The negative prognostic impact of severe aortic stenosis (AS) is unquestioned, and surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI) are well-established, guideline-recommended treatment options for patients with severe, symptomatic AS. In recent years, data have been accumulating which suggest that even moderate AS can have an adverse impact on the clinical outcomes of affected patients, thereby raising the question as to whether treatment indications should be expanded at some point.
The first question that needs to be answered when interpreting the available data on moderate AS is whether moderate AS really is an independent predictor of outcome, or if it is just a surrogate parameter for other prognostically relevant comorbidities. Several studies attempting to answer this question demonstrated a negative prognostic impact of moderate AS on cardiovascular outcomes, independent of any confounding factors1. This was particularly pronounced in patients suffering from symptoms and in those with reduced left ventricular ejection fraction (LVEF). A recently published propensity score-matched analysis by Jean et al comparing the clinical outcomes of patients with heart failure (HF) alone versus HF and moderate AS found a substantial increase in all-cause mortality and HF hospitalisations after 6 years in those patients with HF and moderate AS2.
It can be hypothesised that this additional risk may either be caused by rapid, untreated progression of the disease from moderate to severe AS or that it is due to the haemodynamic compromise mediated by the outflow obstruction through moderate AS itself. While the rapid progression of AS may play an important role in a selected subset of patients with borderline high transvalvular gradients at baseline, current data suggest that the additive haemodynamic burden caused by moderate AS appears to be the driving force leading to a poor outcome in most patients, especially in those with pre-existing cardiac damage3. For patients with severe AS, a landmark study by Généreux et al defined a staging classification based on the extent of concomitant cardiac damage showing a strong association with patient prognosis following aortic valve replacement (AVR)4. A recent study by Amanullah et al found similar associations with poor outcome when applying this classification to patients with moderate AS, further strengthening the hypothesis that the combination of moderate AS and cardiac damage is particularly unfortunate3.
These data raise the question of whether AVR in patients with moderate AS, at least in certain vulnerable patient subsets, may yield prognostic benefit (Figure 1). To address this question, several registry-based studies retrospectively compared patients with moderate AS undergoing AVR to patients who remained on medical therapy, including mostly patients with reduced LVEF25. Given the nature of these studies and the current lack of an indication for AVR in patients with moderate AS, the comparability of the interventional and medical subgroups in these studies is limited per se. Nonetheless, all studies observed a prognostic benefit in patients with moderate AS undergoing AVR. A recent study from the international ATLAS TAVI Registry (ClinicalTrials.gov: NCT04914481) included a cohort of patients with non-severe (i.e., moderate) AS based on computed tomography (CT)-derived aortic valve calcification assessment and reduced LVEF undergoing transfemoral TAVI. Compared to a propensity score-matched medical control group with moderate AS and HF, TAVI treatment was independently associated with significant improvement of cardiovascular survival after 2 years5.
Eventually, the fate of TAVI as a potential treatment option for patients with moderate AS will have to be determined by the results of randomised controlled trials (RCTs). In fact, three currently ongoing RCTs are designed to compare TAVI versus medical therapy in slightly different patient subsets with moderate AS and with different primary effectiveness endpoints at 1- or 2-year follow-up (Table 1):
⢠The PROGRESS trial (ClinicalTrials.gov: NCT04889872) is comparing TAVI, using balloon-expandable devices from the SAPIEN platform (Edwards Lifesciences), to clinical surveillance in patients with either symptomatic moderate AS or evidence of cardiac damage.
⢠The EXPAND TAVR II Pivotal trial (ClinicalTrials.gov: NCT05149755) is comparing TAVI, using the self-expanding Evolut PRO+ and Evolut FX devices (Medtronic), to optimal medical treatment in patients with moderate AS who are either symptomatic, have reduced functional capacity or show signs of cardiac damage.
⢠The TAVR UNLOAD trial (ClinicalTrials.gov: NCT02661451) is comparing the SAPIEN 3 (Edwards Lifesciences) TAVI device to guideline-directed medical therapy in patients with moderate AS and systolic heart failure (LVEF <50%).
By clarifying whether moderate AS should be considered a therapeutic target for interventional treatment, the trade-off between long-term prognostic benefit and the potential harm of an invasive procedure must be acknowledged. Only if ongoing RCTs are able to prove a significant benefit of TAVI compared to medical therapy in patients with moderate AS regarding hard clinical endpoints over a longer-term follow-up, outweighing the elevated short-term risk posed by the procedure, would an introduction into clinical practice seem justifiable. In addition, the issue of cost-effectiveness of a TAVI procedure when expanding the treatment indication towards patients with moderate AS warrants closer attention. Moreover, patients with moderate AS tend to be younger, at lower risk and have longer life expectancy compared to patients with severe AS. Therefore, when considering TAVI for these patients, a potential scenario of aortic valve reintervention at follow-up must be taken into account. Lastly, despite multiple efforts investigating medical treatment targets to address AS progression, there is currently no available medical treatment for AS. The potential future development of a drug that can slow down, inhibit or even reverse aortic stenosis progression could challenge the indication for AVR in some patients, but particularly in those with moderate AS.
In conclusion, the body of evidence demonstrating an additive prognostic impact of moderate AS, especially in symptomatic patients with structural abnormalities, has been growing over the last years, thereby suggesting a potential target for intervention. There are currently three ongoing RCTs investigating whether TAVI is superior to medical therapy in patients with moderate AS, and the results of these trials have the potential to change clinical practice. However, given the substantial differences regarding inclusion criteria and clinical endpoints, the discussion on the role of TAVI in patients with moderate AS will remain far from over, even if a benefit of intervention can be demonstrated. Ultimately, the decision to perform TAVI in patients with AS always needs to consider the relationship between individual risk and long-term benefit. This becomes even more important when considering TAVI as an early intervention for patients suffering from moderate AS.
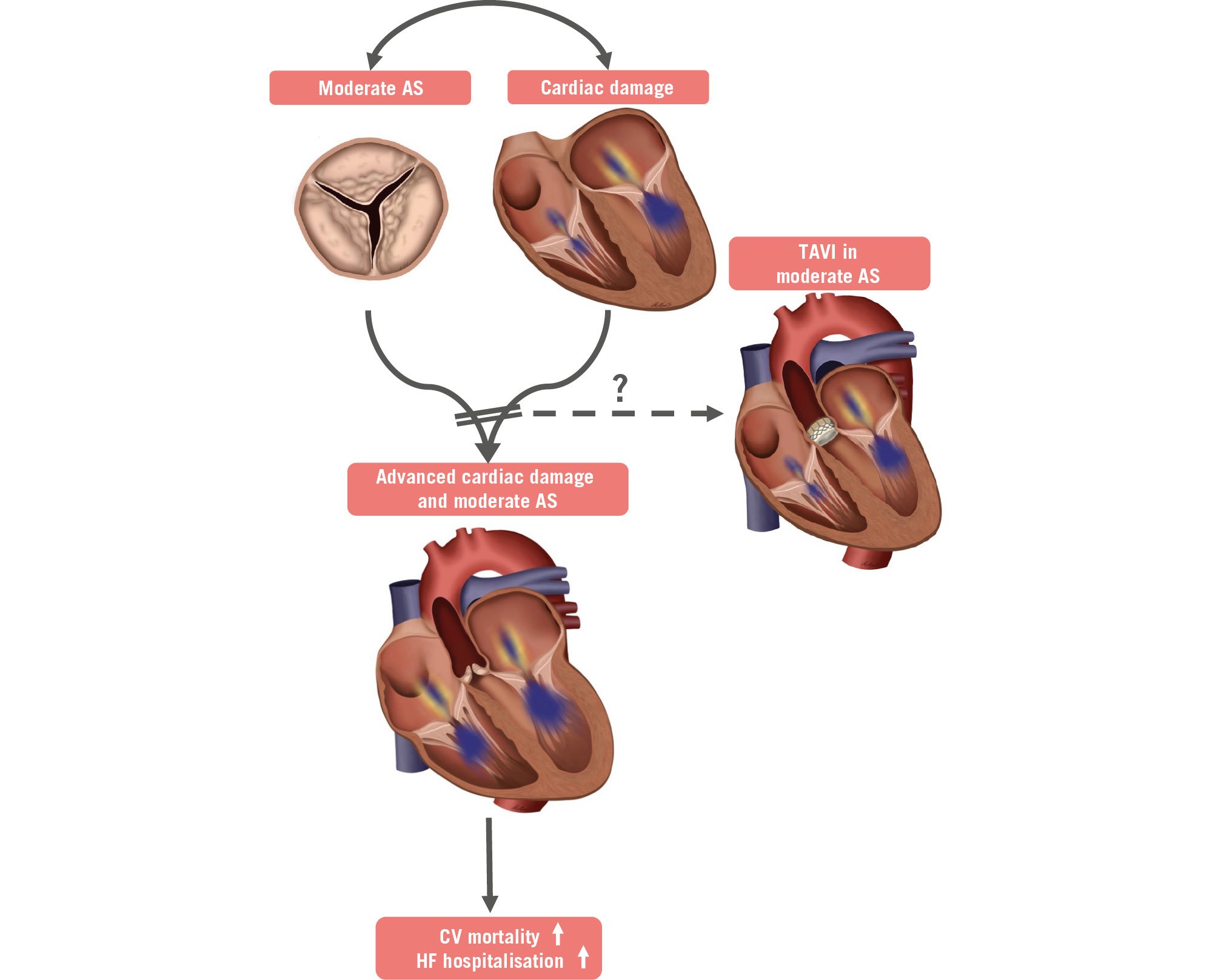
Figure 1. The potential benefit of TAVI in patients with moderate AS and cardiac damage. AS: aortic stenosis; CV: cardiovascular; HF: heart failure; TAVI: transcatheter aortic valve implantation
Table 1. Summary of ongoing prospective randomised controlled trials (RCTs) in patients with moderate aortic stenosis.
| PROGRESS (NCT04889872) | EXPAND TAVR II (NCT05149755) | TAVR UNLOAD (NCT02661451) | |
|---|---|---|---|
| Study population | Moderate AS | Moderate AS | Moderate or pseudo-severe AS and HF (EF <50%) |
| Sponsor | Edwards Lifesciences | Medtronic | Cardiovascular Research Foundation |
| TAVI device | SAPIEN 3 SAPIEN 3 Ultra SAPIEN 3 Ultra RESILIA |
Evolut PRO+Evolut FX | SAPIEN 3 |
| Randomisation | N=750TAVI vs clinical surveillance (1:1) | N=650TAVI+GDMT vs GDMT alone (1:1) | N=600TAVI vs GDMT (1:1) |
| Key inclusion criteria | Symptoms (NYHA ≥II)orevidence of cardiac damage | Symptoms (NYHA ≥II or reduced functional capacity)orHFH in previous year,EF <60%GLS ≤16%E/e’ ≥14, orNT-proBNP ≥600 ng/L | Symptoms (NYHA ≥II)andEF ≥20% and <50% |
| Primary effectiveness endpoint | Composite endpoint (non-hierarchical):- Death,- HFH or HF event | Composite endpoint (non-hierarchical):- Death,- HFH or HF event,- Medical instability leading to AVR or reintervention |
Composite endpoint (hierarchical):1) Death,2) Disabling stroke,3) HFH or HFH equivalent,4) Change in KCCQ |
| Time frame | 2 years | 2 years | 1 year |
| AS: aortic stenosis; AVR: aortic valve replacement; EF: ejection fraction; GDMT: guideline-directed medical therapy; GLS: global longitudinal strain; HF: heart failure; HFH: heart failure hospitalisation; KCCQ: Kansas City Cardiomyopathy Questionnaire; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; TAVI: transcatheter aortic valve implantation | |||
Conflict of interest statement
N. Schofer has received speaker honoraria, proctor fees and travel compensation from Edwards Lifesciences; speaker honoraria and travel compensation from Abbott; speaker honoraria and travel compensation from Boston Scientific; and speaker honoraria from HighLife SAS. S. Ludwig has received a grant from the German Heart Foundation (DHS); speaker honoraria from Abbott; travel compensation from Edwards Lifesciences; advisory fees from Bayer; and is a consultant for NVT.

