Abstract
BACKGROUND: Large datasets of transcatheter aortic valve implantation (TAVI) for pure aortic valve regurgitation (PAVR) are scarce.
AIMS: We aimed to report procedural safety and long-term clinical events (CE) in a contemporary cohort of PAVR patients treated with new-generation devices (NGD).
METHODS: Patients with grade III/IV PAVR enrolled in the FRANCE-TAVI Registry were selected. The primary safety endpoint was technical success (TS) according to Valve Academic Research Consortium 3 criteria. The co-primary endpoint was defined as a composite of mortality, heart failure hospitalisation and valve reintervention at last follow-up.
Results: From 2015 to 2021, 227 individuals (64.3% males, median age 81.0 [interquartile range {IQR} 73.5-85.0] years, with EuroSCORE II 6.0% [IQR 4.0-10.9]) from 41 centres underwent TAVI with NGD, using either self-expanding (55.1%) or balloon-expandable valves (44.9%; p=0.50). TS was 85.5%, with a non-significant trend towards increased TS in high-volume activity centres. A second valve implantation (SVI) was needed in 8.8% of patients, independent of valve type (p=0.82). Device size was ≥29 mm in 73.0% of patients, post-procedure grade ≥III residual aortic regurgitation was rare (1.2%), and the permanent pacemaker implantation (PPI) rate was 36.0%. At 30 days, the incidences of mortality and reintervention were 8.4% and 3.5%, respectively. The co-primary endpoint reached 41.6% (IQR 34.4-49.6) at 1 year, increased up to 61.8% (IQR 52.4-71.2) at 4 years, and was independently predicted by TS, with a hazard ratio of 0.45 (95% confidence interval: 0.27-0.76); p=0.003.
CONCLUSIONS: TAVI with NGD in PAVR patients is efficient and reasonably safe. Preventing the need for an SVI embodies the major technical challenge. Larger implanted valves may have limited this complication, outweighing the increased risk of PPI. Despite successful TAVI, PAVR patients experience frequent CE at long-term follow-up.
Transcatheter aortic valve implantation (TAVI) is a mature technique in the field of aortic stenosis (AS). In contrast, aortic regurgitation (AR) has remained on the margins of percutaneous innovations. Nonetheless, AR frequency appears to increase with age1, and a subgroup of patients with AR displays a prohibitive surgical risk, making the valvular mini-invasive approach the only available therapeutic option2. As stated in European guidelines, this strategy is based on tertiary-centre expertise and cautious patient selection3. Two large registries have been previously published, establishing the feasibility of off-label TAVI in the setting of pure aortic valve regurgitation (PAVR)45. However, most of those reports assessed the added value of new-generation devices (NGD) compared to older versions, which is no longer a clinically pertinent question, given that only the most recent bioprostheses are available. Moreover, both registries were conducted during the period between 2007 and 2017, which does not reflect contemporary practices in interventional cardiology. Additionally, the NGD routinely implanted in our cath labs (SAPIEN 3 [Edwards Lifesciences] and Evolut R [Medtronic]) were used in only a minority of the patients in these studies: 19.3% and 5% for the SAPIEN 3, 23.6% and 15.0% for the Evolut R, respectively45. Although more recent data have been reported, they have been based solely on short-term outcomes, and only half of the studied population were implanted with NGD that are commonly used in clinical practice (Evolut and SAPIEN valves)6. For all these aforementioned reasons, we aimed to analyse contemporary long-term clinical endpoints in patients included in the FRANCE-TAVI Registry and treated with NGD for PAVR.
Methods
REGISTRY
FRANCE-TAVI is a national, prospective, multicentre registry that was initiated in 2013, collecting baseline and procedural characteristics for all patients who undergo TAVI across the 55 active centres in France. All patients provided written informed consent, and the Institutional Review Board of the French Ministry of Health approved the registry. A list of the STOP-AS and FRANCE-TAVI investigators is available in Supplementary Appendix 1.
Clinical outcomes were identified through the single-payer national health data system (SNDS), whose methodology has been described elsewhere7. In brief, the SNDS covers 99% of the French population, and an algorithm has been developed to match registry patients with the national data system.
POPULATION
Eligible patients for our study had grade III or IV native valve AR, without associated stenosis (mean gradient less than 15 mmHg), and comorbidities, making the local Heart Team consider surgical aortic valve replacement (SAVR) a prohibitive option. Only patients implanted with a new-generation bioprosthesis were considered for this analysis. Exclusion criteria were aortic stenosis and valve-in-valve or TAVI-in-TAVI procedures.
ENDPOINTS
The primary safety endpoint was technical success (TS) at the exit from the procedure room, defined by the Valve Academic Research Consortium 3 (VARC-3) criteria8 as the combination of freedom from mortality; successful access, delivery of the device, and retrieval of the delivery system; correct positioning of a single prosthetic heart valve into the proper anatomical location; and freedom from surgery or intervention related to the device or to a major vascular, access-related, or cardiac structural complication. The co-primary endpoint was a composite of VARC-3 major cardiac events, including all-cause death, heart failure hospitalisation (HFH) and valve-related reintervention (surgery or percutaneous) at the last follow-up. Secondary outcomes comprised reported 30-day clinical events and each component of the co-primary composite endpoint. The incidence of the co-primary endpoint was compared between predefined groups: self-expanding valves (SEV) versus balloon-expandable valves (BEV), and patients with and without TS.
STATISTICAL ANALYSIS
Continuous variables are presented as mean±standard deviation (SD) when the distribution was normal and, when non-normal, as median (interquartile range [IQR]; 25th-75th percentiles). Groups were compared using the Student’s t-test or the Wilcoxon rank-sum/Mann-Whitney U test for normally and non-normally distributed continuous variables, respectively. Categorical data are presented as number (percentage), and a comparison between groups was performed using the χ² test.
Activity volume categories (high vs non-high) were defined according to each centre’s total number of TAVI implantations (not limited to PAVR) between 2010 and 2021. High-volume centres corresponded to the fourth quartile.
The adverse event rates were based on Kaplan-Meier estimates, and all comparisons were made using the log-rank test. Cox regression was utilised to establish independent correlates of all-cause death, HFH and valve-related reintervention. All statistical analyses were performed using Python, version 3.8 (Python Software Foundation).
Results
POPULATION
From 2015 to 2021, we included 227 patients (0.3% of the registry) from 41 tertiary centres (Supplementary Figure 1). All baseline characteristics are represented in Table 1. In brief, patients were mostly male (64.3%), with a median age of 81.0 (IQR 73.5-85.0) years and a European System for Cardiac Operative Risk Evaluation (EuroSCORE) II of 6.0% (IQR 4.0-10.9). They were highly symptomatic: 77.3% were in New York Heart Association (NYHA) Class III or IV, and 13.4% of them had a preprocedural critical condition. Comorbidities were frequent: 46.3% had atrial fibrillation, 25.8% had a previous percutaneous coronary intervention, 12.4% had previous coronary artery bypass graft surgery, and 51.6% had chronic kidney failure.
Regarding preprocedural imaging characteristics, 14.9% of the patients had pulmonary hypertension greater than 60 mmHg, aortic annuli tended to be large, with a median diameter of 26.0 (IQR 23.0-29.0) mm, in accordance with an increased median aortic sinotubular junction diameter of 33.0 (IQR 30.0-38.0) mm. Valve cusps had none to mild calcification, consistent with a low aortic valve calcification score of 95.6 (IQR 0.0-508.5).
Table 1. Population characteristics at baseline.
| Total population n=227 | With co-primary composite endpoint at 1 year n=83# |
Free from co-primary composite endpoint at 1 year n=105# |
p-value | |
|---|---|---|---|---|
| Follow-up duration, days | 480.0 (155.5-1,024.0) | 169.0 (50.0-400.5) | 914.0 (657.0-1,381.0) | <0.001 |
| Demography | ||||
| Age, years | 81.0 (73.5-85.0) | 77.0 (69.5-83.0) | 82.0 (77.0-85.0) | <0.001 |
| Male | 146 (64.3) | 59 (71.1) | 66 (62.9) | 0.30 |
| BMI, kg/m² | 24.0 (22.0-27.2) | 25.2 (22.2-27.5) | 23.7 (21.6-27.0) | 0.35 |
| Risk scores | ||||
| EuroSCORE II, % | 6.0 (4.0-10.9) | 5.6 (3.6-10.5) | 6.9 (4.1-10.9) | 0.43 |
| Logistic EuroSCORE I, % | 18.8 (10.1-31.2) | 16.6 (8.3-30.6) | 20.4 (11.0-33.4) | 0.11 |
| Main indication | ||||
| NYHA Class III/IV | 150 (77.3) | 53 (74.6) | 71 (78.9) | 0.66 |
| Non-eligibility for surgery | 0.11 | |||
| Prohibitive risk | 148 (65.2) | 56 (67.5) | 67 (63.8) | |
| Frailty | 33 (14.5) | <11 (≥ 1%) | 21 (20.0) | |
| Hostile chest, porcelain aorta | 17 (7.5) | <11 (≥ 1%) | <11 (≥ 1%) | |
| Other | 14 (6.2) | <11 (≥ 1%) | <11 (≥ 1%) | |
| Medical history | ||||
| Hypertension | 147 (64.8) | 55 (66.3) | 67 (63.8) | 0.84 |
| Diabetes | 37 (16.5) | 12 (14.6) | 16 (15.5) | 1.00 |
| Hypercholesterolaemia | 51 (22.5) | 18 (21.7) | 27 (25.7) | 0.64 |
| >1 pulmonary oedema in the past year | 35 (16.6) | 15 (19.7) | 14 (14.1) | 0.43 |
| Permanent pacemaker | 30 (13.5) | 12 (15.0) | 13 (12.5) | 0.78 |
| Atrial fibrillation | 105 (46.3) | 41 (49.4) | 51 (48.6) | 1.00 |
| Percutaneous coronary intervention | 58 (25.8) | 20 (24.4) | 28 (26.9) | 0.82 |
| Coronary artery bypass graft surgery | 28 (12.4) | 12 (14.5) | 11 (10.6) | 0.56 |
| Transient ischaemic attack/stroke | 34 (15.1) | <11 (≥ 1%) | 16 (15.4) | 0.36 |
| Peripheral artery disease | 43 (19.1) | 16 (19.5) | 20 (19.2) | 1.00 |
| Timing of procedure: urgent (vs elective) | 32 (15.0) | 21 (26.6) | <11 (≥ 1%) | 0.004 |
| Preprocedural critical condition | 30 (13.4) | 15 (18.5) | 13 (12.5) | 0.35 |
| Chronic kidney disease | 0.82 | |||
| None | 108 (48.4) | 37 (45.1) | 47 (45.6) | |
| Moderate | 72 (32.3) | 30 (36.6) | 34 (33.0) | |
| Severe or requiring dialysis | 43 (19.3) | 15 (18.3) | 22 (21.4) | |
| Creatinine, mmol/L | 111.0 (85.0-150.3) | 108.0 (84.9-145.0) | 115.0 (93.0-151.0) | 0.42 |
| Preprocedural imaging | ||||
| Aortic valve area*, cm² | 1.8±1.0 | 1.4±0.9 | 2.1±1.1 | 0.003 |
| Transaortic mean gradient, mmHg | 10.0 (7.0-12.0) | 9.0 (7.0-12.0) | 10.0 (7.0-11.0) | 0.95 |
| Left ventricular ejection fraction, % | 46.0 (35.0-60.0) | 45.0 (35.0-60.0) | 49.0 (40.0-60.0) | 0.04 |
| Annular diameter, mm | 26.0 (23.0-29.0) | 27.0 (23.0-29.3) | 27.0 (24.2-29.0) | 0.75 |
| Aortic valve calcium score (Agatston score)* | 95.6 (0.0-508.5) | 148.0 (0.0-482.0) | 42.5 (0.0-703.2) | 0.65 |
| Maximum diameter at the sinotubular junction*, mm | 33.0 (30.0-38.0) | 36.0 (31.4-39.5) | 32.0 (30.0-39.0) | 0.24 |
| Severe pulmonary hypertension (>60 mmHg) | 27 (14.9) | <11 (≥ 1%) | 15 (17.4) | 0.57 |
| Mitral regurgitation grade ≥2 | 89 (48.1) | 28 (44.4) | 50 (53.8) | 0.33 |
| Data are given as median (interquartile range), n (%) or mean±standard deviation. *More than 50% of data missing. #The combined number of patients in each subgroup is not equal to the total population. P-values in bold indicate statistical significance. BMI: body mass index; EuroSCORE: European System for Cardiac Operative Risk Evaluation; NYHA: New York Heart Association | ||||
PROCEDURAL DETAILS, TECHNICAL SUCCESS AND 30-DAY OUTCOMES
Procedural and periprocedural outcomes are reported in Table 2. Procedures were mostly performed with a transfemoral approach (91.6%). SEV were used slightly more frequently than BEV (55.1% vs 44.9%; p=0.50), and valve sizes were markedly large, with 73.0% of valves equal to or larger than 29 mm. A total of 8.8% of the patients had two devices implanted. The incidence of this aforementioned complication was identical regardless of whether the first implanted valve was a BEV or a SEV: the rates in both cohorts were 7.8% and 9.6%, respectively; p=0.82 (Supplementary Table 1). Of the 20 patients that required a second valve implantation (SVI), the first implanted valve was a SEV in 60% of the cases, and most of the second devices were BEV (70%). Following TAVI, left ventricular ejection fraction (LVEF) decreased from 46.0% (IQR 35.0-60.0) to 42.0% (IQR 30.0-55.0), and valve haemodynamics were as follows: 98.7% of the patients had a mean gradient <20 mmHg, and 98.8% had AR Supplementary Table 1). However, activity volume, a surrogate of centre experience, appeared related to higher TS, despite being statistically non-significant (Figure 1).
At 30 days, reintervention related to the device or vascular access was observed in 4.6% and 4.0% of the cases, respectively. The incidence of bleeding requiring transfusion was 8.8%, and tamponade was 4.0%. A key finding was the increased incidence of permanent pacemaker implantation (PPI), which was as high as 36.0%, with no difference between SEV and BEV, at 37.3% and 34.5%, respectively (p=0.80) (Supplementary Table 1).
Table 2. Procedural characteristics, technical success and 30-day outcomes.
| Total population n=227 | With co-primary composite endpoint at 1 year n=83# |
Free from co-primary composite endpoint at 1 year n=105# |
p-value | |
|---|---|---|---|---|
| Procedural characteristics | ||||
| Predilation | <11 (≥ 1%) | <11 (≥ 1%) | <11 (≥ 1%) | 0.48 |
| Femoral access | 207 (91.6) | 79 (95.2) | 91 (87.5) | 0.12 |
| Need for second valve implantation | 20 (8.8) | 12 (14.5) | 8 (7.6) | 0.08 |
| Proper anatomical position of the first implanted valve | 205 (93.6) | 69 (87.3) | 97 (96.0) | 0.06 |
| Annular rupture | <3 (< 1%) | <3 (≥ 1%) | 0 (0) | 0.90 |
| Coronary obstruction | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Aortic dissection | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Device | 0.58 | |||
| CoreValve Evolut PRO1 | 22 (9.7) | <11 (≥ 1%) | <11 (≥ 1%) | |
| CoreValve Evolut R1 | 103 (45.4) | 45 (54.2) | 49 (46.7) | |
| SAPIEN 32 | 102 (44.9) | 33 (39.8) | 48 (45.7) | |
| Device type | 0.50 | |||
| Self-expanding | 125 (55.1) | 50 (60.2) | 57 (54.3) | |
| Balloon-expandable | 102 (44.9) | 33 (39.8) | 48 (45.7) | |
| Bioprosthesis diameter, mm | 0.06 | |||
| 23.0 | 18 (8.1) | <11 (≥ 1%) | <11 (≥ 1%) | |
| 26.0 | 40 (17.9) | 18 (22.5) | 15 (14.4) | |
| 29.0 | 100 (44.8) | 27 (33.8) | 55 (52.9) | |
| 34.0 | 63 (28.2) | 28 (35.0) | 28 (26.9) | |
| Bioprosthesis diameter ≤26 mm | 58 (26.0) | 23 (28.7) | 21 (20.2) | 0.24 |
| Postprocedural imaging | ||||
| Left ventricular ejection fraction, % | 42.0 (30.0-55.0) | 35.0 (25.0-47.2) | 49.0 (35.0-57.0) | <0.001 |
| Transaortic mean gradient | 8.0 (5.0-10.0) | 8.0 (6.0-11.0) | 8.0 (5.0-10.0) | 0.44 |
| Aortic valve area*, cm² | 2.1±0.6 | 1.9±0.5 | 2.1±0.6 | 0.16 |
| Postprocedural transaortic mean gradient <20 mmHg | 154 (98.7) | 49 (98.0) | 84 (98.8) | 1.00 |
| Postprocedural aortic valve regurgitation grade <3 | 164 (98.8) | 53 (96.4) | 90 (100) | 0.28 |
| Severe pulmonary hypertension (>60 mmHg) | <11 (≥ 1%) | <11 (≥ 1%) | <11 (≥ 1%) | 1.00 |
| Technical success (at exit from procedure room) | ||||
| Technical success | 189 (85.5) | 63 (77.8) | 92 (91.1) | 0.02 |
| Freedom from mortality | 225 (99.1) | 81 (97.6) | 105 (100) | 0.38 |
| Freedom from surgery related to the device | 220 (96.9) | 76 (91.6) | 105 (100) | 0.01 |
| Correct positioning of a single prosthetic heart valve into the proper anatomical location | 199 (90.9) | 67 (84.8) | 95 (94.1) | 0.07 |
| Freedom from surgery/intervention related to a major vascular or access-related complication | 219 (96.5) | 82 (98.8) | 101 (96.2) | 0.52 |
| Freedom from surgery/intervention related to a cardiac structural complication | 227 (100) | 83 (100) | 105 (100) | 1.00 |
| Outcomes at 30 days | ||||
| Freedom from mortality | 208 (91.6) | 64 (77.1) | 105 (100) | <0.001 |
| Freedom from reintervention (surgery or percutaneous) related to the device |
217 (95.6) | 73 (88.0) | 105 (100) | <0.001 |
| Freedom from surgery/intervention related to a major vascular or access-related complication |
218 (96.0) | 82 (98.8) | 101 (96.2) | 0.52 |
| Freedom from intervention for tamponade | 218 (96.0) | 75 (90.4) | 104 (99.0) | 0.02 |
| Bleeding requiring transfusion | 20 (8.8) | 13 (15.7) | <11 (≥ 1%) | 0.01 |
| PM/CRT-D/CRT-P/ICD implantation** | 71 (36.0) | 21 (29.6) | 39 (42.4) | 0.13 |
| Stroke | <11 (≥ 1%) | <11 (≥ 1%) | 0 (0) | 0.01 |
| New-onset renal replacement therapy | <11 (≥ 1%) | <11 (≥ 1%) | 0 (0) | 0.01 |
| Length of stay, days | 6.0 (3.0-9.0) | 7.0 (3.0-12.0) | 5.0 (3.0-7.8) | 0.05 |
| Data are given as n (%), median (interquartile range) or mean±standard deviation. P-values in bold indicate statistical significance. *More than 50% of data. **197 patients without prior pacemakers were analysed. #The combined number of patients in each subgroup is not equal to the total population. 1By Medtronic; 2by Edwards Lifesciences. CRT-D: cardiac resynchronisation therapy-defibrillator; CRT-P: cardiac resynchronisation therapy-pacemaker; ICD: implantable cardioverter defibrillator; PM: pacemaker | ||||
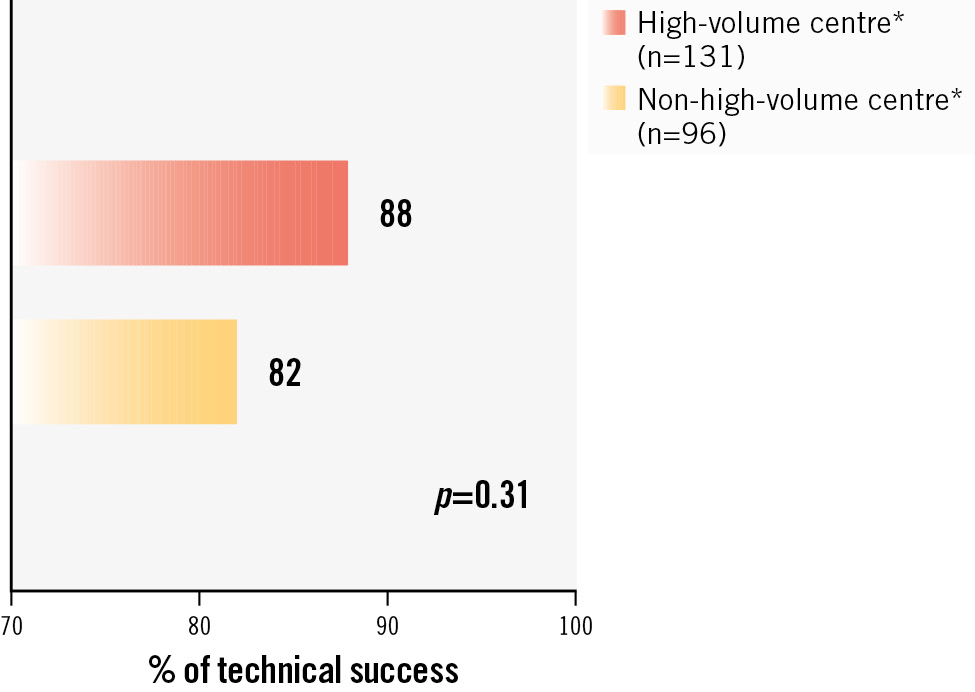
Figure 1. Technical success rates according to centre volume activity. *Definition based on total TAVI procedures between 2010 and 2021: high-volume centre: >250 implantations/year; non-high volume centre: <250 implantations/year (average of 110/year). TAVI: transcatheter aortic valve implantation
CLINICAL OUTCOMES
The incidence of the co-primary endpoint, a composite of all-cause mortality, HFH and device-related reintervention, was 61.8% (95% confidence interval [CI]: 52.4-71.2) at 4-year follow-up; this increased rate was mostly related to high mortality at 4 years, up to 53.5% (Figure 2). At 1 year, the incidence was 41.6% (95% CI: 34.4-49.6), driven by mortality (24.0%), HFH (28.8%) and reintervention (6.2%) (Supplementary Figure 2). At this timepoint, the composite endpoint rate was found to be statistically lower in patients with versus without the need for an SVI: 67.6% (95% CI: 46.5-86.9) and 43.4% (95% CI: 36.4-51.1), respectively; p=0.004 (Central illustration). Neither the type of valve (Figure 2) nor the need for PPI (Supplementary Figure 3) were found to be associated with prognosis.
Factors associated with the occurrence of the co-primary endpoint are presented in Table 3. Due to competing factors, technical success and the need for an SVI were analysed in separate models and were both independent predictors: hazard ratio (HR) 0.45, 95% CI: 0.27-0.76; p=0.003; and HR 1.95, 95% CI: 1.08-3.52; p=0.03, respectively.
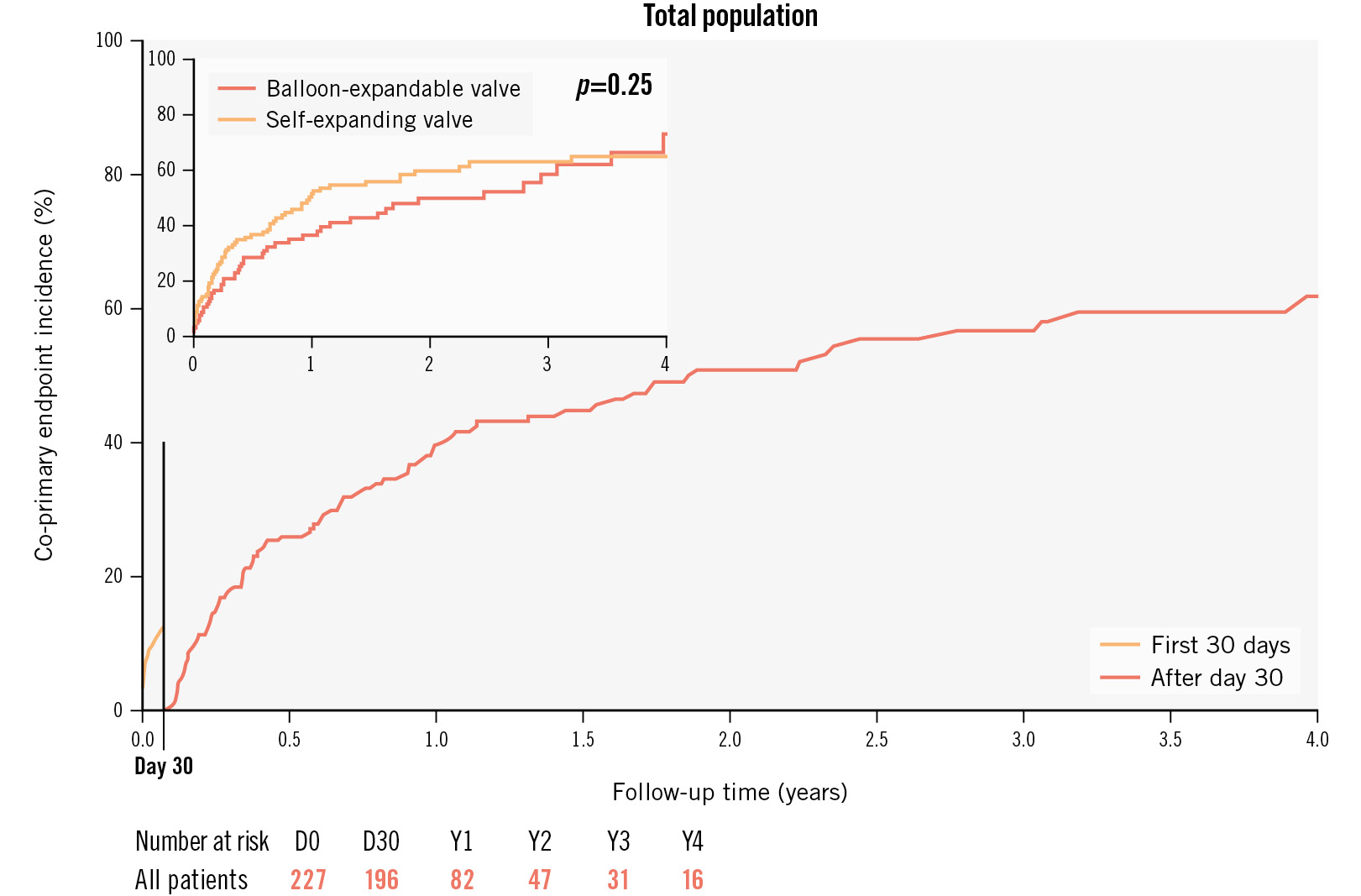
Figure 2. Landmark analysis at day 30 and Kaplan-Meier estimates for the co-primary outcome comprising all-cause mortality, HFH and device-related reintervention for the whole population and according to the type of valve: self-expanding versus balloon-expandable (upper left corner). HFH: heart failure hospitalisation
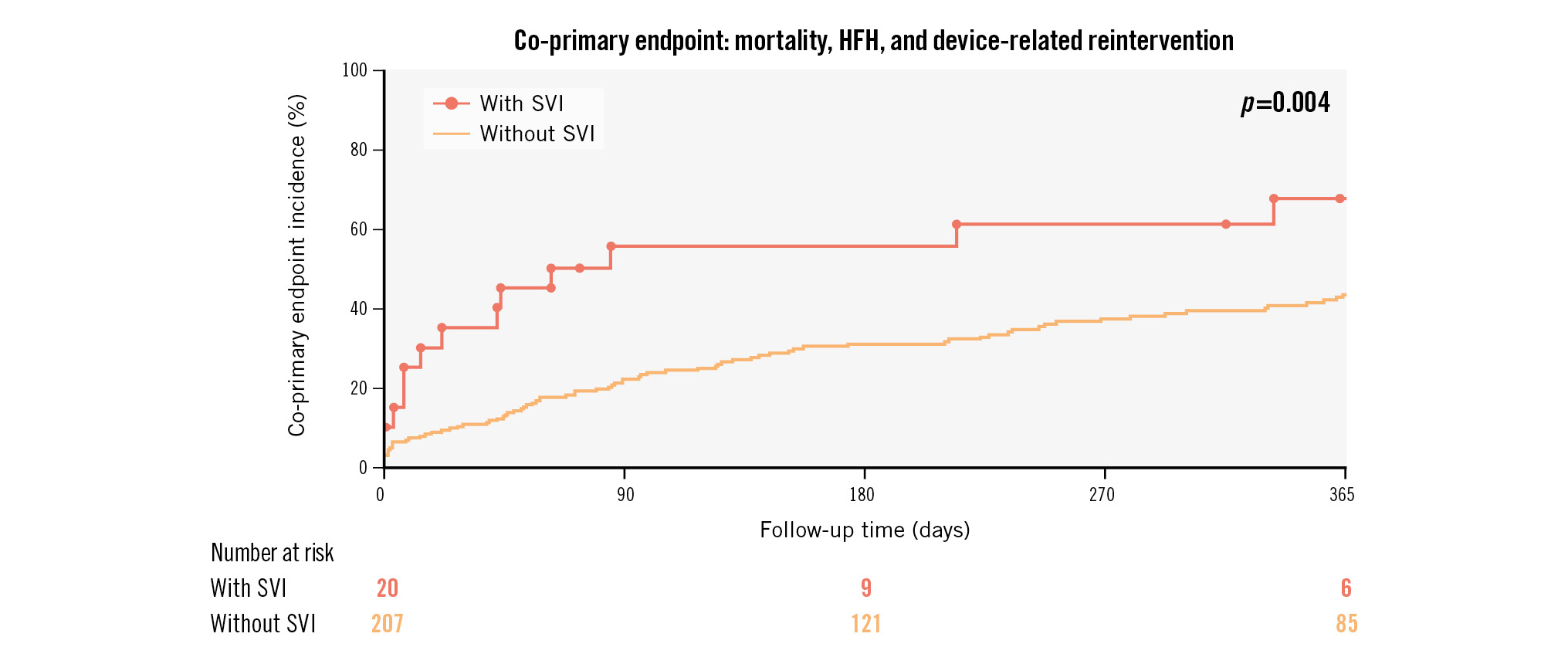
Central illustration. Kaplan-Meier estimates for the co-primary endpoint in patients with and without SVI. HFH: heart failure hospitalisation; SVI: second valve implantation
Table 3. Univariate analysis and multivariate models for the co-primary endpoint.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR [95% CI] | p-value | HR [95% CI] | p-value | |
| Model 1 | ||||
| NYHA III/IV | 1.57 [0.95-2.62] | 0.08 | 1.81 [1.05-3.11] | 0.03 |
| Left ventricular ejection fraction | 0.99 [0.98-1.00] | 0.14 | 1.00 [0.98-1.01] | 0.81 |
| Bioprosthesis diameter >29 mm | 1.52 [1.03-2.24] | 0.04 | 1.69 [1.11-2.56] | 0.01 |
| Technical success | 0.39 [0.24-0.62] | <0.001 | 0.45 [0.27-0.76] | 0.003 |
| Model 2 | ||||
| NYHA III/IV | 1.57 [0.95-2.62] | 0.08 | 1.88 [1.09-3.23] | 0.02 |
| Left ventricular ejection fraction | 0.99 [0.98-1.00] | 0.14 | 0.99 [0.98-1.00] | 0.16 |
| Bioprosthesis diameter >29 mm | 1.52 [1.03-2.24] | 0.04 | 1.65 [1.09-2.50] | 0.02 |
| Need for second valve implantation | 2.00 [1.14-3.50] | 0.02 | 1.95 [1.08-3.52] | 0.03 |
| CI: confidence interval; HR: hazard ratio; NYHA: New York Heart Association | ||||
Discussion
FRANCE-TAVI PAVR is the largest contemporary study reporting long-term morbimortality outcomes in patients implanted with new-generation devices for PAVR. The main findings are enumerated hereinafter:
1) Procedural safety has improved in comparison to previous studies; nonetheless, the need for an SVI remains the major complication, reported in up to 8.8% of cases.
2) BEV and SEV were equally distributed throughout the cohort, and no difference was found between device type and occurrence of the primary outcomes.
3) Nowadays, most of the bioprosthesis sizes implanted for PAVR are the largest ones available, which reflects the intended oversizing required in this setting.
4) This interventional practice had 2 main consequences: an incidence of grade ≥III residual AR as low as 1.2%, and a high rate of cardiac conductance disorders requiring PPI, up to 36.0% at 30 days.
5) Despite a majority of successful interventional treatments, this population of heart failure patients is at high risk of medical events, and particularly all-cause mortality, which reached 53.5% at 4-year follow-up.
PROCEDURAL ASPECTS
In line with modern interventional practices, the transfemoral route was preferred for vascular access; indeed, rates in our contemporary cohort appear much higher than in previous NGD subgroups: 91.6% versus 76%5 or even 61%4. This primordial technical characteristic may have significantly contributed to improving procedural success and outcomes, since in the past, most non-femoral routes were transapical, with a well-known worse prognosis9. Bleeding event frequency at 30 days tended to be lower than previously documented, with 8.8% in this study compared to 9.9% reported in a prior study4. At 30 days, the rate of 4% for reinterventions related to a major vascular complication was encouraging regarding the highly comorbid patients and considering the major vascular complications incidence of 7.9% in the intermediate-risk population in the PARTNER II Trial10.
Our study highlights the feasibility of the procedure, with a rate of 85.5% for VARC-3 TS. Prior registries reported rates of 81.1% and 82% VARC-2 device success in their NGD subgroups45. Considering that the updated VARC-3 composite outcome includes more clinical endpoints, we may assume that global interventional success has largely improved. The need for an SVI was 8.8%, which is less frequent than in the previous largest registry that found an incidence of up to 12.7% despite the newer bioprostheses4. Finally, this result is even lower than the most recent international study that reported a rate of 10.5%6. Provided that TS is associated with later clinical outcomes, and that the main determinant of TS is the need for an SVI, then everything should be done to avoid such prognostic complication. It is worth highlighting that, in our study, this complication rate did not differ between the two types of valve (BEV vs SEV). The need for an SVI is mainly driven by device embolisation or migration. Valve undersizing has been suggested as a reason for the increased risk of such a complication, but so has excessive oversizing, particularly regarding self-expanding devices5. Device dimension is not the only factor to be considered as causal in the occurrence of valve malposition. The increased stroke volume caused by AR, the low implantation height favoured by the absence of fluoroscopic calcific landmarks, and pacing failure are other well-known risk factors. As previously shown, centre experience has great importance in optimising interventional outcomes4. In order to solve these current technical limits, dedicated transcatheter valves have been developed1112. In the recent ALIGN-AR Trial, evaluating the safety and efficacy of the Trilogy transcatheter heart valve (JenaValve), TS increased up to 95%, which represents major progress13. Albeit efficient and promising, this new device will not cover the whole spectrum of anatomies in the field of PAVR14.
Independently of technical innovations, operators may have gained experience in implanting valves in this particular environment that calls for specific precautions, such as greater oversizing15, among other practical considerations. Improvement in computed tomography (CT) scan quality and analysis may have contributed to the refinement of procedural planning, including specific device choice and accurate sizing, to ultimately increase TAVI success in this setting. The incidence of 8.4% mortality at 30 days in our population is lower than the rate of 11.9% (95% CI: 9.4%-14.7%) observed in a recent meta-analysis16 and seems acceptable considering the high-risk features of the patients included in this analysis. Conversion to surgery related to the valve was 3.1%, which is similar to rates previously described: 3.8%4. Put together, these findings suggest an improvement in procedural safety over time.
DEVICE ASPECTS
A major question in clinical practice is whether we should treat PAVR with either SEV or BEV. For the first time, we describe a nationwide cohort of patients implanted exclusively with devices already available in routine practice (SAPIEN 3, Evolut R and Evolut Pro). We report excellent valve haemodynamics and particularly an incidence of significant residual AR that dramatically dropped from an estimated rate of 3.3% in the most recent meta-analysis to 1.2% in our population16. It is necessary to put this latter finding into perspective with the sizes of the implanted valves. Indeed, 73% received a valve of 29 mm or greater, which may reflect the increasing awareness of operators regarding the importance of adequately oversizing bioprostheses15. This, in turn, may have caused more cardiac conductance abnormalities requiring PPI, reaching up to 36.0% at 30 days. This rate is concordant with the recent series of PAVR patients exclusively treated with SAPIEN 3 valves that reported a 35.1% rate of PPI, but it is higher than in the contemporary cohort that had a majority of SEV and found an incidence of 22.3%617. PPI in this context is in part due to device oversizing but is also due to a low implantation height, given that, unlike aortic stenosis, no calcification is present to identify the valvular plan.
CLINICAL OUTCOMES
A PAVR population treated with a percutaneous approach is highly comorbid and frequently suffers from advanced heart failure, as reflected in the preprocedural LVEF of 46.0% (35.0-60.0) in our analysis, corresponding with other registries’ data456. As expected in this setting, restoration of valve function induces an apparent worsening in left ventricular function, in coherence with the LVEF of 42.0% (30.0-55.0) reported after the procedure.
At 1 year, the all-cause death rate of 24% was consistent with the incidence of 24.7% reported in a meta-analysis16. This event rate is comparable to what can be observed in some cohorts of elderly patients suffering from acute decompensated heart failure18. Regarding aortic valve reintervention, the incidence was high, and events occurred exclusively during the 6 months following the procedure. For comparison purposes, we can refer to the 5.9% rate of reintervention in patients undergoing valve-in-ring transcatheter mitral valve implantation. The 4-year co-primary endpoint incidence was 61.8%, and this was despite a successful procedure in the majority of patients. This suggests that poor late prognosis is primarily driven by the evolution of irreversible myocardial damage caused by the regurgitant aortic valvulopathy. In the absence of a control group, the net benefits of the procedure cannot be clearly assessed.
Limitations
Although national, multicentre and prospective, our data suffer the drawbacks of observational reports. Valve haemodynamics and clinical events were based on investigator evaluation, without an independent approach through an imaging core lab or a clinical event adjudication committee. Most of the CT scan parameters were lacking, with more than 50% missing data, particularly regarding sinotubular aortic diameter and aortic valve calcium score. The case report form was standardised and initially designed for AS; therefore, several technical aspects were unavailable, such as the oversizing rate, exhaustive details on procedural complications and other interventional features (general anaesthesia, imaging guidance, rapid pacing modalityâ¦). Due to our internal policy regarding data safety, we were unable to report details on some subgroups with fewer than 11 patients. Despite the aforementioned limitations, ours is the largest cohort of patients treated with NGD, reflecting contemporary practices and outcomes in interventional cardiology. Our analysis provides a comprehensive assessment of long-term outcomes.
Conclusions
TAVI with NGD in PAVR patients is efficient and reasonably safe. Prevention of the need for an SVI remains the major prognostic and technical challenge. Greater valve oversizing may have improved this major procedural complication, despite being associated with increased PPI. Major advances are expected from dedicated devices addressing PAVR-specific features. At long-term follow-up after TAVI, patients remain at high risk of clinical events.
Impact on daily practice
Contemporary off-label transcatheter aortic valve implantation (TAVI) procedures with new-generation devices in pure aortic valve regurgitation (PAVR) patients are still challenging. Technical success is a determinant of patient prognosis. For this reason, expertise is required to accurately select the patients, the device and, ultimately, perform the procedure. Great technical advances are expected from upcoming dedicated valves. The PAVR population treated with TAVI is composed of severe chronic heart failure patients, and therefore, close follow-up should be pursued even after successful valvular disease treatment.
Acknowledgements
The authors thank Nicole Naccache from the Registry Department of the French Society of Cardiology. The authors are also grateful to Helena Fettre, Martine Riou, Johann Cattan, Marie Lebosse, Thibaut Guitteny, Pietro Laforgia, Antonin Trimaille, and Imane Bagdadi for their assistance in data collection.
Funding
This work was supported by the French Government, managed by the National Research Agency (ANR) under the programme “Investissements d’Avenir” with the reference ANR-16-RHU-0003-STOP-AS.
Conflict of interest statement
L. Leroux has served as a proctor for Medtronic. T. Lefèvre has served as a proctor for Edwards Lifesciences and Abbott. D. Tchétché has received honoraria or consultation fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. B. Iung has received consulting fees from Boehringer Ingelheim; and has received speaker fees from Edwards Lifesciences. H. Le Breton has received speaker fees from Edwards Lifesciences and Medtronic. H. Eltchaninoff has served as a proctor for and received lecture fees from Edwards Lifesciences. J.-P. Collet has received research grants from Bristol-Myers Squibb and Medtronic; and lecture fees from Bristol-Myers Squibb, Bayer, Daichii Sankyo, AstraZeneca, and Medtronic. J.-F. Obadia received consulting or speaker fees from Abbott, Delacroix-Chevalier, and Medtronic. E. Teiger has served as a proctor for Medtronic. C. Saint-Etienne has received honoraria from Abbott and Biotronik. The other authors have no conflicts of interest to declare relevant to the contents of this paper.
Supplementary data
To read the full content of this article, please download the PDF.

