Abstract
Background: No data compare newer-generation transcatheter heart valves (THVs) in terms of next-day discharge (NDD) following transfemoral (TF) transcatheter aortic valve implantation (TAVI).
Aims: We aimed to evaluate the safety of NDD in unselected patients who received ACURATE (neo/neo2), Evolut (PRO/PRO+/FX) and the SAPIEN (3/Ultra) THVs.
Methods: This multicentre registry included patients who underwent TF-TAVI without a preprocedural permanent pacemaker implantation (PPI) and were discharged the next day without a new PPI. The primary endpoint was unplanned readmissions at 30 days. Multinomial gradient-boosted inverse probability of treatment-weighted (IPTW) propensity scores (stage 1) followed by the modified Poisson regression (stage 2) approach were used to compare the average effects of the THVs on the primary outcome.
Results: A total of 963 all-comer patients (ACURATE=264, Evolut=306, and SAPIEN=393) were included in this study. ACURATE patients were older (p<0.001) and included a greater proportion of females (p<0.001), whereas Evolut patients had a higher risk profile as assessed by the Society of Thoracic Surgeons score (p=0.01). There were no differences between the groups in terms of right or left bundle branch block (p=0.75). At 30 days, the overall readmission rate was 8%, and there were no differences in cardiac (ACURATE 4.6% vs Evolut 4.2% vs SAPIEN 3.1%; p=0.56) or non-cardiac readmissions (ACURATE 4.6% vs Evolut 3.3% vs SAPIEN 4.6%; p=0.64). Readmission for new PPI was 2.7%, 1.0% and 1.8% (p=0.32) and for heart failure (HF) was 1.5%, 2.0% and 1.3% (p=0.76) in ACURATE, Evolut and SAPIEN patients, respectively. The IPTW propensity score model followed by modified Poisson regression indicate that, using ACURATE as the reference, no significant differences were found in 30-day readmissions (relative risk [RR] 0.76, 95% confidence interval [CI]: 0.38-1.52; p=0.38 for Evolut and RR 0.74, 95% CI: 0.44-1.22; p=0.28 for SAPIEN).
Conclusions: In pacemaker-naïve patients undergoing TF-TAVI with newer-generation THVs, NDD was not associated with a negative impact on overall 30-day readmissions, cardiac or non-cardiac readmissions, readmissions for PPI or HF after discharge, or mortality, regardless of the type of THV.
The minimalist approach for transcatheter aortic valve implantation (TAVI) represents substantial progress in simplifying procedural facets, promoting early ambulation and a prompt return to normal daily living activities. Another important step forward in the management of TAVI patients has been early discharge (ED) protocols12, altogether improving the overall individual experience and reducing healthcare costs34.
Studies have shown promising data related to the safety around ED pathways; however, most of these studies included patients with strict selection criteria lined up with selected transcatheter heart valves (THVs)125. In this regard, the design and mechanism of the THV may preclude opportunities for ED, mainly considering differences in the rates of peri- and postprocedural new conduction abnormalities that warrant extended telemetry monitoring, thereby prolonging the length of stay (LOS). Furthermore, the need for permanent pacemaker implantation (PPI) is another caveat against ED, and this risk continues for the first few days and indeed weeks following discharge.
Importantly, there is no direct comparison of newer-generation THVs regarding next-day discharge (NDD) in patients undergoing TAVI. Hence, we aimed to evaluate the safety of NDD in unselected patients who received ACURATE neo/neo2 (Boston Scientific), Evolut PRO/PRO+/FX (Medtronic), and the SAPIEN 3/Ultra (Edwards Lifesciences) THVs.
Methods
Population
Data from consecutive, all-comer patients who underwent planned outpatient transfemoral (TF) TAVI (TF-TAVI) for native severe symptomatic aortic stenosis at 2 academic centres of excellence for the treatment of valvular heart disease in Canada (University Hospital, London Health Sciences Centre, Western University, London, ON, and St. Paul’s/Vancouver General Hospital, University of British Columbia, Vancouver, BC) and 1 in the USA (University Hospitals Cleveland Medical Center, Cleveland, OH) between January 2020 and June 2023 were prospectively collected in dedicated local databases. Patients with preprocedural PPI (n=139), those who required a new post-TAVI PPI (n=124), and those whose LOS was greater than 1 day (n=225, other than the previously mentioned post-TAVI PPI patients) during the index admission were excluded from the primary analysis (Central illustration). The decision to exclude patients with pre- and post-TAVI PPI was deemed necessary to provide a strong message around readmissions for new PPI early after discharge.
Patient mobilisation was promptly recommended within 4 hours following completion of the procedure, and a transthoracic echocardiogram was performed either the same day or the following morning before discharge. All patients had an electrocardiogram (ECG) soon after TAVI and before discharge. Patients were deemed suitable for the NDD pathway if they were capable (at the time of the discharge) of ambulation and self-care along with the absence of new-onset conduction disturbances on ECG, uncontrolled arrhythmia (i.e., rapid atrial fibrillation) or transient conduction abnormalities on telemetry monitoring, any signs of haemodynamic instability or major adverse events (i.e., stroke), symptoms of heart failure (HF), ischaemic chest pain, suspected infectious disease, and acute kidney injury or decreased urine output, and had stable haemoglobin.
The primary outcome was 30-day unplanned readmissions. The secondary outcome was exploratory, looking at the differences between cardiac and non-cardiac causes of readmission. Cardiac readmission included any conduction disturbances requiring PPI, HF, acute coronary syndrome, arrhythmias, and valve-related complications. Non-cardiac causes included stroke, vascular complications, infections, respiratory problems, gastrointestinal issues, and others. The population of individuals who were excluded from the primary analysis is provided using descriptive data along with a simple statistical analysis against the NDD population only for comparison purposes.
Outcomes were reported according to Valve Academic Research Consortium-3 definitions6. Institutional review board and ethics committee approval was obtained at each participating site. This manuscript conforms to the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines7, and the STROBE checklist is provided in Supplementary Table 1.
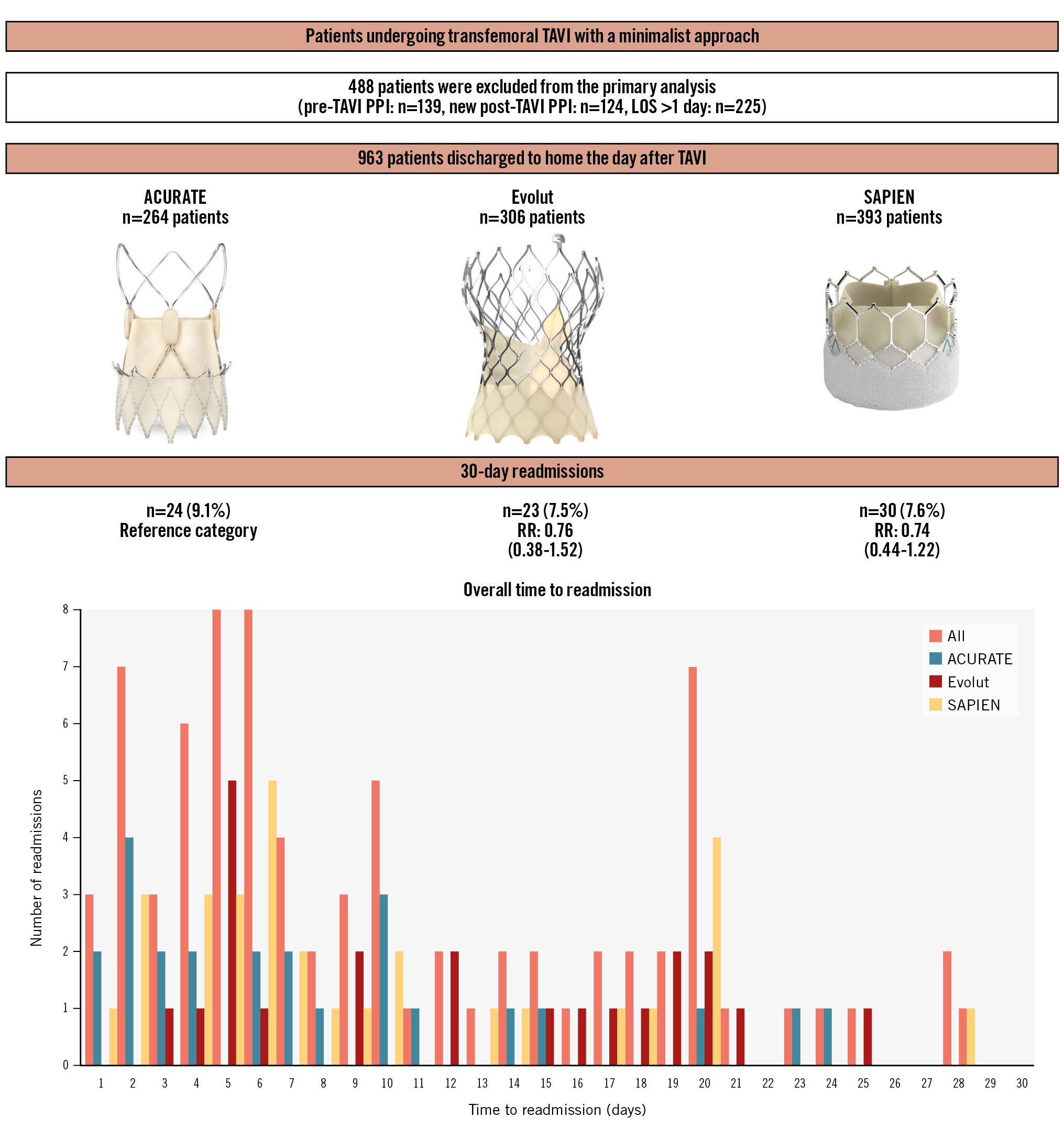
Central illustration. Readmission rates after a next-day discharge pathway following TAVI. An IPTW propensity score model followed by modified Poisson regression indicate that, using ACURATE as the reference, no significant differences were found in 30-day readmissions (RR 0.76, 95% CI: 0.38-1.52; p=0.38 for Evolut and RR 0.74, 95% CI: 0.44-1.22; p=0.28 for SAPIEN). Images were provided and reproduced courtesy of Boston Scientific Corporation ©2024, Medtronic Inc. ©2024 and Edwards Lifesciences LLC, Irvine, CA. ©2024. ACURATE: ACURATE neo/neo2; CI: confidence interval; Evolut: Evolut PRO/PRO+/FX; IPTW: inverse probability of treatment-weighted; LOS: length of stay; PPI: permanent pacemaker implantation; RR: risk ratio; SAPIEN: SAPIEN 3/Ultra; TAVI: transcatheter aortic valve implantation
Statistical analysis
Continuous variables are reported as mean±standard deviation or median (interquartile range), whereas categorical variables are reported as frequencies and percentages. Crude comparisons were performed using Pearson’s chi-square and Fisher’s exact tests for categorical variables, and analysis of variance (ANOVA) for continuous variables, as deemed suitable. To account for imbalances in clinical and anatomical variables (i.e., aortic annulus size) and to address potential biases that may affect the association between valve types and 30-day readmission, we utilised directed acyclic graphs (DAGs) to identify the minimally sufficient set of covariates for generating propensity score (PS) weights (Figure 1A)8. This approach aimed to mitigate causation, mediation, and interaction, thereby providing unbiased estimates while comparing the 3 types of valve. We assessed covariate balance between treatment groups before and after applying the PS weighting using the Kolmogorov-Smirnov (KS) test. The KS test is interpreted as follows: values <0.1 indicate negligible difference or good balance, values between 0.1 and 0.2 indicate some imbalance, and values>0.2 indicate substantial imbalance (Figure 1B). Multinomial gradient-boosted inverse probability of treatment-weighted (IPTW) PS (stage 1) followed by the modified Poisson regression (stage 2) approach were used to compare the average effects of the THVs on the primary outcome. The twang package for R (R Foundation for Statistical Computing) was used for this analysis. Results are reported as a relative risk (RR) with a 95% confidence interval (CI). All statistical analyses are 2-tailed, and a p-value of <0.05 was considered statistically significant. Statistical analyses were performed using SAS version 9.4 (SAS Institute) and R version 3.3.2 (R Foundation for Statistical Computing).
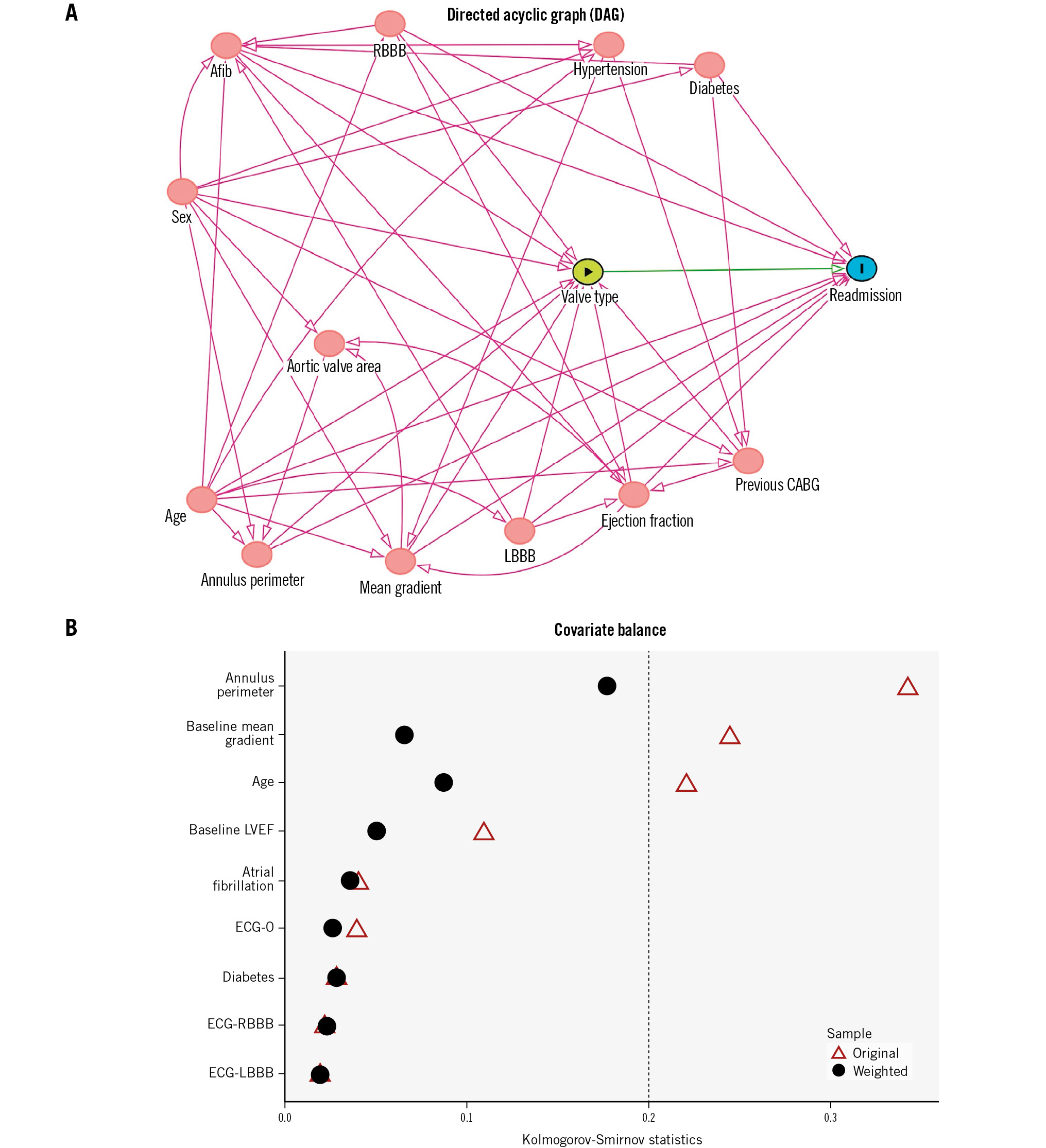
Figure 1. Causal inference and handling of imbalanced data. A) Directed acrylic graph. The DAG represents causal relationships among the set of variables (D: directed − indicates that arrows point in one single direction, A: acyclic − indicates that there is no sequence of arrows forming a closed loop or backwards causation). The variables included in the DAG were age, sex, aortic annulus perimeter, mean gradient, aortic valve area, LVEF, LBBB, RBBB, atrial fibrillation, hypertension, diabetes, and previous CABG. The DAG identified atrial fibrillation, age, LVEF, aortic annulus perimeter, mean gradient, diabetes, and ECG findings (no conduction abnormalities, LBBB, RBBB) as the covariates for adjustment. These selected covariates were then utilised to construct propensity scores, which were employed in inverse probability of treatment-weighted estimation to assess the average effects of the valves, utilising boosted models. B) Covariate balance. This image shows the balance of covariates before and after weighting, for the maximum balance across treatment pairs using Kolmogorov-Smirnov statistics. Afib: atrial fibrillation; CABG: coronary artery bypass graft; ECG-0: no conduction abnormalities; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; RBBB: right bundle branch block
Results
Study population
A total of 963 all-comer patients (ACURATE=264, Evolut=306, and SAPIEN=393) underwent NDD post-TAVI and are the subject of this study. ACURATE patients were older (83.6±5.9 years vs Evolut 79.5±7.3 years vs SAPIEN 81.1±7.7 years; p<0.001) and included a greater proportion of females (61% vs 50% vs 34%; p<0.001). In contrast, Evolut patients had a higher risk profile as assessed by the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score (Evolut 3.5±2.1 vs SAPIEN 3.2±1.4 vs ACURATE 3.0±1.0; p=0.01) (Table 1).
There were no differences between the groups in terms of preprocedural atrial fibrillation (ACURATE 27%, Evolut 22% and SAPIEN 26%; p=0.35), right bundle branch block (RBBB; ACURATE 10%, Evolut 12% and SAPIEN 10%; p=0.75), left bundle branch block (LBBB; ACURATE 7.6%, Evolut 7.2% and SAPIEN 5.6%; p=0.75), left ventricular ejection fraction (LVEF; ACURATE 58±11%, Evolut 57±11% and SAPIEN 57±11%; p=0.45) or the proportion of individuals with an LVEF <35% (ACURATE 6.1%, Evolut 6.9% and SAPIEN 6.1%; p=0.90). Patients who received an ACURATE THV had a higher mean gradient (ACURATE 47±12 mmHg vs Evolut 42±14 mmHg vs SAPIEN 45±14 mmHg; p<0.001) and a smaller aortic annulus perimeter (ACURATE 75.2±5.2 mm vs Evolut 76.7±8.6 mm vs SAPIEN 79.9±7.9 mm; p<0.001). The remaining baseline clinical, electrocardiographic, and echocardiographic characteristics of the study population are summarised in Table 1.
Table 1. Baseline characteristics of the study population according to discharge pathways and type of valve.
| Variables | Next-day discharge | Excluded from the analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| ACURATE neo/neo2 n=264 |
Evolut PRO/PRO+/FX n=306 | SAPIEN3/Ultra n=393 | p-value | ACURATE neo/neo2 n=138 |
Evolut PRO/PRO+/FX n=180 | SAPIEN3/Ultra n=170 | p-value | |
| Baseline characteristics | ||||||||
| Age, years | 83.6±5.9 | 79.5±7.3 | 81.1±7.7 | <0.001# | 84.4±5.9 | 80.6±7.3 | 81.9±7.2 | <0.001# |
| Female sex | 160 (61) | 153 (50) | 132 (34) | <0.001# | 83 (60) | 74 (41) | 56 (33) | <0.001# |
| Hypertension | 226 (86) | 275 (90) | 334 (85) | 0.15 | 123 (89) | 165 (91) | 141 (83) | 0.04# |
| Diabetes | 75 (28) | 99 (33) | 115 (29) | 0.51 | 38 (27) | 66 (36) | 57 (33) | 0.22 |
| Previous CABG | 34 (13) | 39 (13) | 61 (16) | 0.49 | 23 (17) | 20 (11) | 29 (17) | 0.32 |
| STS-PROM score | 3.0±1.0 | 3.5±2.1 | 3.2±1.4 | 0.01# | 3.3±1.1 | 3.6±1.8 | 3.7±1.4 | 0.23 |
| Electrocardiographic data | ||||||||
| Atrial fibrillation | 70 (27) | 67 (22) | 102 (26) | 0.35 | 49 (36) | 46 (26) | 64 (38) | 0.03# |
| No conduction abnormalities | 218 (83) | 248 (81) | 331 (84) | 0.75 | 62 (45) | 83 (46) | 60 (35) | 0.09 |
| Right bundle branch block | 26 (9.9) | 36 (12) | 40 (10) | 21 (15) | 32 (18) | 32 (19) | ||
| Left bundle branch block | 20 (7.6) | 22 (7.2) | 22 (5.6) | 5 (3.6) | 10 (5.5) | 16 (9.4) | ||
| Previous permanent pacemaker | - | - | - | - | 41 (28) | 46 (25) | 52 (30) | 0.54 |
| Echocardiographic data | ||||||||
| Ejection fraction, % | 58±11 | 57±11 | 57±11 | 0.45 | 55±11 | 53±10 | 52±11 | 0.45 |
| Ejection fraction <35% | 16 (6.1) | 21 (6.9) | 24 (6.1) | 0.90 | 14 (10) | 24 (13) | 29 (17) | 0.21 |
| Aortic valve area, cm2 | 0.60±0.16 | 0.71±0.19 | 0.71±0.23 | <0.001# | 0.60±0.17 | 0.68±0.18 | 0.70±0.21 | <0.001# |
| Mean gradient, mmHg | 47±12 | 42±14 | 45±14 | <0.001# | 46±12 | 44±13 | 41±14 | 0.02# |
| Computed tomography data | ||||||||
| Aortic annulus perimeter, mm | 75.2±5.2 | 76.7±8.6 | 79.9±7.9 | <0.001# | 75.3±7.2 | 79.3±8.2 | 78.5±7.9 | 0.01# |
| Data are presented as mean±SD or n (%). Some percentages may not add up to 100% owing to rounding. #Indicates statistical significance, i.e., p<0.05. *Missing data on baseline ejection fraction accounted for 2.7% of the next-day discharge cohort. CABG: coronary artery bypass graft; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | ||||||||
Peri- and postprocedural data
Implantation techniques were left to each of the local Heart Team preferences following the current best practices, such as commissural alignment (for ACURATE and Evolut) and cusp overlap (for Evolut).
Pre- and post-dilation were more frequently performed in ACURATE cases compared with Evolut and SAPIEN (99%, 50%, and 14%; and 33%, 13%, and 9%, respectively; p<0.001 for both). The use of the TAVI wire for left ventricular pacing, as opposed to right ventricular pacing, was most common in ACURATE cases (63% vs 17% in Evolut and 35% in SAPIEN; p<0.001) (Table 2).
Echocardiographic data at hospital discharge were similar between groups in terms of mean gradient and aortic valve area, while the LVEF was slightly, but significantly (p<0.001), higher among individuals who received ACURATE compared to Evolut and SAPIEN THVs. Higher rates of mild and moderate paravalvular leakage (PVL) were observed among those who received ACURATE compared with Evolut and SAPIEN THVs (p<0.001) (Table 2). Of note, there were 186 ACURATE neo valves and 78 ACURATE neo2 valves; 19 patients who had moderate PVL received an ACURATE neo while only 1 received an ACURATE neo2. Taking into consideration just the ACURATE neo patients, for the 19 out of the total 264 ACURATE patients, the rate of moderate PVL would be 7.2%, and this is still slightly lower than the reported 9.4% in the SCOPE I9 and 9.6% in SCOPE II10 trials at 30 days.
Table 2. Periprocedural variables and 30-day readmissions according to discharge pathways and the type of valve.
| Variables | Next-day discharge | Excluded from the analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| ACURATE neo/neo2 n=264 |
Evolut PRO/PRO+/FX n=306 | SAPIEN3/Ultra n=393 | p-value | ACURATE neo/neo2 n=138 |
Evolut PRO/PRO+/FX n=180 | SAPIEN3/Ultra n=170 | p-value | |
| Procedural data | ||||||||
| Conscious sedation | 220 (83) | 292 (95) | 370 (94) | <0.001# | 67 (49) | 119 (66) | 131 (77) | <0.001# |
| Valve size | ||||||||
| Small (23 mm) | 58 (22) | - | - | - | 20 (14) | - | - | - |
| Medium (25 mm) | 102 (39) | - | - | - | 60 (44) | - | - | - |
| Large (27 mm) | 104 (39) | - | - | - | 58 (42) | - | - | - |
| 20 mm | - | - | 8 (2.0) | - | - | - | 4 (2.3) | - |
| 23 mm | - | 21 (6.9) | 87 (22) | - | - | 14 (7.8) | 34 (20) | - |
| 26 mm | - | 94 (31) | 185 (47) | - | - | 40 (22) | 79 (47) | - |
| 29 mm | - | 114 (37) | 113 (29) | - | - | 62 (34) | 53 (31) | - |
| 34 mm | - | 77 (25) | - | - | - | 64 (36) | - | - |
| Predilation | 262 (99) | 153 (50) | 55 (14) | <0.001# | 138 (100) | 94 (52) | 25 (15) | <0.001# |
| Post-dilation | 86 (33) | 41 (13) | 37 (9.4) | <0.001# | 60 (43) | 32 (18) | 14 (8.2) | <0.001# |
| Pacing | ||||||||
| Temporary venous pacing | 98 (37) | 254 (83) | 524 (65) | <0.001# | 94 (68) | 156 (87) | 133 (78) | <0.001# |
| TAVI wire pacing | 166 (63) | 52 (17) | 139 (35) | 44 (32) | 24 (13) | 37 (22) | ||
| In-hospital adverse events | - | - | - | - | 62 (45) | 59 (33) | 69 (40) | 0.07 |
| New permanent pacemaker | - | - | - | - | 28 (7.8)* | 52 (12)* | 44 (8.6)* | 0.52 |
| Vascular complications | - | - | - | - | 13 (9.4) | 2 (1.1) | 8 (4.7) | 0.01# |
| Bleeding | - | - | - | - | 15 (11) | 3 (1.7) | 10 (5.9) | 0.01# |
| Major | - | - | - | - | 5 (3.6) | 0 (0) | 2 (1.2) | 0.01# |
| Minor | - | - | - | - | 9 (6.5) | 2 (1.1) | 4 (2.3) | 0.01# |
| Life-threatening | - | - | - | - | 1 (0.7) | 1 (0.5) | 4 (2.3) | 0.25 |
| Stroke | - | - | - | - | 6 (4.3) | 2 (1.1) | 7 (4.2) | 0.15 |
| Transient ischaemic attack | - | - | - | - | 1 (0.7) | 1 (0.5) | 1 (0.6) | 0.64 |
| Non-disabling | - | - | - | - | 2 (1.4) | 1 (0.5) | 0 (0) | 0.35 |
| Disabling | - | - | - | - | 3 (2.2) | 0 (0) | 6 (3.5) | 0.01# |
| Discharge echocardiographic data | ||||||||
| Ejection fraction, % | 62±10 | 61±10 | 59±10 | 0.02# | 60±8.6 | 54±7.1 | 53±9.2 | 0.01# |
| Aortic valve area, cm2 | 1.70±0.37 | 1.75±0.90 | 1.81±0.43 | 0.09 | 1.66±0.32 | 1.78±0.60 | 1.68±0.35 | 0.07 |
| Mean gradient, mmHg | 8.7±4.0 | 8.6±4.1 | 9.1±3.9 | 0.15 | 8.4±4.0 | 8.3±4.1 | 9.0±3.1 | 0.14 |
| Paravalvular leakage | ||||||||
| None/trace | 124 (47) | 210 (69) | 335 (85) | <0.001# | 65 (47) | 115 (64) | 146 (86) | <0.001# |
| Mild | 120 (46) | 91 (30) | 58 (15) | 63 (46) | 55 (30) | 21 (12) | ||
| Moderate | 20 (7.6) | 5 (1.6) | 0 (0) | 10 (7.2) | 10 (5.5) | 2 (1.2) | ||
| Length of overall stay, days | - | - | - | - | 3 (2-8) | 3 (2-8) | 4 (2-8) | 0.25 |
| Previous permanent pacemaker | - | - | - | - | 1 (1-3) | 2 (1-5) | 2 (1-4) | 0.14 |
| New permanent pacemaker | - | - | - | - | 5 (2-10) | 3 (2-8) | 3 (2-8) | 0.32 |
| 30-day readmissions, overall | 24 (9.1) | 23 (7.5) | 30 (7.6) | 0.74 | 23 (17) | 16 (8.9) | 15 (8.8) | 0.04# |
| Cardiac causes | 12 (4.6) | 13 (4.2) | 12 (3.1) | 0.56 | 11 (8.0) | 3 (1.7) | 7 (4.1) | 0.02# |
| New permanent pacemaker | 7 (2.7) | 3 (1.0) | 7 (1.8) | 0.32 | 1 (1.5)a | 1 (1.2)a | 0 (0)a | 0.19 |
| Congestive heart failure | 4 (1.5) | 6 (2.0) | 5 (1.3) | 0.76 | 8 (5.8) | 2 (1.1) | 5 (2.9) | 0.06 |
| Acute coronary syndrome | 1 (0.4) | 0 (0) | 0 (0) | 0.27 | 0 (0) | 0 (0) | 0 (0) | - |
| Arrhythmias | 0 (0) | 2 (0.7) | 0 (0) | 0.12 | 2 (1.5) | 0 (0) | 2 (1.1) | 0.22 |
| Valve related | 0 (0) | 1 (0.3) | 0 (0) | 0.34 | 0 (0) | 0 (0) | 0 (0) | - |
| Non-cardiac causes | 12 (4.6) | 10 (3.3) | 18 (4.6) | 0.64 | 12 (8.7) | 13 (7.2) | 8 (4.7) | 0.36 |
| Stroke/TIA | 1 (0.4) | 3 (1.0) | 3 (0.8) | 0.70 | 3 (2.1) | 2 (1.1) | 1 (0.6) | 0.44 |
| Vascular complications | 1 (0.4) | 2 (0.7) | 1 (0.3) | 0.71 | 0 (0) | 0 (0) | 0 (0) | - |
| Infections | 3 (1.1) | 2 (0.7) | 3 (0.8) | 0.80 | 5 (3.6) | 3 (1.7) | 4 (2.4) | 0.53 |
| Respiratory | 1 (0.4) | 3 (1.0) | 4 (1.0) | 0.64 | 1 (0.7) | 3 (1.7) | 0 (0) | 0.27 |
| Gastrointestinal | 4 (1.5) | 1 (0.3) | 2 (0.5) | 0.20 | 2 (1.5) | 1 (0.6) | 0 (0) | 0.38 |
| Other | 2 (0.8) | 0 (0) | 5 (1.3) | 0.15 | 1 (0.7) | 4 (2.2) | 3 (1.8) | 0.34 |
| Data are presented as mean±SD, median (interquartile range) or n (%). Some percentages may not add up to 100% owing to rounding. #Indicates statistical significance, i.e., p<0.05. *Patients without previous pacemaker. Given the exclusion of this population from next-day discharge, the proportions of new permanent pacemaker implantation were then calculated using the entire cohort of contemporary counterparts, that is, 7.8% for ACURATE, 12% for Evolut, and 8.6% for SAPIEN (p=0.52). aOut of patients without pre- and postprocedural pacemakers. SD: standard deviation; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack | ||||||||
Thirty-day unplanned readmissions
At 30 days, there were no significant differences in all-cause readmissions (ACURATE 9.1% vs Evolut 7.5% vs SAPIEN 7.6%; p=0.74) (Table 2). Overall, the peak of readmissions was on day 5 (8 out of 77) and day 6 (8 out of 77) followed by day 2 (7 out of 77) and day 20 (7 out of 77), and more than half of the readmissions occurred within 7 days of discharge (Central illustration).
Regarding readmissions for cardiac causes, these occurred in 4.6%, 4.2% and 3.1% of ACURATE, Evolut and SAPIEN patients, respectively (p=0.56) (Table 2). Cardiac readmissions occurred more frequently on days 2, 5 and 6 (5 out of 36 for each day), and more than half of these cardiac readmissions happened within 6 days of discharge (Figure 2A).
Readmissions requiring PPI were all related to complete atrioventricular block (CAVB) and occurred in 2.7%, 1.0% and 1.8% of ACURATE, Evolut and SAPIEN patients, respectively (p=0.32) (Table 2); these readmissions occurred more often on day 3 (3 out of 17), day 4 (4 out of 17) and day 5 (3 out of 17) after discharge (Figure 3A). Among individuals with baseline RBBB, one was readmitted with CAVB on day 3 post-discharge in the ACURATE group and one on day 7 post-discharge in the SAPIEN group.
Readmissions for HF occurred in 1.5%, 2.0% and 1.3% of ACURATE, Evolut and SAPIEN patients, respectively (p=0.76) (Table 2), and these readmissions occurred more frequently on day 2 and day 6 (3 out of 15 for each day) after discharge (Figure 3B).
Non-cardiac causes of readmission occurred in 4.6%, 3.3% and 4.6% of ACURATE, Evolut and SAPIEN patients, respectively (p=0.64) (Figure 2B). Non-cardiac readmissions occurred more frequently on day 20 (6 out of 41) followed by day 10 (4 out of 41), and more than half of these non-cardiac readmissions happened within 10 days of discharge (Figure 2B). After NDD, there were 2 deaths during 30 days of follow-up; however, both of these occurred after 30 days of the index TAVI procedure and were secondary to respiratory failure. More specifically, 1 patient in the Evolut group was readmitted with COVID-19 pneumonia at 25 days post-discharge and died 11 days later (36 days after discharge from TAVI), and 1 patient in the SAPIEN group was also readmitted with pneumonia at 10 days post-discharge and died 23 days later (33 days after discharge from TAVI). Although these deaths occurred after 30 days of discharge, the patients had been readmitted during the 30-day readmission period and were therefore classified as non-cardiac readmissions.
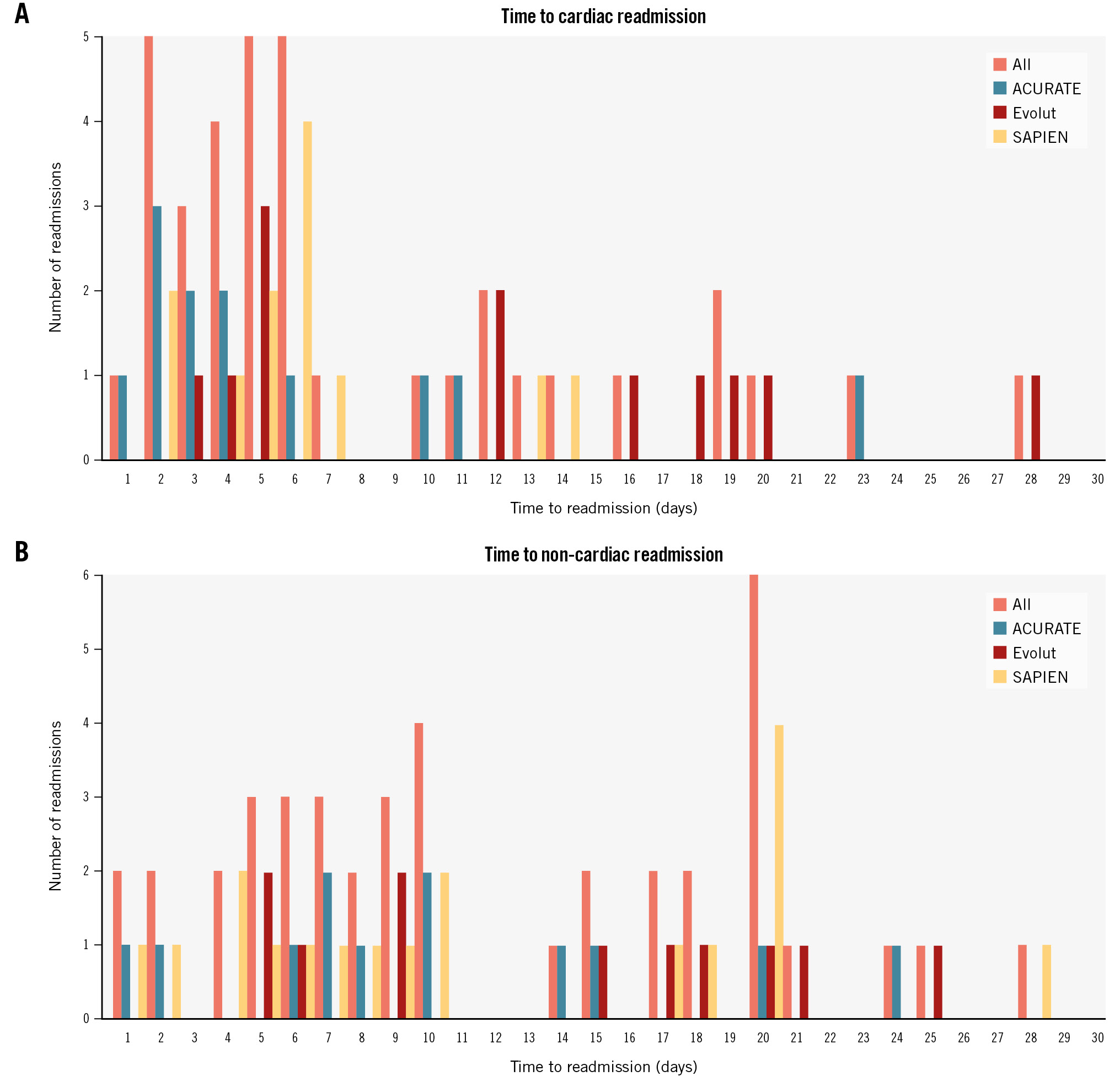
Figure 2. Time to readmission after next-day discharge following TAVI. A) Time to readmission for cardiac causes. B) Time to readmission for non-cardiac causes. ACURATE: ACURATE neo/neo2; Evolut: Evolut PRO/PRO+/FX; SAPIEN: SAPIEN 3/Ultra
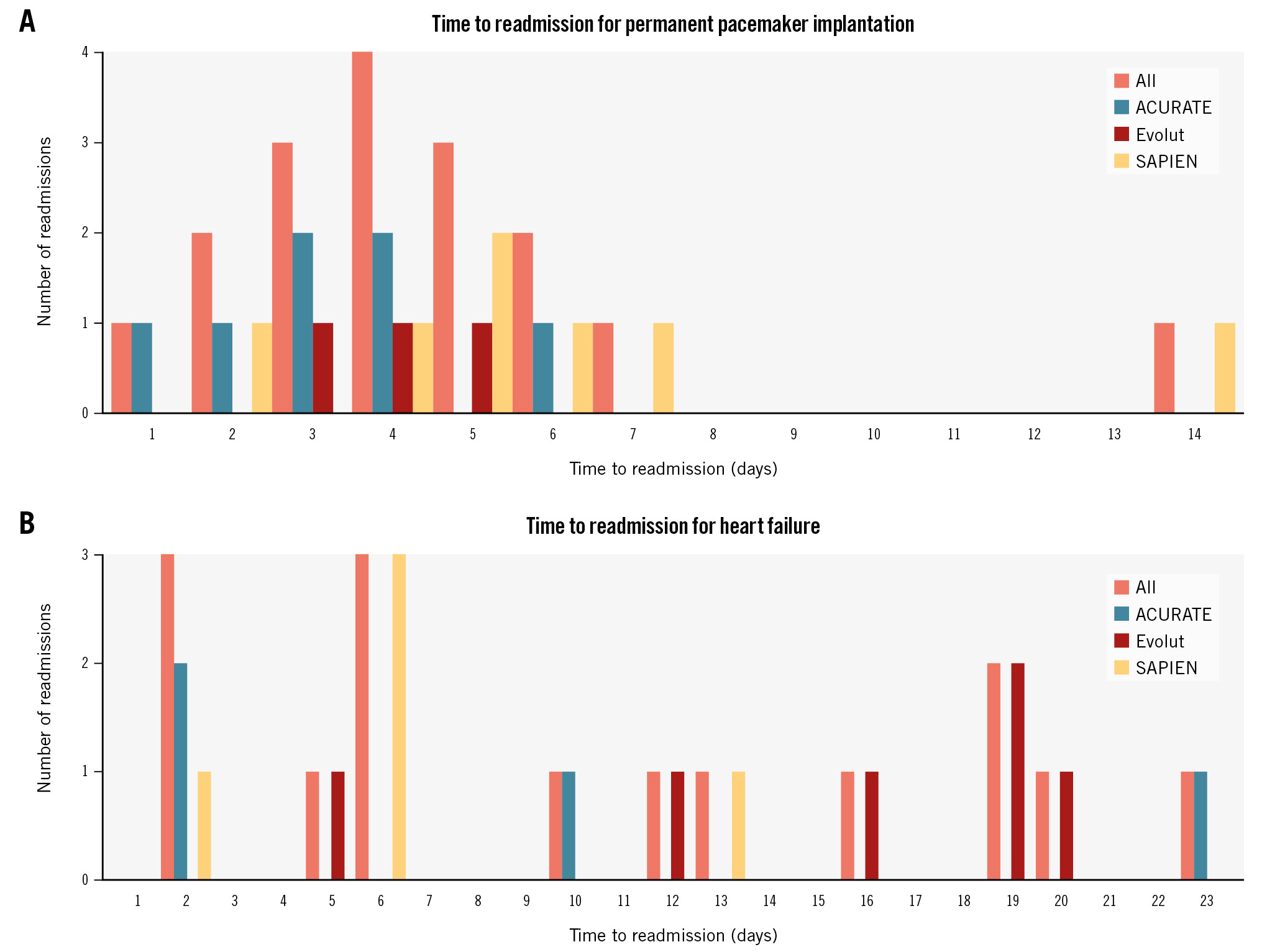
Figure 3. Readmission rates for permanent pacemaker implantation and heart failure. A) Time to readmission for permanent pacemaker implantation. B) Time to readmission for heart failure. ACURATE: ACURATE neo/neo2; Evolut: Evolut PRO/PRO+/FX; SAPIEN: SAPIEN 3/Ultra
Propensity score weighting and IPTW analyses
Because the ACURATE valve cannot accommodate all aortic annulus sizes, potentially leading to confounding, and considering that other clinical and demographic characteristics could introduce a source of bias, a DAG was used to identify the minimally sufficient set of covariates for developing the PS model (Figure 1A). The STS score was excluded from the DAG since it is composed of covariates that are already included for its calculation, hence, avoiding multicollinearity.
The KS tests before and after IPTW for covariates with the most imbalances are shown in Figure 1B and indicate a negligible difference or good balance after IPTW. The findings of the multinomial gradient-boosted IPTW PS model (stage 1) followed by the modified Poisson regression (stage 2) indicate that, using the ACURATE valve as the reference category, the estimated percentage of total readmissions at 30 days was 24% lower (RR 0.76, 95% CI: 0.38-1.52; p=0.38) for those who received an Evolut THV and 26% lower (RR 0.74, 95% CI: 0.44-1.22; p=0.28) for those who received a SAPIEN THV (Central illustration). A sensitivity analysis using a conventional adjusted multivariable linear regression model showed consistent results (RR 0.73, 95% CI: 0.40-1.33; p=0.30 for Evolut, and RR 0.69, 95% CI: 0.40-1.18; p=0.17 for SAPIEN). Despite the lack of statistical significance, the imprecision around the point estimates (wide CIs) must be acknowledged.
Population excluded from the primary analysis
The population of patients excluded from the primary analysis is presented in Table 3 along with statistics compared to the NDD patients. Periprocedural variables are shown in Table 4. As expected, more than 50% of the excluded patients experienced procedure-related complications and the most frequent was the need for new PPI (11.4%). Echocardiographic data at hospital discharge showed a lower LVEF among excluded patients, although no differences were seen in terms of gradients or PVL (Table 4).
The LOS of the excluded population was overall a median of 3 days (interquartile range [IQR] 2-8 days), while the LOS was 2 days (IQR 1-7 days) among the 139 individuals with a pacemaker prior to TAVI. Of these, 90 (65%) patients still followed an NDD pathway, and 23 (14%) patients were discharged 48 hours after TAVI (Table 4).
There was a trend towards a higher 30-day readmission rate among the excluded population compared to the NDD cohort (11% vs 8%; p=0.05). While readmission rates for cardiac causes were similar compared with the NDD cohort (3.7% vs 4.3%; p=0.60), readmissions for non-cardiac causes were significantly higher among excluded patients compared to NDD patients (6.8% vs 4.3%; p=0.04) (Table 4).
The clinical characteristics of the excluded population according to the type of valve are shown in Table 1. The periprocedural aspects of the excluded patients were comparable to those of the NDD cohort (Table 2). In-hospital adverse events were proportionally higher among ACURATE patients, though this did not reach statistical significance (p=0.07) and was driven by vascular and bleeding complications. Patients who received a SAPIEN THV experienced more disabling strokes (p=0.01) (Table 2).
The LOS according to valve type was generally consistent. However, the median LOS among the individuals with a pre-TAVI pacemaker was shorter (1 day [IQR 1-3 days] for ACURATE, 2 days [IQR 1-5 days] for Evolut, and 2 days [IQR 1-4 days] for SAPIEN patients) than those who required new PPI (5 days [IQR 2-10 days] for ACURATE, 3 days [IQR 2-8 days] for Evolut, and 3 days [IQR 2-8 days] for SAPIEN patients) (Table 2).
The 30-day readmission rate was higher among ACURATE patients (17% vs 8.9% in Evolut and 8.8% in SAPIEN patients; p=0.04), and this was driven by cardiac causes (8.0% ACURATE vs 1.7% Evolut and 4.1% SAPIEN; p=0.02), predominantly HF. Readmissions for new PPI were similar (1.5% [n=1] ACURATE vs 1.2% [n=1] Evolut and 0% [n=0] SAPIEN; p=0.19), and these rates are comparable to the NDD cohort and considering the overall cohort of contemporary participants (Table 2).
Table 5 shows the 30-day readmissions among individuals with pre- and post-TAVI permanent pacemakers. Again here, the split of cardiac and non-cardiac readmissions is about 50%, with HF being the leading cause of cardiac readmissions.
The full narrative describing this cohort can be found in Supplementary Appendix 1. The cohort of excluded patients was added upon a peer-review request, therefore, the interpretation of the comparisons warrants caution and is solely provided for a better appreciation of the overall message of the manuscript focused on NDD.
Table 3. Baseline characteristics of the study population according to discharge pathways.
| Variables | Next-day discharge n=963 |
Excluded from the analysis n=488 |
p-value |
|---|---|---|---|
| Baseline characteristics | |||
| Age, years | 81.0±5.2 | 82.1±6.3 | 0.21 |
| Female sex | 445 (46) | 213 (43) | 0.09 |
| Hypertension | 834 (87) | 429 (88) | 0.18 |
| Diabetes | 289 (30) | 161 (33) | 0.24 |
| Previous CABG | 134 (14) | 72 (15) | 0.39 |
| STS-PROM score | 3.2±1.2 | 3.6±1.6 | 0.04# |
| Electrocardiographic data | |||
| Atrial fibrillation | 239 (25) | 159 (33) | 0.01# |
| No conduction abnormalities | 797 (88) | 205 (42) | <0.001# |
| Right bundle branch block | 102 (10) | 85 (17) | |
| Left bundle branch block | 64 (6.6) | 30 (6.1) | |
| Previous permanent pacemaker | - | 139 (29) | - |
| Echocardiographic data | |||
| Ejection fraction*, % | 58±11 | 53±12 | 0.04# |
| Ejection fraction <35% | 64 (6.6) | 67 (14) | <0.001# |
| Aortic valve area, cm2 | 0.66±0.12 | 0.70±0.19 | 0.02# |
| Mean gradient, mmHg | 44±12 | 43±13 | 0.32 |
| Computed tomography data | |||
| Aortic annulus perimeter, mm | 78.1±5.2 | 79.2±7.6 | 0.12 |
| Data are presented as mean±SD or n (%). Some percentages may not add up to 100% owing to rounding. #Indicates statistical significance, i.e., p<0.05. *Missing data on baseline ejection fraction accounted for 2.7% of the next-day discharge cohort. CABG: coronary artery bypass graft; SD: standard deviation; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | |||
Table 4. Periprocedural variables and 30-day outcomes according to discharge pathways.
| Variables | Next-day discharge n=963 |
Excluded from the analysis n=488 |
p-value |
|---|---|---|---|
| Procedural data | |||
| Conscious sedation | 882 (92) | 317 (65) | <0.001# |
| Valve size | |||
| Small (23 mm) | 58 (6.0) | 20 (4.1) | 0.07 |
| Medium (25 mm) | 102 (10) | 60 (12) | 0.34 |
| Large (27 mm) | 104 (11) | 58 (12) | 0.60 |
| 20 mm | 8 (0.8) | 4 (0.8) | 0.67 |
| 23 mm | 108 (11) | 48 (9.8) | 0.42 |
| 26 mm | 279 (29) | 119 (24) | 0.06 |
| 29 mm | 227 (24) | 114 (23) | 0.92 |
| 34 mm | 77 (7.9) | 63 (13) | 0.01# |
| Predilation | 470 (49) | 257 (53) | 0.32 |
| Post-dilation | 164 (17) | 106 (22) | 0.03# |
| Pacing | |||
| Temporary venous pacing | 606 (63) | 382 (78) | <0.001# |
| TAVI wire pacing | 357 (37) | 106 (22) | |
| In-hospital adverse events | |||
| New permanent pacemaker | - | 124 (36)* | - |
| Vascular complications | - | 23 (4.7) | - |
| Bleeding | - | 28 (5.7) | - |
| Major | - | 7 (1.4) | - |
| Minor | - | 15 (3.1) | - |
| Life-threatening | - | 6 (1.2) | - |
| Stroke | - | 15 (3.1) | - |
| Transient ischaemic attack | - | 3 (0.6) | - |
| Non-disabling | - | 3 (0.6) | - |
| Disabling | - | 9 (1.8) | - |
| Discharge echocardiographic data | |||
| Ejection fraction, % | 60±9 | 55±7 | 0.01# |
| Aortic valve area, cm2 | 1.80±0.37 | 1.81±0.42 | 0.25 |
| Mean gradient, mmHg | 8.6±4.0 | 8.8±4.1 | 0.18 |
| Paravalvular leakage | |||
| None/trace | 669 (70) | 326 (67) | 0.14 |
| Mild | 269 (28) | 139 (28) | |
| Moderate | 25 (2.6) | 22 (4.5) | |
| Length of overall stay, days | - | 3 (2-8) | - |
| Previous permanent pacemaker | - | 2 (1-7)a | - |
| New permanent pacemaker | - | 3 (2-8) | - |
| 30-day readmissions, overall | 77 (8.0) | 54 (11) | 0.05 |
| Cardiac causes | 36 (3.7) | 21 (4.3) | 0.60 |
| New permanent pacemaker | 17 (1.7) | 2 (0.9)b | 0.33 |
| Congestive heart failure | 15 (1.6) | 15 (3.1) | 0.04# |
| Acute coronary syndrome | 1 (0.1) | 0 (0) | 0.34 |
| Arrhythmias | 2 (0.2) | 4 (0.8) | 0.86 |
| Valve related | 1 (0.1) | 0 (0.3) | 0.34 |
| Non-cardiac causes | 41 (4.3) | 33 (6.8) | 0.04# |
| Stroke/TIA | 7 (0.7) | 6 (1.2) | 0.33 |
| Vascular complications | 4 (0.4) | 0 (0) | 0.07 |
| Infections | 8 (0.8) | 7 (1.4) | 0.28 |
| Respiratory | 8 (0.8) | 4 (0.8) | 0.64 |
| Gastrointestinal | 7 (0.7) | 3 (0.6) | 0.27 |
| Others | 7 (0.7) | 8 (1.6) | 0.10 |
| Data are presented as mean±SD, median (interquartile range), or n (%). Some percentages may not add up to 100% owing to rounding. #Indicates statistical significance, i.e., p<0.05. *Patients without previous pacemaker. This percentage of new permanent pacemaker implantation appears to be high; however, one must bear in mind that this is among the population that was excluded for NDD; therefore, when the proportion is calculated using the whole cohort of contemporary patients, the true rate of new pacemaker implantation was 11.4%. The same comment applies for the remaining in-hospital complications, which are therefore much lower when applied to the overall cohort. a90 and 23 out of the 139 individuals with previous permanent pacemaker were discharged the next day (65%) or within 48 hours (14%), respectively. bOut of patients without pre- (n=139) and postprocedural (n=124) pacemakers. NDD: next-day discharge; SD: standard deviation; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack | |||
Table 5. Thirty-day readmissions among individuals with pre- and post-TAVI permanent pacemaker.
| Pre-TAVI permanent pacemaker cohort | ACURATE neo/neo2 n=41 |
Evolut PRO/PRO+/FX n=46 |
SAPIEN 3/Ultra n=52 |
p-value |
|---|---|---|---|---|
| 30-day readmissions, overall | 7 (17) | 5 (11) | 7 (14) | 0.70 |
| Cardiac causes | 4 (9.8) | 1 (4.2) | 3 (5.8) | 0.32 |
| Congestive heart failure | 3 (7.3) | 1 (2.2) | 3 (5.8) | 0.52 |
| Acute coronary syndrome | 0 (0) | 0 (0) | 0 (0) | - |
| Arrhythmias | 1 (2.4) | 0 (0) | 0 (0) | 0.12 |
| Valve-related | 0 (0) | 0 (0) | 0 (0) | - |
| Non-cardiac causes | 3 (7.3) | 4 (8.7) | 4 (7.7) | 0.96 |
| Stroke/TIA | 1 (2.4) | 1 (2.2) | 0 (0) | 0.21 |
| Access site complication | 0 (0) | 0 (0) | 0 (0) | - |
| Infections | 1 (2.4) | 1 (2.2) | 2 (3.9) | 0.82 |
| Respiratory | 0 (0) | 2 (4.4) | 0 (0) | 0.19 |
| Gastrointestinal | 1 (2.4) | 0 (0) | 0 (0) | 0.20 |
| Others | 0 (0) | 0 (0) | 2 (3.9) | 0.15 |
| Post-TAVI permanent pacemaker cohort | ACURATE neo/neo2 n=28 |
Evolut PRO/PRO+/FX n=52 |
SAPIEN 3/Ultra n=44 |
p-value |
| 30-day readmissions, overall | 6 (21) | 5 (9.6) | 3 (6.8) | 0.14 |
| Cardiac causes | 3 (11) | 1 (2.2) | 2 (4.6) | 0.21 |
| Congestive heart failure | 3 (11) | 1 (2.2) | 1 (2.3) | 0.12 |
| Acute coronary syndrome | 0 (0) | 0 (0) | 0 (0) | - |
| Arrhythmias | 0 (0) | 0 (0) | 1 (2.3) | - |
| Valve-related | 0 (0) | 0 (0) | 0 (0) | - |
| Non-cardiac causes | 3 (11) | 4 (7.7) | 1 (2.3) | 0.32 |
| Stroke/TIA | 1 (3.6) | 1 (2.2) | 0 (0) | 0.23 |
| Access site complication | 0 (0) | 0 (0) | 0 (0) | - |
| Infections | 0 (0) | 1 (2.2) | 1 (2.3) | 0.21 |
| Respiratory | 0 (0) | 0 (0) | 0 (0) | - |
| Gastrointestinal | 1 (3.6) | 0 (0) | 0 (0) | 0.18 |
| Others | 1 (3.6) | 2 (3.9) | 0 (0) | 0.11 |
| Data are presented as n (%). Some percentages may not add up to 100% owing to rounding. TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack | ||||
Discussion
The present multicentre study including 963 all-comers discharged to home the day after TF-TAVI underscores the safety of an NDD pathway, even more so considering a population of pacemaker-naïve patients and using 3 different commercially available THVs. The NDD strategy was not associated with an increased risk of rehospitalisation for cardiac or non-cardiac causes, nor deaths during 30 days of follow-up, irrespective of the utilisation of 3 different types of THV. Importantly, similar rates of readmission for conduction disturbances requiring PPI and HF were observed among the 3 types of THVs (Central illustration).
Length of stay after TAVI
Although the LOS after TAVI has been decreasing in recent years, the average LOS remains 5-6 days in contemporary studies111213. Larger-volume TAVI centres have been contributing to a greater extent to the adoption of ED protocols relative to low- and intermediate-volume centres314. Indeed, early adopter studies have shown that NDD is attainable in about 23%15 and 29%5 of patients; however, 11% to 15% of them had preprocedural PPI and received a SAPIEN THV515. Our report contributes to the literature with relevant data showing that, in contemporary all-comers, NDD is attainable in 65% of patients without pre- or post-TAVI PPI; hence, this proportion would be much higher if we consider the above-mentioned 90 patients with preprocedural pacemakers or those who needed a PPI after TAVI yet followed an NDD pathway.
A prolonged LOS after TAVI is most commonly attributed to conduction disturbances requiring in-hospital telemetry monitoring or waiting for PPI, vascular complications/bleeding, and stroke. Other than these, unnecessarily prolonged immobilisation, or bed rest, leads to rapid deconditioning, mainly in the elderly, and this practice should be changed. Early mobilisation soon (2-4 h) after TAVI is the strategy for a prompt return to baseline status, specifically in elderly patients.
Patients with high-risk features on their baseline ECG (i.e., RBBB) may benefit from early morning scheduling for TAVI, such that if PPI is required, this can be done in the afternoon, allowing for NDD.
Unplanned readmissions
The safety of NDD and ED with regard to discharge-to-30-day outcomes after TAVI has been explored in the Vancouver Multidisciplinary, Multimodality, But Minimalist Approach to Transfemoral Transcatheter Aortic Valve Replacement (3MTAVR) and Feasibility And Safety of Early Discharge After Transfemoral Transcatheter Aortic Valve Implantation (FAST-TAVI) studies; however, the 3MTAVR study included selected patients, and both studies used a SAPIEN THV516.
Albeit in an all-comers population of mainly octogenarians, the overall all-cause readmission rate at 30 days was 8% following an NDD pathway, which compares favourably with the 11% observed in the excluded population (Table 4). Only 2 (0.2%) patients died, 1 at 33 days and 1 at 36 days after discharge from TAVI, and these deaths occurred during readmissions for pneumonia (1 COVID-19 related). Hence, there were no deaths attributable to cardiac causes. The overall all-cause readmission rate at 30 days was 11% among the excluded population, while 2 deaths (0.4%) occurred (1 cardiac and 1 non-cardiac). Our results following an NDD pathway compare favourably with previous studies that showed rates of mortality between 0% and 2.2% and readmissions between 9% and 10%2516 among patients who followed an ED strategy. About half of the readmissions were for cardiac causes, and these were almost evenly distributed among those presenting with conduction disturbances that required PPI or HF.
The present multicentre study also shows the absence of differences in overall all-cause unplanned readmissions across the 3 valve groups, regardless of procedural differences. This finding is relevant because, as one would expect, pre- and post-dilation were more frequently performed in self-expanding THVs than balloon-expandable THVs (Table 2) (p<0.001), yet, for instance, the rate of stroke was similar after discharge up to 30 days, as was also observed in the population excluded for NDD (Table 2, Table 4).
Readmissions for complete heart block
One of the Achilles’ heels of TAVI remains the need for periprocedural PPI, and this matter also extends to patients who did not require PPI during the index admission for TAVI but who still carry the potential risk in the following days and weeks after discharge171819202122. This is the main reason we decided to include only pacemaker-naïve patients in the analysis, again, providing a better understanding of this matter.
The ACURATE neo/neo2 THVs have been associated with a low incidence of conduction disturbances leading to PPI232425; however, this device has been underrepresented in studies of ED and NDD pathways. Interestingly, even though not statistically significant, there was a higher proportion of ACURATE patients (2.7%) who required PPI from discharge to 30 days, as compared to their Evolut (1.0%) and SAPIEN (1.8%) counterparts, thereby highlighting the safety of NDD with the Evolut platform using current best practices, most precisely, the cusp-overlap technique22. These results are particularly noteworthy when compared to the rates observed in the excluded population (Table 2).
The proportion of patients with baseline RBBB was similar among the 3 types of THVs, and only 2 patients with baseline RBBB were readmitted with CAVB, 1 with a large ACURATE neo2 on day 3 and 1 with a 29 mm SAPIEN 3 on day 7 after discharge.
The paradigm shift of ED/NDD pathways after TAVI has raised some concerns about shifting the ultra-short LOS at the cost of early readmissions for PPI – in other words, shifting the timing of PPI. Ream et al20 discharged pacemaker-naïve patients after TAVI with a real-time ambulatory event monitor and found delayed (>48 hours) high-grade atrioventricular block (AVB) that required PPI in 10% of patients, and the median time to high-degree AVB was 6 days (range 3 to 24 days) after TAVI20. From this perspective, our time to readmission for PPI shows a peak time on day 4 after discharge, and, again, only 1 patient was readmitted with CAVB in the afternoon of day 1 (day 2, strictly speaking for administrative purposes) after discharge (Figure 3A). This patient had a normal ECG at baseline and no periprocedural conduction abnormalities or abnormalities on telemetry monitoring (criteria for NDD). In other words, only 1 (out of the 963 patients, 0.1%) readmission would have been avoided if the patient had remained in the hospital for 48-72 hours following TAVI.
Regardless of the absence of all the well-known predictors for AVB and the need for PPI, there appears to be a rather small proportion of patients (like the one just described above) without conduction disturbances at baseline or after TAVI that will still develop high-degree AVB and require PPI after discharge up to 30 days181920. Furthermore, many patients with delayed AVB captured by an ambulatory event monitor may not develop symptoms and, therefore, may not seek attention1920.
Readmissions for heart failure
Among the total 30-day readmissions, HF-related readmissions accounted for 19.5%, and one should bear in mind that we did not exclude patients with an impaired LVEF (~7% patients with LVEF <35%), which has been a key exclusion criterion in previous ED pathways22. The nature of this issue is complex and appears to be beyond changes in medication pre-/post-TAVI, highlighting the impact of cardiac damage and long-term HF phenotype in post-TAVI patients2627. As a matter of fact, the readmission rates for HF were lower in the NDD cohort compared to the non-NDD group (Table 2, Table 4), making our results even more compelling.
Limitations
The main limitation of this study is related to the non-randomised nature of the analysis; however, although a randomised controlled trial would help determine the ideal pathway after TAVI, in the absence of periprocedural complications that lead to a clinically indicated prolonged LOS, this type of trial would be difficult to undertake. Second, even though the ACURATE valve cannot be used in the larger range of aortic annulus sizes − the upcoming iteration of ACURATE, the Prime XL (Boston Scientific), may add further information with regard to larger-size THVs − the results of the IPTW propensity score model analysis is consistent with the overall cohort. Third, missing data on baseline ejection fraction accounted for 2.7% of the data. While the IPTW approach used in this manuscript could address this missingness under the assumption that the data are “missing at random”, we chose not to proceed with this assumption due to concerns about potential biases it could introduce in the estimates (because we are unable to verify the validity of this assumption based on the information we have). Fourth, even though we provide enough granularity of data, which is what we believe is one of the major strengths of this manuscript, we lack information in terms of medications pre- and post-TAVI; nonetheless, this information is scarcely available in the literature for comparison purposes. Finally, this study was conducted in high-volume centres of excellence for the treatment of valvular heart disease; therefore, bed turnover is of paramount importance for efficiency in healthcare deliverables.
Conclusions
In pacemaker-naïve patients undergoing TF-TAVI with newer-generation THVs, NDD was not associated with a negative impact on overall 30-day readmissions, cardiac or non-cardiac readmissions, readmissions for PPI or HF after discharge, or mortality, regardless of the type of THV. Our results may help the expansion of knowledge around NDD pathways in order that they may become standard practice.
Impact on daily practice
The present study further supports next-day discharge pathways in all-comer pacemaker-naïve patients undergoing transfemoral transcatheter aortic valve implantation with current commercially available ACURATE, Evolut and SAPIEN valves. Next-day discharge was safe and not associated with a negative impact on overall 30-day readmissions, cardiac or non-cardiac readmission rates, readmissions for a new pacemaker or heart failure, or mortality after discharge. These results may help the expansion of knowledge around next-day discharge pathways in order that they may become standard practice.
Funding
This investigator-initiated study was carried out without funding, and none of the TAVI companies contributed to any steps of the process, including study conception and design, data collection, data analysis, data interpretation, and writing and reporting of the results of this manuscript.
Conflict of interest statement
R. Bagur is a consultant and proctor for Medtronic. M.W.A. Chu has received speaker honoraria from Medtronic, Edwards Lifesciences, Terumo Aortic, and Artivion Inc. P. Diamantouros is a consultant and proctor for Boston Scientific. J.G. Webb is a consultant to Edwards Lifesciences; and receives research funding from Edwards Lifesciences, Boston Scientific, and Medtronic. G.F. Attizzani is a consultant, proctor and is on the advisory board for and receives research grants from Medtronic; he is also a consultant for Abbott and Boston Scientific. The other authors have no conflicts of interest relevant to the content of this manuscript to declare.
Supplementary data
To read the full content of this article, please download the PDF.

