min/1.73 m2 vs 59.0 [IQR 42.0-80.0] ml/min/1.73 m2; p=0.076). Pre-existing right bundle branch block (RBBB) and first-degree atrioventricular (AV) block were significantly more prevalent among patients requiring PPI, while no significant difference was observed in the incidence of left bundle branch block (Figure 1).
The unadjusted and body surface area-adjusted diameters of the sinus of Valsalva, sinotubular junction, and ascending aorta were larger in patients requiring PPI. Aortic annular and LVOT perimeters were similar between groups. The MS length was also comparable in both groups (Table 2). In the subgroup of patients with prior RBBB and first-degree AV block, the median MS length was similar between patients requiring PPI and those who did not (new PPI: 2.2 [IQR 0.8-2.8] mm vs no PPI: 1.4 [IQR 0.7-2.3] mm; p=0.43).
Table 1. Baseline characteristics.
| Overall N=141 | No PPI N=107 | New PPI N=34 | p-value | |
|---|---|---|---|---|
| Age, years | 81.0 (76.0-84.8) | 80.7 (74.0-84.2) | 82.0 (79.7-85.0) | 0.12 |
| Female sex | 58 (41) | 46 (43) | 12 (35) | 0.43 |
| Body mass index, kg/m² | 24.8 (21.9-27.8) | 24.7 (21.3-27.8) | 25.1 (23.0-28.7) | 0.24 |
| New York Heart Association Functional Class | 0.80 | |||
| I | 5 (3.5) | 3 (2.8) | 2 (5.9) | |
| II | 37 (26) | 28 (26) | 9 (26) | |
| III | 88 (62) | 68 (64) | 20 (59) | |
| IV | 11 (7.8) | 8 (7.5) | 3 (8.8) | |
| EuroSCORE II, % | 3.6 (2.0-6.4) | 3.4 (1.8-6.5) | 3.8 (2.3-5.1) | 0.95 |
| Diabetes mellitus | 21 (15) | 15 (14) | 6 (18) | 0.60 |
| Hypertension | 118 (84) | 86 (80) | 32 (94) | 0.059 |
| Previous stroke | 15 (11) | 11 (10) | 4 (12) | 0.76 |
| Chronic obstructive pulmonary disease | 17 (12) | 12 (11) | 5 (15) | 0.56 |
| Extracardiac arteriopathy | 12 (8.5) | 9 (8.4) | 3 (8.8) | >0.99 |
| Coronary artery disease | 58 (41) | 47 (44) | 11 (32) | 0.23 |
| Previous cardiac surgery | 12 (8.5) | 12 (11) | 0 (0) | 0.070 |
| Glomerular filtration rate, ml/min/1.73 m² | 56.4 (39.0-79.0) | 59.0 (42.0-80.0) | 48.5 (36.0-71.0) | 0.076 |
| Chronic renal replacement therapy | 2 (1.6) | 1 (1.0) | 1 (3.2) | 0.42 |
| Sinus rhythm at baseline | 107 (76) | 82 (77) | 25 (74) | 0.71 |
| History of atrial fibrillation | 55 (39) | 42 (39) | 13 (38) | 0.92 |
| PR duration, ms; N missing | 184.0 (167.0-218.0); 36 | 181.0 (166.0-212.0); 26 | 215.5 (182.5-236.0); 10 | 0.010* |
| QRS duration, ms | 105.0 (94.0-128.0) | 104.0 (94.0-118.0) | 114.0 (96.0-152.0) | 0.081 |
| Prior right bundle branch block | 8 (5.7) | 2 (1.9) | 6 (18) | 0.003* |
| Prior left bundle branch block | 22 (16) | 17 (16) | 5 (15) | 0.87 |
| First-degree AV block | 37 (26) | 23 (21) | 14 (41) | 0.023* |
| Left ventricular ejection fraction | 0.57 | |||
| >50% | 71 (50) | 57 (53) | 14 (41) | |
| 41-50% | 44 (31) | 31 (29) | 13 (38) | |
| 31-40% | 17 (12) | 13 (12) | 4 (12) | |
| <31% | 9 (6.4) | 6 (5.6) | 3 (8.8) | |
| Aortic regurgitation | 0.17 | |||
| Moderate | 15 (11) | 14 (13) | 1 (2.9) | |
| Moderate-severe | 53 (38) | 37 (35) | 16 (47) | |
| Severe | 73 (52) | 56 (52) | 17 (50) | |
| Data are presented as median (IQR) or frequency (%). *Indicates statistical significance. AV: atrioventricular; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; PPI: permanent pacemaker implantation | ||||
Central illustration. New permanent pacemaker implantation following TAVI with a dedicated device for aortic regurgitation. A) Study details. B) Predictors of PPI after TAVI. Reproduced with permission from JenaValve Technology for providing the valve image of the JenaValve Trilogy System. AR: aortic regurgitation; AV: atrioventricular; BSA: body surface area; CI: confidence interval; OR: odds ratio; PPI: permanent pacemaker implantation; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
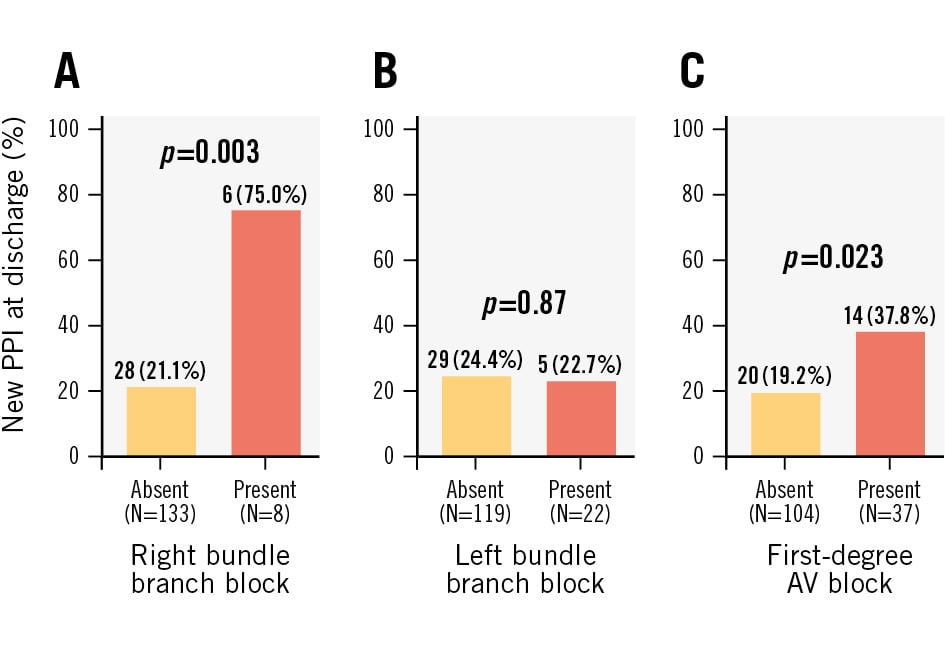
Figure 1. Rates of PPI in non-pacemaker-dependent patients following transcatheter aortic valve implantation with prior conduction abnormalities. New PPI in patients with prior right bundle branch block (A), prior left bundle branch block (B) and prior first-degree AV block (C). AV: atrioventricular; PPI: permanent pacemaker implantation
Table 2. Computed tomographic characteristics.
| Overall N=141 | No PPI N=107 | New PPI N=34 | p-value | |
|---|---|---|---|---|
| Annular perimeter, mm | 83.8 (76.4-86.7) | 83.3 (76.4-86.9) | 84.3 (74.7-86.1) | 0.81 |
| Minimum annular perimeter, mm | 22.9 (21.4-24.3) | 23.1 (21.4-24.4) | 22.6 (21.2-23.9) | 0.35 |
| Maximum annular perimeter, mm | 29.4 (26.8-30.8) | 29.0 (26.8-30.7) | 29.9 (26.8-31.3) | 0.23 |
| Annular area, mm² | 534.1 (446.0-578.1) | 533.8 (446.0-578.1) | 540.9 (438.4-571.4) | 0.97 |
| LVOT perimeter, mm | 85.8 (80.7-91.0) | 85.6 (81.3-91.0) | 87.4 (78.7-91.4) | 0.88 |
| Minimum LVOT perimeter, mm | 21.6 (19.6-23.0) | 21.6 (19.5-23.2) | 21.3 (19.7-22.5) | 0.45 |
| Maximum LVOT perimeter, mm | 31.1 (29.2-33.3) | 31.1 (29.3-33.1) | 31.4 (28.6-33.8) | 0.78 |
| Sinus of Valsalva diameter, mm | 37.2 (33.2-39.3) | 36.7 (32.9-38.4) | 38.5 (36.4-41.8) | 0.005* |
| Sinus of Valsalva diameter/BSA, mm/m² | 20.0 (18.4-21.8) | 19.7 (17.9-21.7) | 20.9 (19.2-22.4) | 0.044* |
| Sinotubular junction diameter, mm | 35.3 (32.2-39.9) | 34.4 (30.9-38.7) | 38.8 (35.2-41.9) | 0.001* |
| Sinotubular junction diameter/BSA, mm/m² | 19.0 (17.3-21.8) | 18.7 (17.0-21.2) | 21.1 (18.4-23.1) | 0.007* |
| Sinotubular junction height, mm | 29.6 (26.1-33.8) | 29.2 (25.9-33.2) | 31.6 (28.3-35.3) | 0.027* |
| Sinotubular junction height/BSA, mm/m² | 16.2 (14.6-18.3) | 15.9 (14.2-18.2) | 17.0 (15.8-18.7) | 0.057 |
| Maximum ascending aorta diameter, mm | 40.3 (37.0-44.7) | 38.8 (36.5-42.7) | 43.0 (39.9-46.9) | 0.003* |
| Maximum ascending aorta diameter/BSA, mm/m² | 21.9 (19.4-24.7) | 21.2 (19.0-24.7) | 23.4 (21.0-24.5) | 0.062* |
| Aortic annulus-to-left coronary artery distance, mm | 15.3 (13.0-18.1) | 15.6 (13.4-18.2) | 14.7 (12.7-16.8) | 0.24 |
| Aortic annulus-to-right coronary artery distance, mm | 20.3 (18.2-22.9) | 20.4 (18.0-22.8) | 20.2 (18.3-22.9) | 0.98 |
| Morphology of the LVOT | 0.46 | |||
| Tubular | 19 (13) | 16 (15) | 3 (8.8) | |
| Tapered | 93 (66) | 71 (66) | 22 (65) | |
| Flared | 29 (21) | 20 (19) | 9 (26) | |
| Aortic root angle, ° | 55.0 (49.0-63.0) | 54.0 (49.0-63.0) | 57.0 (51.0-62.0) | 0.30 |
| LVOT calcification | 6 (4.3) | 5 (4.7) | 1 (2.9) | >0.99 |
| Annular calcification | 0.76 | |||
| No calcification | 126 (89) | 96 (90) | 30 (88) | |
| Mildly calcified (small, isolated spots) | 15 (11) | 11 (10) | 4 (12) | |
| Membranous septum length, mm | 2.1 (1.2-3.0) | 2.1 (1.2-3.0) | 2.3 (1.1-3.0) | 0.70 |
| Membranous septum length <2 mm | 63 (45) | 49 (46) | 14 (41) | 0.64 |
| Data are presented as median (IQR) or frequency (%). *Indicates statistical significance. BSA: body surface area; IQR: interquartile range; LVOT: left ventricular outflow tract; PPI: permanent pacemaker implantation | ||||
Procedural characteristics and clinical outcomes
Procedural characteristics, as displayed in Table 3, showed no significant differences between groups. Implantation depths at the non-coronary cusp, left coronary cusp, and the deepest implantation point did not differ significantly between the groups. Oversizing at the annular level was 11.5% (IQR 8.3-14.1%), and at the LVOT level, 14.9% (IQR 10.3-20.9%); there were no significant differences between groups. An analysis of various degrees of valve oversizing at the annular (Figure 2A) and LVOT (Figure 2B) levels did not reveal a trend towards increased PPI rates in patients with greater valve oversizing.
Other procedural parameters, including contrast usage, fluoroscopy time, and type of anaesthesia, were comparable between groups. Complication rates – including stroke, valve implantation success, and the need for a second valve – showed no significant differences. However, patients requiring PPI had a significantly longer hospital stay (new PPI: 8 [IQR 6.5-12] days vs no PPI: 7 [IQR 5-9] days; p=0.012).
PPIs were performed at a median of 3 (IQR 1-6) days after the TAVI procedure. Pacing data beyond 30 days were available for 21 patients, with device interrogation conducted at a median of 111 (IQR 93-360) days, revealing a median right ventricular pacing rate of 92% (IQR 30-99%).
Haemodynamic performance was excellent in both groups, characterised by low valve gradients, large orifice areas, and over 90% of patients showing no or trace PVR. At follow-up, 29 patients (85%) in the PPI group were alive (median follow-up: 342 [IQR 100-534.5] days), compared to 95 patients (89%) in the non-PPI group (median follow-up: 363 [IQR 122.5-716] days; p=0.55).
Table 3. Procedural/in-hospital characteristics.
| Overall N=141 | No PPI N=107 | New PPI N=34 | p-value | |
|---|---|---|---|---|
| Implantation date | 0.89 | |||
| ≤2022 | 74 (52) | 57 (53) | 17 (50) | |
| 2023/2024 | 67 (48) | 50 (47) | 17 (50) | |
| Valve size | 0.61 | |||
| 23 mm | 23 (16) | 19 (18) | 4 (12) | |
| 25 mm | 28 (20) | 22 (21) | 6 (18) | |
| 27 mm | 90 (64) | 66 (62) | 24 (71) | |
| Anaesthesia | 0.28 | |||
| General anaesthesia | 39/140 (28) | 32/106 (30) | 7/34 (21) | |
| Conscious sedation | 101/140 (72) | 74/106 (70) | 27/34 (79) | |
| Procedure duration, min; N missing | 66.0 (57.0-84.0); 12 | 68.5 (54.0-86.0); 9 | 66.0 (59.0-78.0); 3 | 0.98 |
| Contrast, ml; N missing | 155.0 (120.0-209.0); 16 | 157.0 (120.0-209.0); 10 | 143.0 (122.5-207.5); 6 | 0.81 |
| Fluoroscopy time, min; N missing | 16.0 (12.4-21.8); 17 | 16.0 (13.0-22.0); 10.0 | 16.0 (11.1-21.5); 7 | 0.65 |
| Post-dilatation performed | 1 (0.7) | 0 (0) | 1 (2.9) | 0.24 |
| LVOT oversizing, % | 14.9 (10.3-20.9) | 14.5 (9.9-20.4) | 15.7 (11.3-24.0) | 0.55 |
| Annular oversizing, % | 11.5 (8.3-14.1) | 11.5 (8.3-14.1) | 11.5 (9.2-13.4) | 0.89 |
| Annular minimal perimeter/valve size | 0.9 (0.8-0.9) | 0.9 (0.8-0.9) | 0.9 (0.8-0.9) | 0.11 |
| Eccentricity index | 0.2 (0.2-0.2) | 0.2 (0.2-0.2) | 0.2 (0.2-0.3) | 0.10 |
| Cover index | 0.0 (−3.0 to 2.2) | 0.0 (−3.0 to 2.2) | 0.4 (−2.2 to 2.6) | 0.55 |
| Non-coronary cusp ID, mm; N missing | 6.6 (5.7-7.4); 40 | 6.5 (5.8-7.4); 32 | 6.6 (5.2-7.7); 8 | 0.95 |
| Left coronary cusp ID, mm; N missing | 6.6 (6.0-7.4); 40 | 6.6 (6.0-7.4); 32 | 6.7 (5.9-7.4); 8 | 0.76 |
| Deepest ID, mm; N missing | 7.2 (6.6-7.9); 40 | 7.1 (6.7-7.9); 32 | 7.3 (6.4-7.9); 8 | 0.77 |
| New stroke | 2 (1.4) | 1 (0.9) | 1 (2.9) | 0.43 |
| Acute kidney injury stage | 0.45 | |||
| None | 132 (94) | 101 (94) | 31 (91) | |
| Stage 1 | 6 (4.3) | 3 (2.8) | 3 (8.8) | |
| Stage 2 | 2 (1.4) | 2 (1.9) | 0 (0) | |
| Stage 3 | 1 (0.7) | 1 (0.9) | 0 (0) | |
| Vascular complication | 0.79 | |||
| None | 133 (94) | 101 (94) | 32 (94) | |
| Minor vascular complication | 6 (4.3) | 4 (3.7) | 2 (5.9) | |
| Major vascular complication | 2 (1.4) | 2 (1.9) | 0 (0) | |
| Bleeding complication | 0.64 | |||
| None | 128 (91) | 98 (92) | 30 (88) | |
| Type 1 | 10 (7.1) | 7 (6.5) | 3 (8.8) | |
| Type 2 | 3 (2.1) | 2 (1.9) | 1 (2.9) | |
| Type 3/4 | 0 (0) | 0 (0) | 0 (0) | |
| Second valve implanted | 2 (1.4) | 1 (0.9) | 1 (2.9) | 0.43 |
| Conversion to open surgery | 1 (0.7) | 1 (0.9) | 0 (0) | >0.99 |
| Total length of hospital stay, days | 7.0 (6.0-9.0) | 7.0 (5.0-9.0) | 8.0 (7.0-11.0) | 0.012* |
| Mean aortic valve gradient at discharge, mmHg; N missing | 4.0 (3.0-5.0); 8 | 4.0 (3.0-5.0); 7 | 5.0 (4.0-6.0); 1 | 0.006* |
| Aortic valve area at discharge, cm²; N missing | 2.4 (2.0-2.9); 58 | 2.4 (2.0-2.9); 43 | 2.5 (2.0-2.9); 15 | 0.54 |
| Paravalvular regurgitation | >0.99 | |||
| None-trace | 127/138 (92) | 96/105 (91) | 31/33 (94) | |
| Mild | 11/138 (8.0) | 9/105 (8.6) | 2/33 (6.1) | |
| Moderate-severe | 0/138 (0) | 0/105 (0) | 0/33 (0) | |
| Technical success | 139 (99) | 106 (99) | 33 (97) | 0.43 |
| Indication for PPI | ||||
| AV block III° | - | - | 25 (74) | |
| Bradyarrhythmia absoluta | - | - | 0 (0) | |
| AV block I°+left bundle branch block | - | - | 4 (12) | |
| Sinus arrest/sick sinus syndrome | - | - | 0 (0) | |
| AV block II°+left bundle branch block | - | - | 3 (8.8) | |
| Other indication | - | - | 2 (5.9) | |
| Data are presented as median (IQR) or frequency (%). *Indicates statistical significance. AV: atrioventricular; ID: implantation depth; IQR: interquartile range; LVOT: left ventricular outflow tract; N: number; PPI: permanent pacemaker implantation | ||||
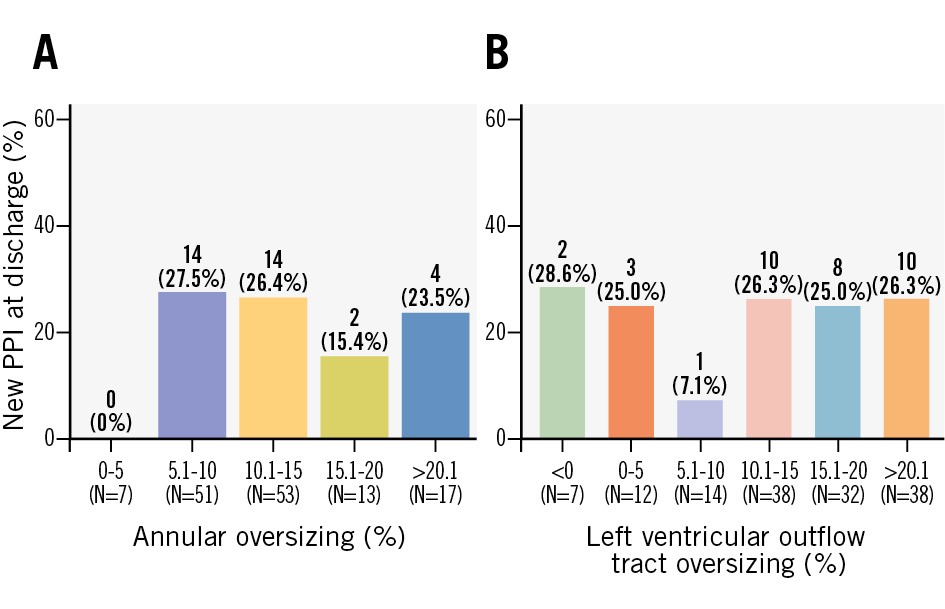
Figure 2. Degree of oversizing at the annular and LVOT levels and its impact on rates of PPI. A) Annular oversizing and new PPI at discharge. B) LVOT oversizing and new PPI at discharge. LVOT: left ventricular outflow tract; PPI: permanent pacemaker implantation
Predictors for PPI
Table 4 presents the univariable and multivariable analyses for predictors of PPI. In the univariable analyses, the diameter of the sinus of Valsalva and the sinotubular junction exhibited statistically significant trends with p-values<0.10, but these did not reach statistical significance in the multivariable analysis. RBBB and first-degree AV block were statistically significant predictors of PPI in the multivariable analysis (Central illustration). Notably, valve oversizing at the annular or LVOT level and implantation depth were not significant predictors of PPI.
Table 4. Univariable and multivariable analysis for new PPI.
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age, years | 1.03 (0.98-1.09) | 0.31 | ||
| Female sex | 0.72 (0.32-1.59) | 0.43 | ||
| Body mass index, kg/m² | 1.04 (0.96-1.13) | 0.33 | ||
| New York Heart Association Functional Class III/IV vs I/II | 0.85 (0.38-2.01) | 0.71 | ||
| EuroSCORE II, % | 0.95 (0.85-1.02) | 0.25 | ||
| Left ventricular ejection fraction <50% vs ≥50% | 1.63 (0.75-3.61) | 0.22 | ||
| Diabetes mellitus | 1.31 (0.43-3.58) | 0.61 | ||
| Atrial fibrillation | 0.96 (0.43-2.10) | 0.92 | ||
| Prior right bundle branch block | 11.3 (2.44-79.7) | 0.004* | 13.6 (2.69-102) | 0.001* |
| Prior left bundle branch block | 0.91 (0.28-2.54) | 0.87 | ||
| AV block I° | 2.56 (1.11-5.84) | 0.026* | 3.60 (1.45-9.13) | 0.006* |
| Annular perimeter, mm | 1.01 (0.96-1.07) | 0.65 | ||
| Annular area, mm² | 1.00 (1.00-1.01) | 0.80 | ||
| Cover index | 1.02 (0.93-1.10) | 0.71 | ||
| Left ventricular outflow tract oversizing, % | 1.01 (0.97-1.05) | 0.62 | ||
| Annular oversizing, % | 1.00 (0.93-1.06) | 0.95 | ||
| Minimum annular perimeter/valve size | 0.01 (0.00-2.94) | 0.12 | ||
| Sinus of Valsalva diameter/BSA, mm/m2 | 1.14 (0.99-1.31) | 0.074* | 1.07 (0.86-1.34) | 0.53 |
| Sinotubular junction diameter/BSA, mm/m2 | 1.10 (0.99-1.21) | 0.065* | 1.07 (0.92-1.24) | 0.37 |
| Sinotubular junction height/BSA, mm/m2 | 1.08 (0.97-1.21) | 0.17 | ||
| Maximum ascending aorta diameter/BSA, mm/m2 | 1.05 (0.97-1.14) | 0.21 | ||
| Membranous septum length, mm | 1.02 (0.77-1.32) | 0.90 | ||
| Morphology of the left ventricular outflow tract | ||||
| Tubular | — | |||
| Tapered | 1.65 (0.49-7.57) | 0.46 | ||
| Flared | 2.40 (0.60-12.2) | 0.24 | ||
| Aortic root angle | 1.02 (0.98-1.06) | 0.38 | ||
| Left ventricular outflow tract calcification | 0.62 (0.03-4.02) | 0.67 | ||
| Annular calcification: no vs mild | 1.16 (0.30-3.69) | 0.81 | ||
| Implantation date: ≤2022 vs 2023/2024 | 1.14 (0.52-2.48) | 0.74 | ||
| Valve size | ||||
| 23 mm | — | |||
| 25 mm | 1.30 (0.32-5.72) | 0.72 | ||
| 27 mm | 1.73 (0.58-6.41) | 0.36 | ||
| Non-coronary cusp ID, mm (N=101) | 0.98 (0.68-1.42) | 0.93 | ||
| Left coronary cusp ID, mm (N=101) | 0.86 (0.54-1.34) | 0.51 | ||
| Deepest ID, mm (N=101) | 0.93 (0.63-1.34) | 0.70 | ||
| *Indicates statistical significance. AV: atrioventricular; BSA: body surface area; CI: confidence interval; EuroSCORE: European System for Cardiac Operative Risk Evaluation; ID: implantation depth; OR: odds ratio; PPI: permanent pacemaker implantation | ||||
Discussion
In this study, the PPI rate with the dedicated TAVI device (JVTS) was 24.1%. The main findings can be summarised as follows (Central illustration): (1) patients with prior RBBB had a significantly increased risk of new PPI; (2) a similar trend towards new PPI was observed for patients with first-degree AV block, whereas left bundle branch block showed no such association; and (3) modifiable risk factors such as implantation depth and valve oversizing did not differ between the groups with or without new PPI.
Cardiac conduction disturbances requiring PPI are frequently observed in patients undergoing TAVI for AS111213. PPI following TAVI may yield limited benefits due to the impact on AV synchrony and right ventricular pacing14. While rates are lower with contemporary valves used in the treatment of AS (ACURATE neo2 [Boston Scientific]: 8.0%, SAPIEN 3 Ultra [Edwards Lifesciences]: 9.9%, Evolut FX [Medtronic]: 11.9%, Navitor [Abbott]: 19.0%), they remain clinically significant121315. Similarly, low-risk patients undergoing surgical aortic valve replacement for AS have reported PPI rates of 4.0-6.1% in the Evolut Low Risk and PARTNER 3 trials1116. However, studies also indicate that surgical aortic valve replacement in AR patients is associated with PPI rates exceeding 10%1718, suggesting a potentially more vulnerable interaction with the conduction system when treating AR compared to AS; this might be due to anatomical and pathophysiological differences between these two aortic valve diseases. In patients with AR, the aortic annulus and LVOT tract are rarely calcified, which means there is less restriction on THV expansion and greater interaction between the THV and the conduction system.
The PPI rates exceeding 20% in our study are consistent with previous findings for this device as well as for non-dedicated devices used in the treatment of AR1234. The underlying mechanism for conduction system disorders may be attributed to the proximity of the aortic valve complex to the cardiac conduction system. Interaction between the stent frame of the THV and the conduction system can lead to compression, haemorrhage, ischaemia, trauma, and infarction19.
Over recent years, intensive research has focused on identifying predictors for PPI in AS patients. Baseline conduction disturbances are recognised as the most significant predictors of PPI following TAVI520. Consistent with recent studies in patients with severe AS, RBBB emerged as the strongest predictor52122, while first-degree AV block also demonstrated an impact, aligning with the current literature23.
Several studies have demonstrated that a shallower implantation depth for self-expanding valves is associated with fewer conduction disturbances and lower rates of PPI in AS patients521. However, our analysis found no difference in implantation depth between groups, which may be related to technical factors inherent to the JVTS, where the locators are positioned at the nadir of the annulus129 and thereby might predefine the implant depth to a relevant extent. In addition, the MS length was not associated with new PPI following TAVI with the JVTS, whereas a relationship between the MS length and new PPI has been identified in patients with AS1024. Notably, in our study, we observed a shorter MS length in both groups compared to these prior studies1024, which might help to identify a high-risk population for new PPI.
Moreover, we observed no differences in valve oversizing between the groups, a factor with a controversial role regarding PPI in patients with severe AS2526. Furthermore, we did not find any difference in LVOT morphology related to PPI. In contrast, Zhang et al reported higher rates of PPI in patients with non-tubular LVOT shapes undergoing TAVI with the VitaFlow Liberty device (MicroPort)27.
Surprisingly, we identified a difference in anatomical characteristics related to the diameters of the sinus of Valsalva and the sinotubular junction, which appeared to have a relevant impact; however, this effect did not persist in the multivariable model.
A historical electrophysiological study indicated that patients with aortic valve disease have a high incidence of atrioventricular and intraventricular conduction disorders, which may occur without a clear pattern on the surface electrocardiogram in some cases28. Therefore, one could hypothesise that in AR patients with a large aortic root, sinotubular junction, and ascending aorta, the increase in wall stress with more pronounced dilatation might render the conduction system increasingly vulnerable to mechanical stress during valve implantation.
PPI was associated with a longer in-hospital stay, and the high right ventricular pacing burden suggests that earlier PPI may be warranted in clinical practice. During follow-up, most patients exhibited high percentages of right ventricular pacing rates, indicating a potential and ongoing requirement for PPI. However, due to limited data on pacing modes, we could not precisely define pacemaker dependency. Furthermore, higher rates of right ventricular pacing might be associated with poorer long-term outcomes14.
Recently, Chang et al introduced a novel system utilising a temporary-permanent pacemaker as a 1-month bridge therapy to differentiate between reversible and persistent conduction disturbances. In their study of 688 patients with severe AS, 70 patients received a temporary-permanent pacemaker, and at 1 month, 17 required a PPI. This resulted in a PPI rate of 2.5% in this cohort29.
In light of these developments, future investigations are needed to further understand the requirement for PPI in this vulnerable group of patients and to better identify those who may persistently need PPI.
Limitations
The findings of our study must be interpreted considering several limitations inherent to its retrospective design and the relatively small sample size compared to contemporary studies involving patients with AS. This limited cohort may result in statistical underpowering, thereby affecting the generalisability of our results. Additionally, the outcomes reported are specific to a single device type, which limits the applicability of our findings to other THV systems. The decision regarding PPI was at the physician’s discretion, in accordance with European guidelines, thereby introducing the potential for unmeasured confounding factors that may have influenced the PPI rates30. Moreover, the lack of a standardised assessment protocol for implantation depth could have resulted in inaccuracies in the estimation of procedural parameters. The evaluation of PPI rates was limited to in-hospital outcomes, raising concerns that rates at follow-up, such as at 30 days or 1 year, may be underestimated. Finally, the JVTS is currently not indicated for use in patients with bicuspid valves, which further restricts the broader applicability of our findings.
Conclusions
In this multicentre study, 24.1% of patients without prior PPI who underwent TAVI with the JVTS required new PPI. Baseline RBBB and first-degree AV block were independent predictors of PPI requirement, and no modifiable risk factors for PPI were identified in this analysis. Our findings highlight a higher incidence of conduction disturbances and new PPI in AR patients compared to those with AS, underscoring the importance of further research on the optimal timing of AR interventions, PPI outcomes, and tailored management strategies.
Impact on daily practice
Despite excellent technical outcomes and favourable valve haemodynamics, permanent pacemaker implantation (PPI) remains a frequent complication after transcatheter aortic valve implantation for aortic regurgitation, even with a dedicated device. This study identified prior right bundle branch block and first-degree atrioventricular block as independent predictors of PPI with the JenaValve Trilogy System, while procedural factors such as implantation depth and valve oversizing were not associated with PPI. Careful baseline conduction assessment and further research on optimal timing, long-term outcomes, and tailored pacing strategies are essential to optimise patient care.
Acknowledgements
The authors acknowledge the use of automated assistive writing technologies for proofreading and optimising code for statistical analysis.
Conflict of interest statement
H. Wienemann reports consultancy fees and travel grants from JenaValve Technology. M. Geyer received proctoring honoraria from JenaValve Technology. M. Stukenberg conducted blinded computed tomography data analysis and was an employee of JenaValve Technology at the time of this study. E.W. Kuhn reports consultancy and personal fees from Medtronic, Edwards Lifesciences, Boston Scientific, and Abbott. D.S. Pinto serves as the Chief Medical Officer of JenaValve Technology; and is a consultant for Abiomed, Abbott, Magenta, NuPulseCV, and Terumo. L. Conradi is an advisory board member for Medtronic, Abbott, and JenaValve Technology; and a consultant for Boston Scientific, Edwards Lifesciences, Venus MedTech, MicroPort, Micro Interventions, SmartCanula SARL, Neovasc, Pi-Cardia, and 4C Medical. S. Bleiziffer reports personal fees from Abbott, Edwards Lifesciences, Medtronic, and Boston Scientific. S. Baldus reports lecture fees from Abbott, Edwards Lifesciences, AstraZeneca, and JenaValve Technology; he received research grants from Abbott and AstraZeneca; and participates as a principal investigator in clinical trials sponsored by AstraZeneca and Abbott. A. Baumbach reports consultation or speaker fees from AstraZeneca, SINOMED, MicroPort, Medtronic, Faraday, Pi-Cardia, JenaValve Technology, and Meril Life Sciences. T.K. Rudolph reports institutional fees from Amgen, Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and JenaValve Technology. M. Adam reports grants and personal fees from Medtronic; and personal fees from Abbott, JenaValve Technology, Edwards Lifesciences, Boston Scientific, and Meril Life Sciences. The other authors have no conflicts of interest to declare regarding this project.

