Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Percutaneous large-bore arteriotomy closure devices are either suture- or plug-based. The comparative efficacy and safety of both techniques and optimal patient selection remain controversial.
Aims: We aimed to conduct a patient-level meta-analysis of randomised trials comparing suture-based ProGlide versus plug-based MANTA large-bore vascular closure devices (VCDs).
Methods: We searched PubMed, the Cochrane Central Register of Controlled Trials, and Google Scholar for randomised controlled trials comparing vascular closure with the ProGlide-based and the MANTA-based technique. The primary endpoint of this analysis was access site-related vascular complications defined according to the Valve Academic Research Consortium-3 criteria.
Results: We identified 2 trials that enrolled a total of 722 patients undergoing transcatheter aortic valve implantation. The primary endpoint was significantly less common after vascular closure with the ProGlide-based technique (odds ratio [OR] 0.54, 95% confidence interval [CI]: 0.35-0.82). Access site-related bleeding events were also less common with the ProGlide-based technique (OR 0.41, 95% CI: 0.18-0.94). Prespecified subgroup analyses did not reveal any subgroup favouring the plug-based technique. Clinical outcomes with the MANTA-based technique were better in larger-sized vessels. Patients who received the ProGlide-based technique were less likely to undergo endovascular stenting or vascular surgery (OR 0.22, 95% CI: 0.06-0.79).
Conclusions: In this patient-level meta-analysis of randomised trials, the ProGlide-based technique for large-bore arterial access was superior to the MANTA-based technique in terms of vascular and bleeding complications.
Vascular closure is an integral aspect of percutaneous procedures requiring large-bore arterial access, and closure device failure resulting in vascular complications is associated with increased morbidity and mortality123. The need for safe and effective percutaneous closure techniques following large-bore arterial access is expanding, particularly with the increasing volumes of transcatheter aortic valve implantation (TAVI), endovascular aortic repair, and temporary transcatheter mechanical circulatory support4567. For many years, suture-based vascular closure devices (VCDs) were the only available option for large-bore arteriotomy closure. Closure techniques utilising the ProGlide VCD (Abbott) demonstrated fewer vascular and bleeding complications compared to other commercially available suture-based devices89. For closing large-bore vascular access, two ProGlide VCDs are typically applied in a crossed configuration. However, excellent outcomes have also been reported with parallel deployment10, and the combination of one or more ProGlide VCDs with a small plug-based VCD (e.g., Angio-Seal [Terumo]) has also demonstrated considerable success and is gaining wider adoption1112. Recently, a variety of dedicated non-suture-based large-bore vascular closure techniques have been developed, and among these, the plug-based MANTA VCD (Teleflex) has emerged as the most extensively studied and widely used device. The MANTA VCD utilises a sandwich technique, combining intraluminal polymer toggles with an extraluminal collagen plug to close the arterial puncture site13. Two randomised controlled trials (RCTs) have compared the suture-based ProGlide and the plug-based MANTA techniques in patients undergoing transfemoral TAVI. The MASH-TAVI Trial found no significant differences between the two techniques in terms of access site bleeding or vascular complications14. However, the trial may have been underpowered. The larger CHOICE-CLOSURE trial reported a higher incidence of access site-related vascular complications with the MANTA-based technique15. Several meta-analyses combining both RCTs and observational studies found no significant differences between the two closure techniques in terms of access site-related vascular complications91617. However, a recent meta-analysis of published results from RCTs and observational studies yielded conflicting findings. Observational studies reported fewer vascular complications with the MANTA-based technique, while RCTs favoured the ProGlide-based technique18. This discordant outcome suggests the presence of relevant confounding factors in the published observational studies. To date, a patient-level meta-analysis of published RCTs allowing meaningful subgroup comparisons has not been performed. Additionally, CHOICE-CLOSURE and MASH-TAVI used partially different endpoint definitions, and neither assessed endpoints according to the most recent Valve Academic Research Consortium (VARC)-3 criteria19. To address these limitations, we conducted an individual patient-level meta-analysis of RCTs comparing the ProGlide-based technique and the MANTA-based technique in large-bore (≥12 Fr) arterial access procedures utilising contemporary endpoint definitions.
Methods
Search strategy and selection criteria
The reporting of this collaborative meta-analysis follows the Preferred Reporting Items for Systematic reviews and Meta-Analyses of Individual Patient Data (PRISMA-IPD) statement (PRISMA-IPD Checklist is provided in Supplementary Appendix 1)20. We searched PubMed, the Cochrane Central Register of Controlled Trials, and Google Scholar for trials registered or published between 1 January 2016 and 1 September 2024. The search period started in the year of the European Conformity (CE) certification of the MANTA VCD. The online search was performed in September 2024. Specific search strategies are detailed in Supplementary Appendix 2. Eligible for inclusion in this meta-analysis were registered or published RCTs comparing the ProGlide-based with the MANTA-based technique in patients undergoing procedures with large-bore (≥12 Fr) arterial access. Only trials whose investigators agreed to collaborate and provide individual patient data were included. All included trials had individual ethical approval.
Data extraction
Two independent investigators (O. Dumpies and A. Jobs) conducted a literature search using the pre-established search terms. All studies that did not meet the eligibility criteria were excluded. In cases of discrepancy, the results were discussed with a third investigator (M. Abdel-Wahab). Patient-level data were requested for studies that met the eligibility criteria. A prepared datasheet was provided to the coordinating investigators for data extraction to ensure uniform coding of data, and all trial data were then combined into a single database. Discrepancies, inconsistencies, and incomplete data were checked against published reports to ensure the integrity of the combined database. In addition, missing data were retrospectively extracted from the original trial documentation by the respective coordinating investigator and their trial team. The risk of bias was assessed by two independent investigators (O. Dumpies and A. Jobs) according to the scheme provided by the Cochrane Handbook for Systematic Reviews of Interventions21 (Supplementary Table 1).
Study outcomes
The primary endpoint of this analysis was in-hospital major and minor main access site-related vascular complications, defined according to VARC-319. A detailed definition of access site-related vascular complications with the respective components is given in Supplementary Table 2. The primary endpoint was also analysed in predefined subgroups: sex (female vs male), age (75 years or younger vs older than 75 years), body mass index, anticoagulation (no vs yes), peripheral artery disease (no vs yes), access site common femoral artery diameter (less than 7 mm vs at least 7 mm), distribution of access site vascular calcification (non-anterior vs anterior), severity of access site vascular calcification (none vs mild vs moderate vs severe [according to the MASH-TAVI classification14], puncture height (at or above the centre of the femoral head vs below the centre of the femoral head), and access site puncture guidance (ultrasound vs angiographic guidance). Secondary endpoints included all-cause mortality; the individual endpoints of access-related major and minor vascular complications (VARC-3); bleeding events (VARC-3); vascular closure device failure according to VARC-3 – with the exception that the single use of a small plug-based VCD (Angio-Seal) after ProGlide use was not considered a VCD failure but part of the vascular closure strategy; and vascular stenting or vascular surgery due to VCD failure. Endpoints were evaluated based on the fulfilment of the individual VARC-3 criteria, without additional adjudication from the initial clinical event committees.
Statistical analysis
All analyses were conducted following the intention-to-treat principle. The primary analyses were based on a one-stage meta-analysis. Binary clinical outcomes were assessed using a logistic regression model. Due to the anticipated low number of studies and patients, a less complex model with the trial as a dummy variable was initially prespecified. However, EuroIntervention required that we adjust the primary analysis to a model with stratified intercepts to account for baseline risk differences across trials. Subgroup analysis was performed according to the previously mentioned, prespecified dichotomous or dichotomised baseline factors. The interpretation of these analyses was based on an interaction test, with a p-value<0.05 considered significant. Treatment effects were estimated as odds ratios (ORs) with their respective 95% confidence intervals (CIs) for each subgroup. Continuous baseline variables such as age, body mass index, and femoral artery diameter were additionally assessed as continuous variables in logistic regression models with a treatment-by-baseline factor interaction. The predicted probability of the primary endpoint derived from these models was plotted over the range of the respective continuous baseline factor for each group. As a secondary analysis, conventional two-stage meta-analyses were performed. Random-effects meta-analyses were conducted using the Mantel-Haenszel method with the Paule-Mandel estimator of between-study variance. Heterogeneity was assessed using Cochran’s Q statistics and Higgins and Thompson’s I². R, version 4.4.1 (R Foundation for Statistical Computing) was used for all statistical analyses.
Registration and funding source
This meta-analysis is registered with PROSPERO (CRD42022335295). There was no external funding source for this meta-analysis.
Results
Included studies and meta-analysis population
Our search identified 871 records; after excluding duplicates and screening the titles, abstracts and full texts, four RCTs remained. One RCT was still recruiting and one RCT did not meet the inclusion criteria, and these were therefore excluded. The study selection process is outlined in Figure 1. The investigators of the two remaining RCTs agreed to collaborate on a meta-analysis. Overall, the analysis included 362 patients treated with the ProGlide-based technique and 360 patients treated with the MANTA-based technique. The main study design features are summarised in Table 1. Both RCTs compared one MANTA with two ProGlides for large-bore arteriotomy closure after transfemoral TAVI. In both studies, the two ProGlide VCDs were implanted in a rotated position relative to each other. One of the main differences between the two trials was the use of ultrasound. In the MASH-TAVI Trial, access site puncture procedures were exclusively performed under ultrasound guidance, while in the CHOICE-CLOSURE trial, the choice of puncture guidance technique was at the discretion of the operator. Additionally, in the CHOICE-CLOSURE trial, ultrasound assessments were conducted post-procedure to assess potential vascular complications, whereas the MASH-TAVI Trial relied solely on clinical follow-up for this purpose. The ProGlide-based and MANTA-based technique groups were well balanced in terms of baseline characteristics, as illustrated in Table 2. Most patients in both groups were male (55.5% vs 54.4% in the ProGlide-based and MANTA-based technique groups, respectively) and elderly (81.0 [interquartile range [IQR] 76.0-84.0] vs 81.0 [IQR 77.0-84.0] years), at intermediate surgical risk as predicted by the Society of Thoracic Predicted Risk of Mortality score (3.2 [IQR 2.1-4.9] vs 3.1 [IQR 2.1-4.8]) and the presence of frailty and other comorbidities. Prediagnosed peripheral arterial disease was not common in either group (6.6% vs 6.7%), and vascular access site characteristics were similar (Supplementary Table 3). The only noticeable difference was observed in the external iliac artery diameter, which was smaller in the ProGlide-based technique cohort compared to the MANTA-based technique cohort (7.0 [IQR 6.3-8.4] vs 8.0 [IQR 7.0-9.0] mm). Vascular access was mostly guided by road mapping (52.1%) followed by ultrasound guidance (41.6%), with no difference between the suture-based and plug-based cohorts. The groups were largely comparable regarding main access sheath size, valve type, heparin and protamine use, as well as activated clotting time at vascular closure (Supplementary Table 4). Short manual compression of less than 3 minutes was more common in the MANTA-based technique group (49.9% vs 80.3%), whereas compression of 3-10 minutes was more common in the ProGlide-based technique group (45.4% vs 13.2%) (Table 3). The use of additional VCDs was exclusive to the ProGlide-based technique cohort, where they were needed in 50.0% of patients, with 45.0% of patients receiving an additional small plug as an adjunct to the suture-based vascular closure technique. The completeness of data, as well as distribution by trial for baseline characteristics, access site characteristics, procedural and vascular closure characteristics are shown in Supplementary Table 5-Supplementary Table 6-Supplementary Table 7-Supplementary Table 8. 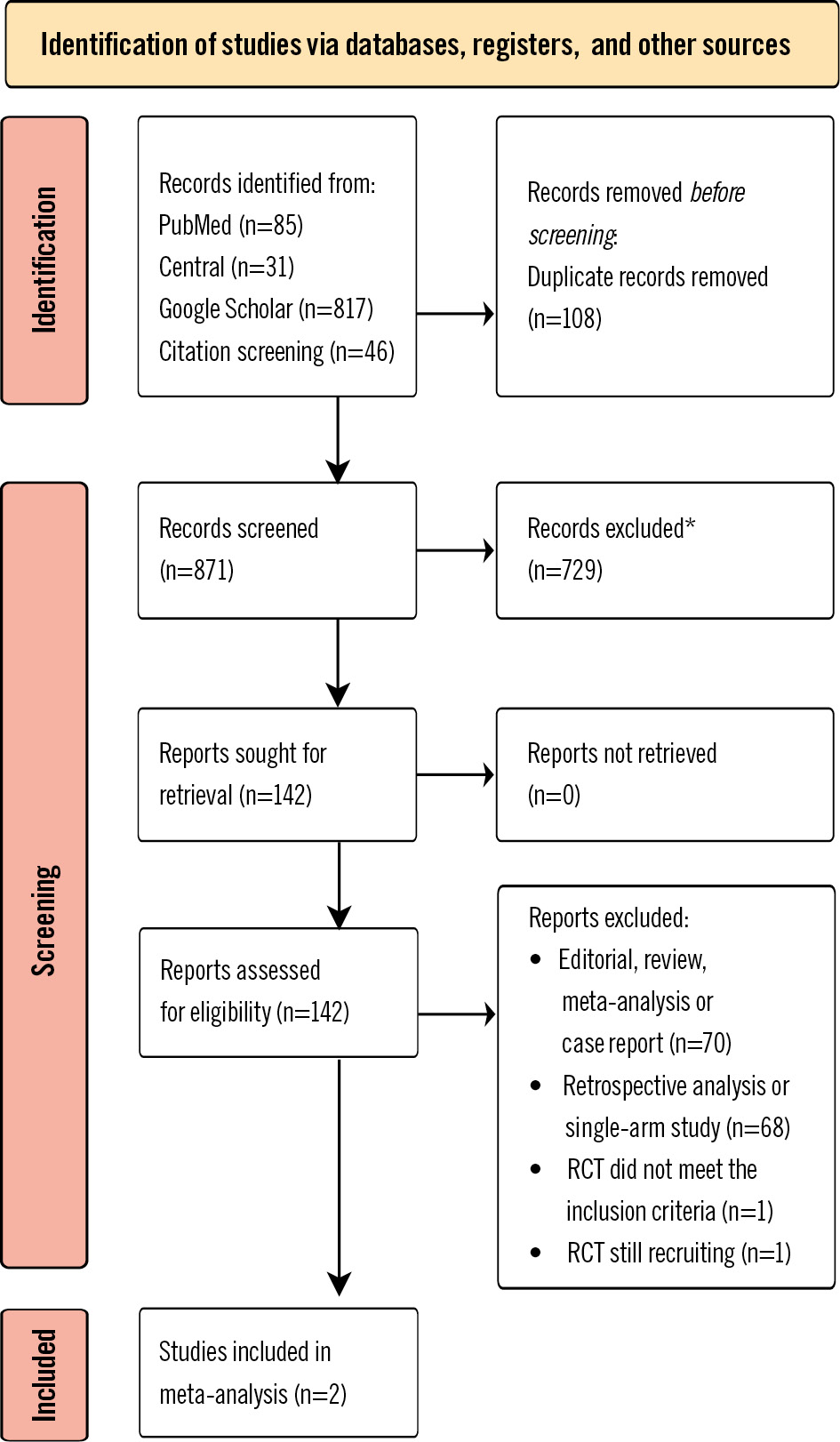
Figure 1. PRISMA flowchart of trial identification and selection. *Different topic33. RCT: randomised controlled trial
Table 1. Key design features of included trials.
| MASH-TAVI | CHOICE-CLOSURE | |
|---|---|---|
| Identifier | NCT03811119 | NCT04459208 |
| Enrolment period | 10/2018-01/2020 | 06/2020-06/2021 |
| Multicentre study | Yes (2 centres) | Yes (3 centres) |
| Key inclusion criteria | Patient undergoing elective transfemoral TAVI with a commercially available device Common femoral artery diameter >5.0 mm (14-22 Fr compatible) |
Patient undergoing elective transfemoral TAVI with a commercially available device |
| Key exclusion criteria | Vascular access site anatomy not suitable for percutaneous vascular closure with study VCDs Absence of preprocedural computed tomography data of the access site Morbidly obese or cachectic (BMI >40 kg/m2 or <20 kg/m2) | Vascular access site anatomy not suitable for percutaneous vascular closure with study VCDs Absence of preprocedural computed tomography data of the access site |
| Patients with MANTA/ProGlide VCD | 102/104 | 258/258 |
| Procedure | TAVI | TAVI |
| Used size of MANTA VCD | 18 Fr | 18 Fr |
| One or two ProGlide VCDs | 2 ProGlides | 2 ProGlides |
| Methods of vascular access | Ultrasound-guided | Angiography- or ultrasound-guided |
| Anticoagulation during procedure | UFH | UFH |
| Anticoagulation reversal | Protamine | Protamine |
| Primary endpoint | Access site-related major and minor vascular complications | Access site-related major and minor vascular complications |
| Outcome assessment | Clinical | Clinical and ultrasound |
| Vascular complications and bleeding definition | VARC-2 | VARC-2 |
| Vascular closure device failure definition | Failure of the vascular closure device to achieve haemostasis within 5 min or requiring additional endovascular manoeuvres (endovascular stenting, surgical techniques, or additional closure devices) | VARC-2 |
| Follow-up | 30 days | 30 days |
| BMI: body mass index; Fr: French; TAVI: transcatheter aortic valve implantation; UFH: unfractionated heparin; VARC: Valve Academic Research Consortium; VCD: vascular closure device | ||
Table 2. Baseline characteristics.
| Variable | Total (n=722) | ProGlide-based technique (n=362) | MANTA-based technique (n=360) | p-value |
|---|---|---|---|---|
| Age, years | 81.0 [76.0-84.0] | 81.0 [76.0-84.0] | 81.0 [77.0-84.0] | 0.67 |
| Male sex | 397 (55.0) | 201 (55.5) | 196 (54.4) | 0.83 |
| Body mass index, kg/m² | 27.1 [24.4-30.8] | 27.4 [24.0-31.0] | 26.9 [24.6-30.5] | 0.95 |
| Logistic EuroSCORE I | 2.9 [1.9-5.0] | 3.0 [1.9-4.9] | 2.9 [1.9-5.0] | 0.60 |
| STS-PROM score, % | 3.1 [2.1-4.9] | 3.2 [2.1-4.9] | 3.05 [2.1-4.8] | 0.66 |
| Hypertension | 616 (85.3) | 310 (85.6) | 306 (85.0) | 0.89 |
| Diabetes mellitus | 248 (34.3) | 126 (34.8) | 122 (33.9) | 0.86 |
| Current smoker | 56 (8.35) | 29 (8.76) | 27 (7.94) | 0.81 |
| Previous CABG | 62 (8.6) | 31 (8.6) | 31 (8.6) | 1.00 |
| Previous PCI | 192 (26.6) | 93 (25.7) | 99 (27.5) | 0.64 |
| Previous valve surgery | 57 (7.9) | 29 (8.0) | 28 (7.8) | 1.00 |
| Previous stroke | 102 (14.1) | 46 (12.7) | 56 (15.6) | 0.33 |
| Peripheral arterial disease | 48 (6.7) | 24 (6.6) | 24 (6.7) | 1.00 |
| Atrial fibrillation | 225 (31.2) | 123 (34.0) | 102 (28.3) | 0.12 |
| Baseline eGFR, | 58.0 [43.0-73.0] | 57.0 [43.0-70.0] | 60.0 [43.8-76.2] | 0.17 |
| Baseline creatinine level, μmol/L | 95.5 [75.0-120.0] | 96.0 [75.0-120.0] | 95.0 [74.0-120.0] | 0.74 |
| Baseline haemoglobin level, mmol/L | 6.9 [6.1-7.7] | 6.8 [6.0-7.7] | 7.0 [6.1-7.7] | 0.34 |
| Baseline platelet count (109/l) | 166 [135-205] | 169 [132-207] | 164 [137-202] | 0.67 |
| Antithrombotic therapy | ||||
| Oral anticoagulation | 256 (35.8) | 140 (39.3) | 116 (32.3) | 0.06 |
| Antiplatelet therapy | 0.29 | |||
| None | 351 (49.1) | 180 (50.6) | 171 (47.6) | |
| Aspirin | 220 (30.8) | 109 (30.6) | 111 (30.9) | |
| Clopidogrel | 59 (8.3) | 33 (9.3) | 26 (7.2) | |
| Other single antiplatelet therapy | 2 (0.3) | 1 (0.3) | 1 (0.3) | |
| Dual antiplatelet therapy | 83 (11.6) | 33 (9.27) | 50 (13.9) | |
| Values are median [interquartile range] or n (%). CABG: coronary artery bypass grafting; eGFR: estimated glomerular filtration rate; EuroSCORE: European System for Cardiac Operative Risk Evaluation; PCI: percutaneous coronary intervention; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality | ||||
Table 3. Vascular closure characteristics.
| Variable | Total (n=722) | ProGlide-based technique (n=362) | MANTA-based technique (n=360) | p-value |
|---|---|---|---|---|
| Use of protamine | 0.15 | |||
| None | 130 (18.1) | 74 (20.4) | 56 (15.6) | |
| Less than full dose | 475 (66.0) | 227 (62.7) | 248 (69.3) | |
| Full dose | 115 (16.0) | 61 (16.9) | 54 (15.1) | |
| Manual compression | <0.01 | |||
| Less than 3 minutes | 462 (65.1) | 177 (49.9) | 285 (80.3) | |
| Between 3 and 10 minutes | 208 (29.3) | 161 (45.4) | 47 (13.2) | |
| More than 10 minutes | 40 (5.6) | 17 (4.8) | 23 (6.5) | |
| Additional VCD | 181 (25.1) | 181 (50.0) | 0 (0) | <0.01 |
| Type of additional VCD | ||||
| MANTA | - | 7 (1.9) | - | |
| ProGlide | - | 11 (3.0) | - | |
| Small plug-based VCD | - | 163 (45.0) | - | |
| Angio-Seala 6 Fr | - | 89 (24.6) | - | |
| Angio-Seal 8 Fr | - | 71 (19.6) | - | |
| FemoSeala | - | 1 (0.3) | - | |
| ProGlideb and Angio-Seal | - | 2 (0.6) | - | |
| Endovascular ballooning | 50 (6.9) | 16 (4.4) | 34 (9.4) | 0.01 |
| Stent or stent graft | 19 (2.6) | 4 (1.1) | 15 (4.2) | 0.02 |
| Unplanned vascular surgery | 2 (0.3) | 0 (0) | 2 (0.6) | 0.25 |
| Values are n (%). aBy Terumo; bby Abbott. Fr: French; VCD: vascular closure device | ||||
Primary and SECONDARY endpoints
The primary endpoint of in-hospital main access site-related major and minor vascular complications was observed in 107 of 722 patients (14.8%); this was less common in the ProGlide-based technique group (40 of 362 patients [11.0%]) than in the MANTA-based technique group (67 of 360 patients [18.6%]; OR 0.54, 95% CI: 0.35-0.82) as depicted in the Central illustration and Table 4. These results were also consistent for the individual endpoints of access site-related major vascular complications (5 of 362 patients [1.4%] vs 14 of 360 patients [3.9%]; OR 0.35, 95% CI: 0.12-0.97) and access site-related minor vascular complications (35 of 362 patients [9.7%] vs 53 of 360 patients [14.7%]; OR 0.62, 95% CI: 0.39-0.98). The individual types of main access site vascular complications are illustrated in the Central illustration. Overall, main access site-related bleeding events according to the VARC-3 definition were reported in 27 of 722 patients (3.7%) and were also significantly less common with the ProGlide-based technique (8 of 362 patients [2.2%] vs 19 of 360 patients [5.3%]; OR 0.41, 95% CI: 0.18-0.94). Type 2 and 3 bleeding events occurred numerically more frequently with the MANTA-based technique, but the difference between groups was not statistically significant (Central illustration). There were no fatal bleeding events (type 4 bleeding), and only one type 1 bleeding event was adjudicated. In-hospital all-cause mortality was infrequent (10 of 722 patients [1.4%]), and there was no significant difference between the treatment groups (2 of 362 patients [0.6%] vs 8 of 360 patients [2.2%]; OR 0.24, 95% CI: 0.05-1.16). No patient died because of an access site-related vascular complication or an access site-related bleeding event. Among all 10 patients who died, only one patient had a bleeding complication, and one patient had a vascular closure device failure. A detailed description of all deceased patients and the respective cause of death is provided in Supplementary Table 9. Access site VCD failure according to VARC-3 was evenly distributed between both closure techniques (17 of 362 patients [4.7%] vs 18 of 360 patients [5.0%]; OR 0.94, 95% CI: 0.47-1.85), but vascular surgery or stenting due to VCD failure was more common with the MANTA-based technique (3 of 362 patients [0.8%] vs 13 of 360 patients [2.8%]; OR 0.22, 95% CI: 0.06-0.79). 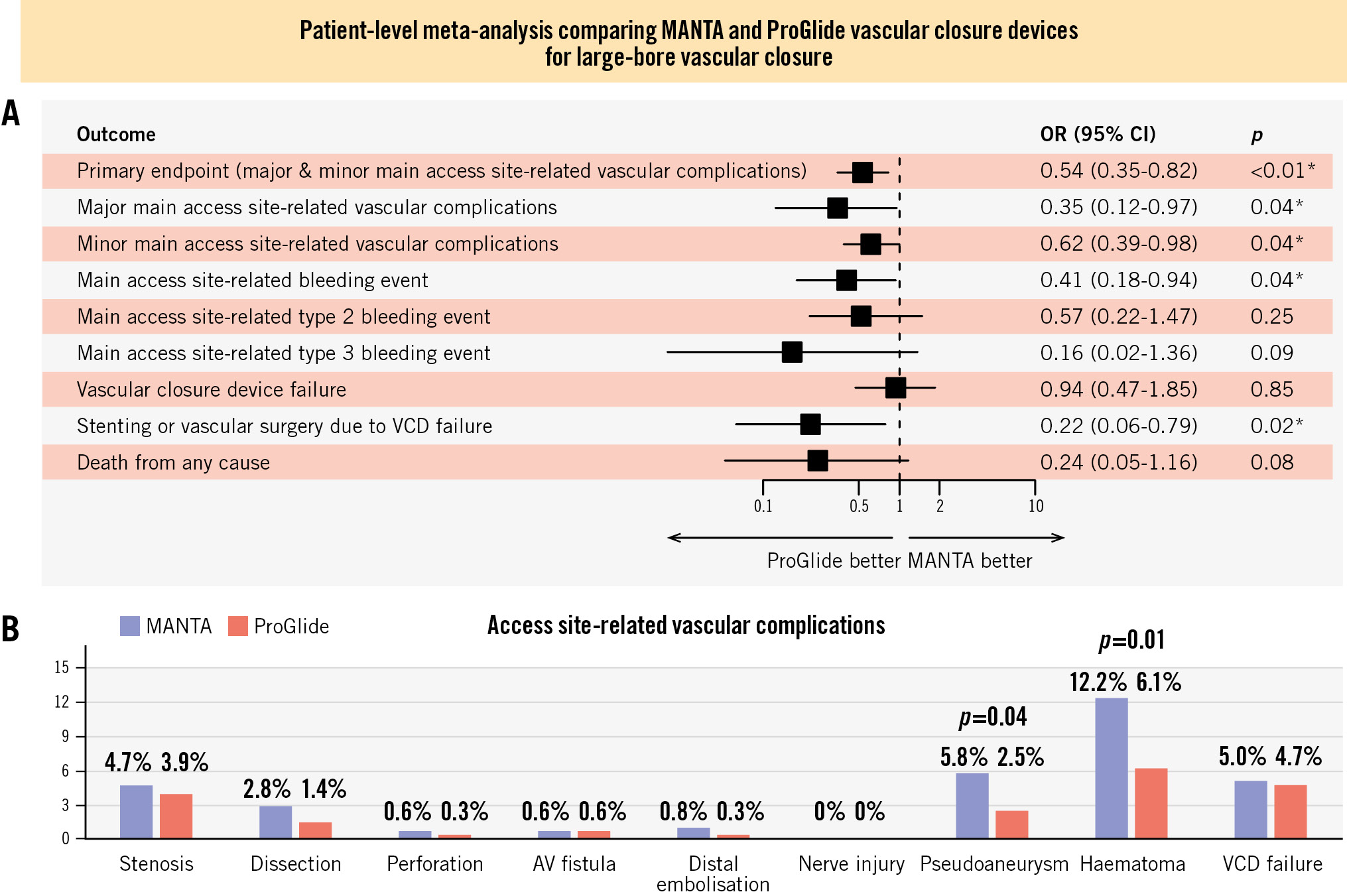
Central illustration. ProGlide- versus MANTA-based vascular closure technique for large-bore arterial access: a patient-level meta-analysis of two randomised trials including 722 patients. Outcome assessment according to Valve Academic Research Consortium-3 criteria. A) Primary and secondary endpoints. B) Details of access site-related vascular complications. *Indicates statistical significance. AV: arteriovenous; CI: confidence interval; OR: odds ratio; VCD: vascular closure devic
Subgroup and sensitivity analyses
Nearly all prespecified subgroups, with respect to the primary endpoint, consistently suggested a benefit of the ProGlide-based technique, without a statistically significant interaction (Figure 2). Since only a few patients had a high femoral bifurcation above the femoral head, it is difficult to draw any conclusions about this subgroup. Analysis of continuous variables revealed a steady decline of the probability of the primary endpoint for the MANTA-based technique in larger femoral arteries (Figure 3). A femoral artery diameter >9.5 mm was associated with a trend towards fewer major and minor access site-related vascular complications with the MANTA-based technique than with the ProGlide-based technique. All other interactions of continuous variables were in favour of the ProGlide-based technique (Supplementary Figure 1, Supplementary Figure 2). The primary endpoint and the individual endpoint of access site-related minor vascular complications was significantly more common in the CHOICE-CLOSURE trial (Supplementary Table 10). Furthermore, we analysed the non-prespecified subgroups of compression duration and the additional use of a small plug-based VCD in the ProGlide cohort. We categorised the compression duration into three groups: less than 3 minutes, 3-10 minutes, and more than 10 minutes. A longer compression duration was associated with a higher likelihood of the primary endpoint (OR 3.55, 95% CI: 1.29-9.77). However, the used VCD did not significantly modify the effect of compression duration on the primary endpoint (OR 0.79, 95% CI: 0.39-1.64; p for interaction=0.53) (Supplementary Figure 3). The combination of two ProGlide VCDs with a small plug-based VCD demonstrated a significant reduction of the primary endpoint compared to the MANTA-based technique (OR 0.46, 95% CI: 0.26-0.82), whereas the pure ProGlide-based technique showed no significant difference compared to the MANTA-based technique (OR 0.61, 95% CI: 0.37-1.03). The results of the different statistical models for the prespecified primary and secondary endpoints, as well as measurements for heterogeneity (I² and Tau) and model diagnostic parameters are summarised in Supplementary Table 11. There were no relevant outcome differences between the statistical models. Furthermore, the heterogeneity in the other statistical models was generally low.
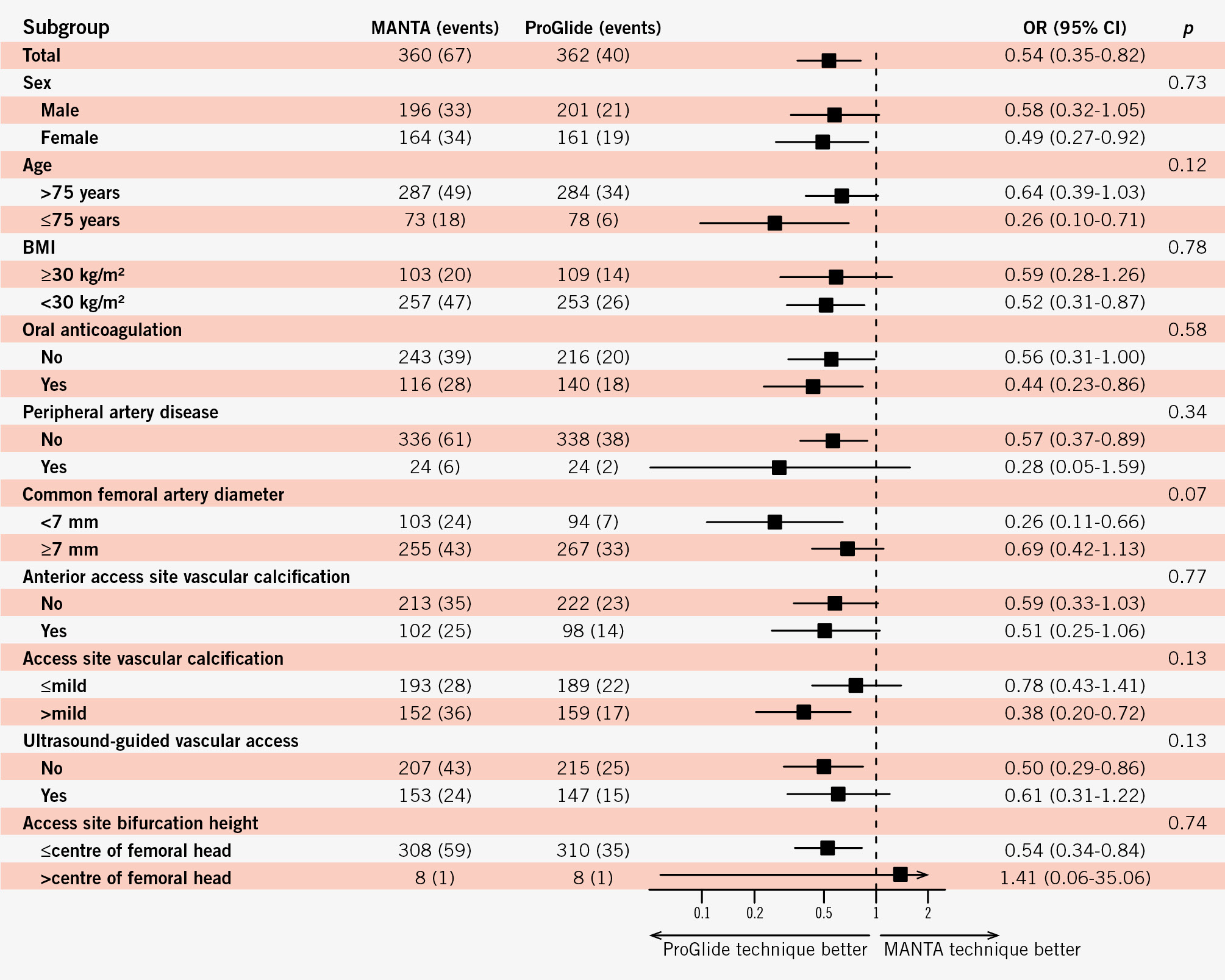
Figure 2. Odds ratios for the primary endpoint in the intention-to-treat population in prespecified subgroups. BMI: body mass index; CI: confidence interval; OR: odds ratio
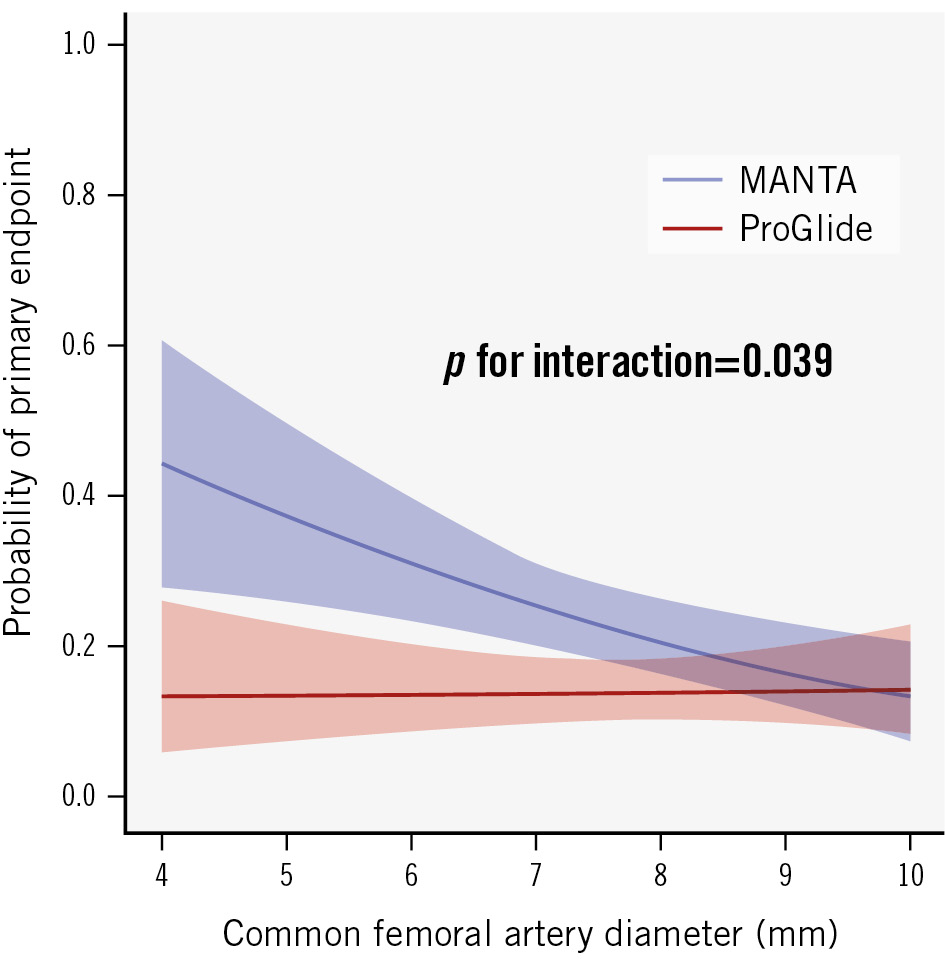
Figure 3. Interaction of access site femoral artery diameter and the primary endpoint. Interaction is adjusted for the CHOICE-CLOSURE trial.
Discussion
This is the first patient-level meta-analysis of RCTs comparing the ProGlide-based and MANTA-based techniques in patients with large-bore arterial access. The main findings of the study are as follows: (1) overall access site-related vascular complications were more common with the MANTA-based than with the ProGlide-based technique; (2) major access site complications were uncommon (<4%) but more frequent with the MANTA-based technique; (3) the MANTA-based technique seemed to perform better in large femoral arteries (>9.5 mm); and (4) VCD failure was comparable between both groups – the MANTA-based technique required more covered stents or vascular surgery, while the ProGlide-based technique needed more additional VCDs. Overall, vascular complication rates were lower than expected based on previous studies, especially with the suture-based technique82223. This may be an effect of increasing operator experience, but it may also be related to the technical improvements of the latest TAVI generation. The recently published EASIER registry reported comparable low rates of major vascular complications with the new-generation TAVI devices24. Furthermore, it must be noted that the results of this individual patient-level meta-analysis differ from two previous study-level meta-analyses of RCT data1825. Some endpoints, such as major access site-related vascular complications and major access site-related bleeding events, showed different significance levels. The harmonisation of endpoint definitions allowed us to change from VARC-2 to VARC-3. This patient-level meta-analysis employed a multilevel model with stratified intercepts, whereas the previous meta-analyses were study-level analyses. One fundamental difference between the two vascular closure strategies is the fact that, in the ProGlide-based technique, wire access is maintained even after the sheath is removed and the sutures are tightened. This allows a wider and simpler variety of bailout strategies, such as additional VCDs. In this way, a suboptimal closure of the ProGlide VCD can be addressed directly and easily, whereas this is not possible with the MANTA VCD. In this meta-analysis, this advantage is reflected in the lower rate of vascular stenting or vascular surgery. The use of an additional small plug-based VCD after application of the ProGlide VCD has been established as part of the ProGlide-based technique1112 and was therefore not considered a VCD failure. In fact, the non-prespecified subgroup analysis showed that this group achieved particularly favourable outcomes regarding the primary endpoint compared to the MANTA-based technique. These findings highlight the advantages of this hybrid vascular closure approach. In this context, the recently published randomised ACCESS-TAVI Trial, found significantly less vascular complications after TAVI when using a combination of one ProGlide and one Angio-Seal compared to vascular closure with two ProGlides26. The use of additional VCDs may also be the reason for the lower incidence of bleeding complications in the ProGlide-based technique group. Remarkably, short manual compression times (1-3 minutes) were more common in the MANTA-based group than in the ProGlide-based group but so were the number of haematomas, pseudoaneurysms, and overall access site bleeding events. The typically rapid, visually assessed haemostasis provided by the MANTA VCD could lead to an underestimation of residual bleeding or an underappreciation of VCD failure, potentially resulting in inappropriately short manual compression times. It is reasonable to assume a higher likelihood of subacute or clinically inapparent microbleeds within the MANTA-based technique treatment group. In contrast, the ProGlide VCD provides early warning of potential haemostatic problems, both during preclosure and during final suture tightening. However, in a subgroup analysis of compression duration, a higher incidence of the primary endpoint was observed with longer compression, regardless of the VCD used. It is plausible that a problematic vascular closure leads to longer compression. However, the extent to which compression duration influenced the results cannot be definitively determined. Subgroup analysis suggests a consistent tendency of lower vascular complications with the ProGlide-based technique. Only patients with a large access site diameter (>9.5 mm) seemed to have similar complications with the MANTA-based technique. Previous retrospective studies have shown that vascular diameter is a predictor for vascular complications when using the MANTA-based technique2728. It is possible that larger vessels promote better device deployment and adaptation. Meticulous assessment and accurate puncture of the femoral arterial access site are essential elements for successful vascular closure. A growing body of evidence suggests that ultrasound-guided puncture may be superior in this context29. However, the advantage of the ProGlide-based technique in this meta-analysis was independent of the method used for guiding arterial access. Moriyami et al observed a significant reduction in major vascular and bleeding complications in a retrospective study using ultrasound-guided MANTA VCD deployment30. A comparison of this strategy with the ProGlide-based technique has not been performed. The ProStyle VCD (Abbott) was recently introduced as the successor to the ProGlide VCD. It features several design enhancements for improved handling, as well as stronger needles and an additional hydrophilic coating. Although the basic principles of suture-based VCD remain unchanged, the impact of these changes on VCD performance has not been adequately studied. A retrospective, non-randomised, propensity-matched analysis by Barbash et al found comparable rates of major and minor vascular complications between the new ProStyle and the MANTA-based technique31. Numerically, major vascular complications were more common with the ProStyle-based technique, which is an unexpected finding, given the minor improvements in the ProStyle’s design. Looking at the endpoints by trial, the MASH-TAVI study showed a significantly lower incidence of the primary endpoint, driven by a lower rate of minor access site vascular complications. In particular, haematomas and stenoses were more common in the CHOICE-CLOSURE trial15. A possible reason may be the exclusive use of ultrasound-guided puncture in the MASH-TAVI study. However, the subgroup analysis of our study showed no significant effect of the puncture technique on the outcomes. Another explanation for the higher rate of complications could be the significantly higher dose of heparin administered in the CHOICE-CLOSURE study. This was likely related to the higher body weight of the included patients and, therefore, had no significant effect on the activated clotting time at vascular closure. However, the most likely reason for the differences between the two trials is a detection bias. In the CHOICE-CLOSURE trial, an ultrasound examination of each access site was performed after the procedure, which revealed complications that may not have been seen on clinical examination. Since the current VARC-3 criteria consider all findings (e.g., haematoma, vascular stenosis) related to the device insertion, delivery, and removal to be access site complications (regardless of clinical significance), the number of minor vascular complications is relatively higher than reported in the original publications19. To highlight these definition-related changes in the event numbers, we have displayed them in Supplementary Figure 4. Conversely, the number of minor bleeding events (type 1) was lower than in the original publications. This is because the new definition demands “a higher level of care or medical evaluation” (VARC-3) and no longer includes “any bleeding worthy of clinical mention” (VARC-2)1932. Only one type 1 bleeding event was identified in our patient cohort under the VARC-3 criteria, whereas 47 events would have been recorded using the VARC-2 criteria. Moreover, it seems difficult to accurately document a higher level of care or medical evaluation in a study, as there are no specific parameters. This lack of precision may contribute to potential underreporting of type 1 bleeding events. It remains to be seen whether these small, but important, changes in the definitions will be reflected in the results of other studies as well. The higher rate of overall main access site-related bleeding in the MANTA-based technique group was thus mainly driven by severe (type 2) and life-threatening (type 3) bleeding events. Overall, it is important to emphasise that the superior outcome of the ProGlide-based technique clearly extends to major bleeding and major vascular complications. The results are not solely attributable to minor events, which may be more variable than major complications due to detection bias or changed definitions. Therefore, further research is necessary to help identify patients who are vulnerable to complications with the MANTA-based technique. This would ensure an individual choice of closure technique for each patient.
Limitations
This meta-analysis has several limitations. First, the study only includes two RCTs and a relatively small number of patients. Second, as previously mentioned, the two trials differed with respect to access site guidance and access site follow-up. Third, only the 18 Fr MANTA VCD was used. Fourth, both trials only included patients receiving transfemoral TAVI. Other large-bore procedures, such as extracorporeal membrane oxygenation or endovascular aortic repair, are often characterised by a different clinical setting, and the results of this analysis may not be extended to these procedures. Furthermore, the weighting of clinically questionable minor vascular complications may also be a point of criticism in the comparison of the two devices. Finally, this meta-analysis only included the ProGlide VCD and not the new suture-based ProStyle VCD.
Conclusions
Main access site-related vascular complications and bleeding events are less common with the ProGlide-based technique than with the MANTA-based technique in this individual patient-level meta-analysis of two RCTs. The differences between both techniques in terms of main access site vascular complications are consistent among various subgroups. As the diameter of the femoral artery increases, outcomes with the plug-based technique improve.
Impact on daily practice
Percutaneous large-bore arteriotomy closure methods are either suture-based (ProGlide vascular closure device) or plug-based (MANTA vascular closure device), but the efficacy and safety of both techniques remain controversial. In this patient-level meta-analysis of randomised trials, the ProGlide-based technique for large-bore arterial access was superior to the MANTA-based technique in terms of vascular and bleeding complications. Clinical outcomes with the MANTA-based technique improved as the vessel size increased. Further studies are needed to enable individualised selection of the vascular closure technique based on specific patient characteristics.
Conflict of interest statement
M. Abdel-Wahab reports that his hospital receives speaker honoraria and/or consultancy fees on his behalf from Medtronic and Boston Scientific. N. Van Mieghem received research grant support from Abbott, Boston Scientific, Edwards Lifesciences, Biotronik, Medtronic, Daiichi Sankyo, Abiomed, PulseCath BV, and Pie Medical Imaging. D. Tchétché received consultant fees from Abbott, Boston Scientific, Edwards Lifesciences, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

