More than 12% of Americans ≥65 years old suffer from aortic stenosis (AS)1. Transcatheter aortic valve (TAV) implantation (TAVI) has become an established treatment for severe AS and is performed in growing numbers of patients. It is estimated that >300,000 TAVI procedures are performed globally each year. Nowadays, low-risk patients who undergo TAVI have a longer life expectancy than the implanted valve’s lifespan. Therefore, more and more valve deterioration cases are expected to be seen, in which redo-TAVI will be required.
A high percentage (35-71%) of patients are unable to undergo a valve-in-valve TAVI procedure due to the high associated risks of sinus sequestration, coronary occlusion, or prevention of future coronary reaccess2. If the orifice of the two coronaries is located behind the leaflets of the aortic valve, there is an immediate risk of obstruction of the blood flow into the coronaries when the cusps of the replaced valve are pushed by the new implant34.
The projection for the next 10 years is for the number of valve-in-valve procedures to increase by 400% (courtesy of P. Généreux, et al). It is expected that once the device is approved, cardiologists will opt to cut out a “window” for blood flow in all “critical” cases of TAV-in-TAV (where there is a clear risk of obstruction) as well as in many “marginal” cases in order to ensure adequate blood flow to the coronaries.
The Splitter device (HVT Medical) performs leaflet modification by cusp splitting and partial leaflet excision before TAVI in surgical aortic valve (SAV) and TAV procedures to reduce the risk of coronary occlusion (Moving image 1). The Splitter creates an intentional U-shaped excision of the valve’s cusp tissue by a steerable catheter with an electrocutting wire loop running inside a cutting head that mimics alligator jaws. The U-shaped track is 12x3 mm; however, the actual opening in the leaflet is somewhat larger (Figure 1F). It is operated by two handles (Figure 1A) placed on the bedside and connected to any standard electrosurgical generator.
The procedure is guided by fluoroscopy and echocardiography (Moving image 2 and Moving image 3). The catheter rides a standard guidewire for TAVI; hence, it is streamlined and completely integrated into the TAVI workflow (Figure 1A, Figure 1B, Figure 1F). The device is designed to steer through the aortic arch curvature (Figure 1C, Figure 1G, Figure 1H, Moving image 3).
The Splitter was tested for proof-of-concept by utilising 3 methods. First, bench tests of the cutting mechanism were carried out on various types of tissues, such as processed pericardium, ex vivo calf/pig valve leaflets, an ex vivo human heart (Figure 1F) and explanted calcified human cusps (2-4 mm thick) with several calcification “bumps” (Figure 1I, Figure 1J). Second, bench testing took place on a pulsating silicone human heart model (Figure 1C). Third, in vivo testing with a longer Splitter device was carried out via the abdominal aorta to test the steering of the cutting head and grasping of the selected cusp in a live animal (Figure 1D, Figure 1E, Figure 1G, Figure 1H).
The next set of tests are planned with the full-size system in pigs via femoral access to demonstrate device safety and usability.
Currently, there are few other devices for valve leaflet modification in development or in clinical phases. The Splitter offers several advantages over these devices, including: (1) precise control of the cutting site, (2) stationary and stable grasping jaws during excision (no pulling forces), while a running wire with electrical energy performs a precise, U-shaped excision; (3) the excised tissue is safely trapped in the jaws and removed through the catheter without embolisation; (4) it confirms a successful cut; (5) it can be fully integrated into the TAVI workflow.
The Splitter offers a novel mechanism for the leaflet modification armamentarium prior to TAV-in-TAV/SAV and enables expansion of the options for structural interventions for AS patients.
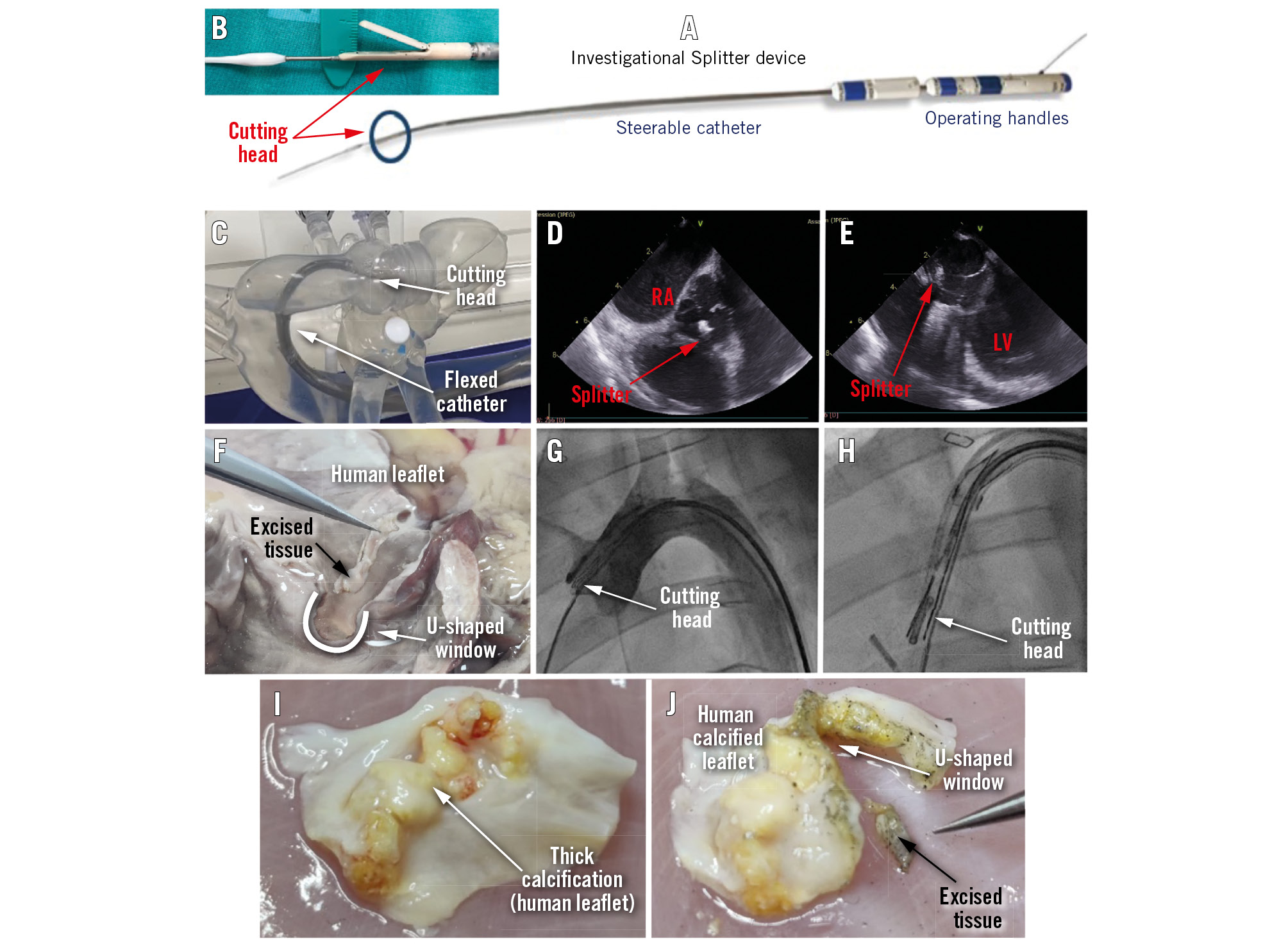
Figure 1. The Splitter system. A) An image of the Splitter system. B) A close-up image of the Splitter's cutting head. C) The Splitter inside a human heart silicone model, showing how the cutting head reaches the valve. D) The Splitter's cutting head as demonstrated by ICE during a porcine study (short-axis view). E) The Splitter's cutting head echo image during a porcine study (long-axis view). F) Precise excision of leaflet tissue and creation of a large U-shaped window in the cusp, demonstrated during an ex vivo test on human heart tissue. G) The Splitter's cutting head placed on the right coronary cusp as demonstrated by fluoroscopy imaging during a porcine study. H) Fluoroscopy image of the cutting head grasping a pigtail next to the right coronary cusp during a porcine study. I) Explanted human calcified leaflet (native). J) The same human calcified leaflet after cutting with the Splitter; the U-shaped window and the excised tissue are clearly observed. ICE: intracardiac echocardiography; LV: left ventricle; RA: right atrium
Conflict of interest statement
A. Kerner and Y. Feld are the founders of HVT Medical. S. Lewkowicz is CEO of HVT Medical. T. Fogel is an employee of HVT Medical.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Animation of Splitter delivery, deployment and cutting procedure.
Moving image 2. Echocardiography video of the leaflet captured by the Splitter in an animal study on a porcine model (with Doppler of aortic flow demonstrating AR due to cusp grasping).
Moving image 3. Cine-fluoroscopy video of the Splitter’s cutting head reaching the right cusp via abdominal aortic access during a porcine animal study.
