With an increasing choice of transcatheter aortic valve implantation (TAVI) to treat younger aortic valve stenosis (AS) patients, it can be anticipated that (1) TAVI will be used more often to treat bicuspid AS, as the latter is more often encountered in young patients, and (2) there will be an increasing need for redo-TAVI in the future. In general, there is more limited evidence on the performance of TAVI in bicuspid AS, as this has been kept out of the large randomised trials. Still, there are relatively good registry data on the safety and efficacy of TAVI with the balloon-expandable SAPIEN (Edwards Lifesciences) and self-expanding Evolut (Medtronic) transcatheter aortic valves (TAVs) to treat severe bicuspid AS12. Unfortunately, there are no data available on redo-TAVI feasibility in patients with bicuspid AS. Redo-TAVI in the self-expanding Evolut can present challenges because of the supra-annular leaflet position, which may result in a higher functional neoskirt in case of redo-TAVI. This study aimed to assess redo-TAVI feasibility and coronary accessibility following index TAVI with an Evolut TAV in patients with bicuspid AS using post-TAVI computed tomography (CT) data.
In this retrospective analysis, we included 175 patients with native bicuspid AS who were treated with an Evolut TAV in 10 centres in Europe between March 2017 and February 2023; it was necessary for the patients to have a post-TAVI CT available in order to be eligible for this study. Of these patients, 105 were prospectively enrolled in the BIVOLUTX trial and the additional 70 patients were retrospectively added to this cohort. For the redo-TAVI simulations, virtual SAPIEN 3 (S3) TAVs were implanted at 3 different positions in the functional leaflet region of the Evolut, namely with the S3 outflow positioned at Evolut nodes 4, 5 and 6. The virtual implantation of the S3 within the Evolut used the expanded Evolut diameters published in a recent in vitro redo-TAVI study3. The risks of coronary flow compromise (low, high) and coronary inaccessibility (low, challenging, high) were assessed for each coronary artery, according to the previously published algorithm by Grubb et al4. The CT images were analysed with 3Mensio software (Pie Medical Imaging).
The study population mainly included type 1 bicuspid aortic valves (93%) with a mean aortic annulus diameter of 26.0±2.4 mm, a sinotubular junction (STJ) diameter of 32.2±3.9 mm and a left and right coronary artery height of 16.8±3.8 mm and 18.3±3.8 mm, respectively. The predicted risk of coronary flow compromise (high risk: red; low risk: green) and coronary inaccessibility (high risk: red; challenging access: yellow; low risk: green) are shown in the Central illustration. As expected, the simulations showed that an increasingly higher S3 implantation is associated with an increasing risk of coronary flow compromise and coronary inaccessibility. Based on our CT analysis and simulations, redo-TAVI would be unfeasible in 15%, 26% and 38% of all Evolut-in-bicuspid AS cases with an S3 outflow implantation at nodes 4, 5 and 6, respectively. In comparison, Grubb et al previously assessed redo-TAVI to be unfeasible in 20%, 47% and 75% of all Evolut-in-tricuspid AS cases with similar S3 outflow simulations at nodes 4, 5 and 64.
Clearly, multiple factors will determine these risks in case of redo-TAVI: (1) the underlying anatomy, (2) the choice of the first TAV type, size and implant depth, (3) the choice of the second TAV type, size and implant depth, and (4) the possible use of leaflet modification techniques (although the latter would not be useful in case of severe commissural misalignment, i.e., 24.8% of cases in this cohort).
The explanation for the better redo-TAVI feasibility outcomes in this study as compared to Grubb et al4 lies in the fact that native bicuspid AS is often associated with a larger aortic root and, consequently, larger valve-to-aorta distances (STJ/aortic annulus diameter ratio: 1.24 in this bicuspid study versus 1.14 in the tricuspid study by Grubb et al). In addition, a calcified raphe in type 1 bicuspid AS may act as a barrier and hold the TAV stent frame at a distance from the coronary ostium (average raphe length: 13.2 mm vs 11.4 mm for patients in the green/yellow versus red risk category, respectively). On the other hand, TAVI operators may aim for a slightly higher Evolut implant position in bicuspid AS (mean implant depth 3.8 mm in this cohort) and sometimes “downsize” the Evolut TAV in excessively calcified anatomies. All these aspects should be weighed against each other when planning (redo-) TAVI in a young patient with an anticipated long(er) life expectancy. As bicuspid AS is more prevalent in young patients, these study results are encouraging when considering TAVI expansion to these younger patients. More data on the durability of TAVs in bicuspid AS are still pending.
In conclusion, redo-TAVI feasibility and coronary accessibility following index TAVI with an Evolut valve are more favourable in patients with bicuspid AS compared to tricuspid AS. The most favourable outcome regarding redo-TAVI feasibility was obtained with a low(er) S3 implantation.
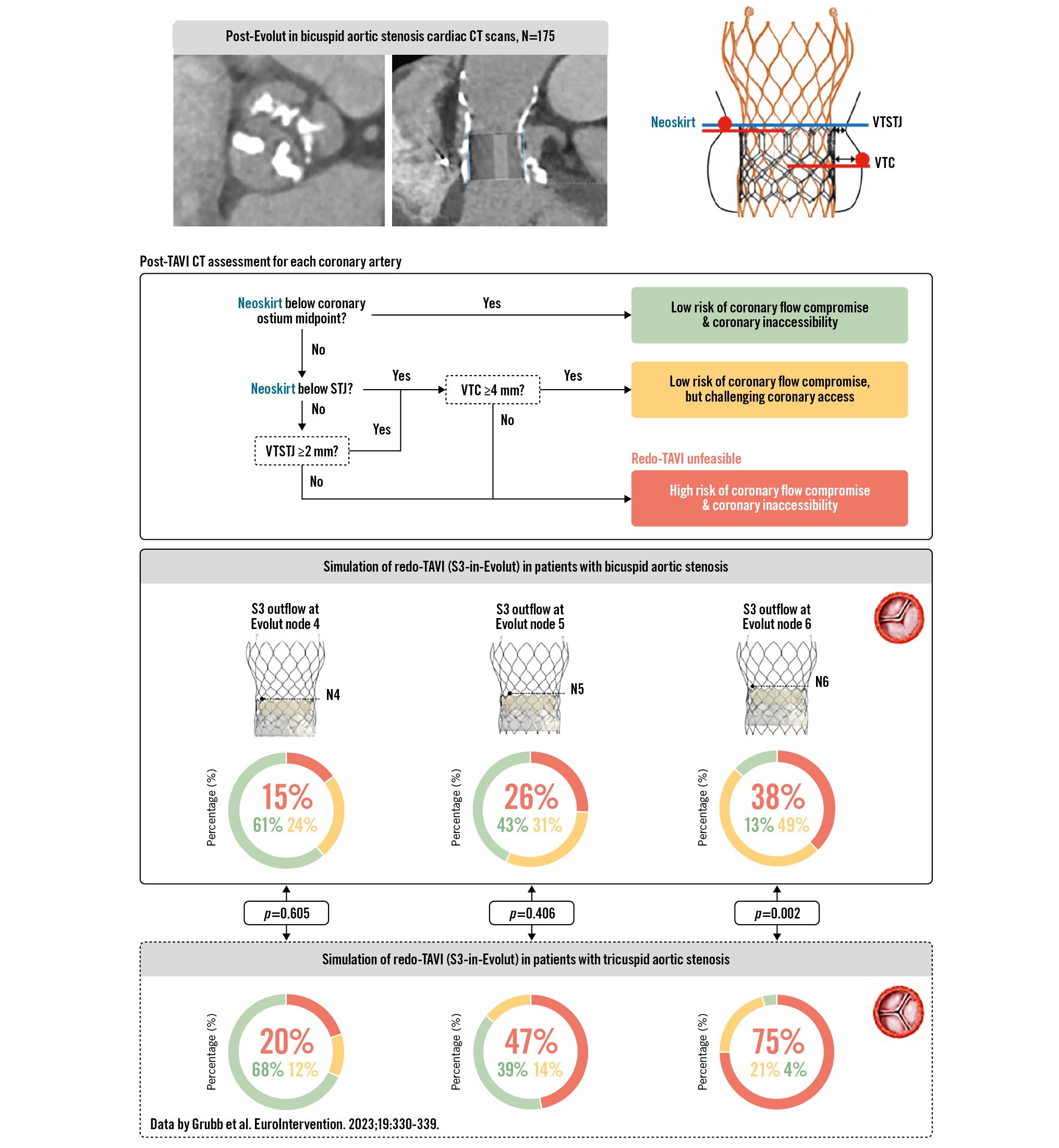
Central illustration. Redo-TAVI feasibility and coronary accessibility following index TAVI with Evolut in bicuspid AS. For the redo-TAVI simulations, virtual SAPIEN 3 (S3) TAVs were implanted at 3 different positions in the index Evolut TAV, namely with the S3 outflow at Evolut nodes 4, 5 and 6. The risks of coronary flow compromise and inaccessibility following redo-TAVI in our 175 bicuspid AS patients were assessed and compared with the risks of redo-TAVI in tricuspid AS, as previously reported by Grubb et al4. Statistical comparisons for redo-TAVI unfeasibility (red) were performed for all three S3 positions using a chi-square test. AS: aortic stenosis; CT: computed tomography; N: node; TAV: transcatheter aortic valve; TAVI: transcatheter aortic valve implantation; VTC: valve-to-coronary distance; VTSTJ: valve-to-sinotubular junction distance
Conflict of interest statement
K. Arslani has received a research grant from the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner Foundation and the Swiss National Science Foundation (P500PM_202963), Switzerland, outside of the submitted work. O. De Backer received institutional research grants and consulting fees from Abbott, Boston Scientific, and Medtronic. G.H.L. Tang has received speaker honoraria and served as a physician proctor, consultant, advisory board member, TAVR publications committee member, RESTORE study steering committee member, APOLLO trial screening committee member and IMPACT MR steering committee member for Medtronic; has received speaker honoraria and served as a physician proctor, consultant, advisory board member and TRILUMINATE trial anatomic eligibility and publications committee member for Abbott; has served as an advisory board member for Boston Scientific and JenaValve; a consultant and physician screening committee member for Shockwave Medical; a consultant for NeoChord, Peija Medical, and Shenqi Medical Technology; and has received speaker honoraria from Siemens Healthineers. K.J Grubb is a consultant for or has received an honorarium from Medtronic, Abbott, Boston Scientific, Ancora, 4C Medical, and OpSens. N.M. Van Mieghem has received research grant support from Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Teleflex, and PulseCath BV; also advisory fees from Antares, JenaValve, Polares Medical, Siemens, Abbott, Boston Scientific, Medtronic, Teleflex, and PulseCath BV. D. Mylotte is a consultant for Medtronic, Boston Scientific, and MicroPort; and received institutional research grants from Boston Scientific. D. Tchétché is a consultant for Abbott Vascular, Edwards Lifesciences, Medtronic, Boston Scientific, T-Heart, and Caranx Medical. V.N. Bapat is a consultant for Abbott Structural, Medtronic, Boston Scientific, and Edwards Lifesciences. L. Leroux is a proctor for Medtronic. G. Tirado-Conte has no conflicts of interest to declare.

