Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Short dual antiplatelet therapy (DAPT) followed by ticagrelor monotherapy may be a valuable therapeutic option for patients with chronic coronary syndrome (CCS) and high ischaemic risk (HIR) undergoing percutaneous coronary intervention (PCI).
Aims: We aimed to compare ticagrelor monotherapy with ticagrelor-based DAPT in CCS patients with and without HIR undergoing PCI.
Methods: The present analysis included the CCS cohort of the TWILIGHT trial, which randomised PCI patients to ticagrelor alone or in combination with aspirin for 12 months after 3 months of ticagrelor-based DAPT. Patients were stratified into HIR and non-HIR based on the 2019 European Society of Cardiology (ESC) CCS guidelines definition. Outcomes of interest were major adverse cardiac and cerebrovascular events (MACCE), a composite of death, myocardial infarction or stroke, and Bleeding Academic Research Consortium (BARC) Type 2-5 bleeding at 1 year.
Results: Of the 2,503 CCS patients who underwent randomisation, the ESC definition classified 1,264 (50.5%) as HIR and 1,239 (49.5%) as non-HIR. HIR patients displayed a higher risk of MACCE (3.9% vs 2.3%; p=0.015) and similar rates of BARC Type 2-5 bleeding (5.1% vs 5.7%; p=0.455) as compared to non-HIR patients. Ticagrelor monotherapy and ticagrelor-based DAPT were associated with similar risks of MACCE (HIR: 4.0% vs 3.8%, hazard ratio [HR] 1.06, 95% confidence interval [CI]: 0.60-1.85; non-HIR: 2.1% vs 2.6%, HR 0.80, 95% CI: 0.38-1.66, pinteraction=0.553) and bleeding (HIR: 4.7% vs 5.7%, HR 0.82, 95% CI: 0.50-1.33; non-HIR: 4.9% vs 6.7%, HR 0.71, 95% CI: 0.44-1.14; pinteraction=0.684) in both the HIR and non-HIR groups.
Conclusions: In a post hoc analysis of the TWILIGHT trial that included CCS patients undergoing PCI, ticagrelor monotherapy after 3 months of DAPT appeared to be safe and was not associated with increased risks of ischaemic or bleeding events, regardless of baseline HIR status, compared with standard ticagrelor-based DAPT. These findings suggest the potential to expand guideline recommendations for ticagrelor monotherapy in CCS.
In the elective patient population undergoing percutaneous coronary intervention (PCI) for chronic coronary syndrome (CCS), the optimal dual antiplatelet therapy (DAPT) is aimed to minimise stent-related thrombotic events and, at the same time, avoid excess bleeding complications1. Clopidogrel, in combination with aspirin, is generally the preferred antithrombotic agent, mainly because of the paucity of clinical trial data testing potent P2Y12 inhibitors (ticagrelor and prasugrel) in the CCS setting23. However, despite successful PCI, the residual risk of atherothrombotic events in CCS patients is not negligible, with cardiac death, myocardial infarction (MI) or urgent revascularisation occurring in up to 10% of patients at 1 year and 30% at 5 years45. Prolonged and/or more potent P2Y12 inhibition might be beneficial to improve prognosis and reduce repeat hospitalisations in selected high-risk subsets67. The European Society of Cardiology (ESC) guidelines recommended considering a CCS patient at high ischaemic risk (HIR) in the presence of multivessel coronary artery disease (CAD) with at least one additional clinical risk factor among diabetes, recurrent MI, peripheral artery disease (PAD) and chronic kidney disease (CKD)8. Additionally, prolonged DAPT or ticagrelor-based DAPT are recommended as potential alternatives to standard clopidogrel-based DAPT in CCS patients undergoing PCI89. However, despite being effective in terms of ischaemic event reduction, standard or prolonged DAPT regimens with ticagrelor in combination with aspirin have been associated with a high risk of bleeding complications, which carry an even worse prognostic impact1011.
Among patients who remained event free after an initial 3-month course of ticagrelor-based DAPT, the TWILIGHT trial compared 12 months of ticagrelor monotherapy with 12 months of continued ticagrelor-based DAPT, showing a reduction in Bleeding Academic Research Consortium (BARC) Type 2-5 bleeding without an associated increase in death, MI or stroke12. In a prespecified subgroup analysis, ticagrelor monotherapy was associated with similar ischaemic event rates compared with ticagrelor plus aspirin in patients with either acute coronary syndrome (ACS) or CCS, while the bleeding risk reduction with ticagrelor monotherapy was significant only in the ACS subgroup13. The aim of the present analysis was to compare ticagrelor monotherapy and ticagrelor-based DAPT after PCI in CCS patients with or without HIR according to the 2019 ESC guidelines definition8.
Methods
Trial design and study population
This was a post hoc analysis of the TWILIGHT trial, whose design and principal results have been published previously1214. In brief, TWILIGHT was a multicentre randomised, double-blind, placebo-controlled trial with 187 enrolling institutions across 11 countries. The study enrolled patients undergoing PCI with drug-eluting stent (DES) implantation and who met at least one clinical and one angiographic feature of high ischaemic and/or bleeding risk. Clinical features included age ≥65 years, female sex, troponin-positive ACS, vascular disease (including previous MI, previous coronary revascularisation or PAD), diabetes mellitus requiring medication, and stage ≥3 CKD (estimated glomerular filtration rate <60 ml/min). Angiographic features were multivessel CAD, total stent length >30 mm, thrombotic target lesion, bifurcation lesion treated with two stents, obstructive left main (LM; ≥50% stenosis) or proximal left anterior descending artery (LAD; ≥70% stenosis) disease, and calcified target lesion requiring atherectomy. Key exclusion criteria were ST-segment elevation MI presentation, cardiogenic shock, prior stroke, end-stage CKD on permanent dialysis and chronic anticoagulation. Patients with ACS or with missing data on the indication for PCI were also excluded from the present analysis.
All included patients received DAPT with open-label ticagrelor (90 mg bid) and enteric-coated aspirin (81 or 100 mg daily) for up to 3 months following the index PCI. Patients who were adherent to DAPT and free from major bleeding or ischaemic events (BARC Type ≥3b bleeding, stroke, MI or coronary revascularisation) were randomised in a 1:1 double-blind fashion to aspirin or matching placebo for a further 12 months, in combination with open-label ticagrelor. Clinical follow-up was performed via telephone call at 1 month and in-person visits at 6 and 12 months following randomisation. The study protocol was approved by national regulatory agencies, institutional review boards and ethics committees at the enrolling institutions.
Endpoints
Outcomes of interest for the present analysis included BARC Type 2-5 bleeding and major adverse cardiac and cerebrovascular events (MACCE), defined as the composite of all-cause death, MI or stroke. All additional endpoints were defined as previously reported12. All clinical endpoints were adjudicated by an independent external committee that was blinded to treatment allocation.
Statistical analysis
The 2019 ESC guidelines definition was applied to stratify the study population into two groups, defined as HIR and non-HIR8. Accordingly, patients were identified as HIR if they had multivessel CAD and at least one concomitant condition among diabetes mellitus, prior MI, CKD and PAD. Supplementary Table 1 details the definitions of the ESC HIR criteria in the TWILIGHT trial protocol.
Baseline demographic, clinical, and procedural characteristics were reported as mean and standard deviation for continuous variables and absolute number and frequency for categorical variables and were compared using the Student’s t-test or the chi-square test, respectively. An UpSet plot was created to quantitatively display the most frequent combinations (i.e., exclusive intersections) of the clinical and angiographic study inclusion criteria, excluding troponin-positive ACS and thrombotic target lesion, in the CCS population.
The cumulative incidence of each study endpoint in the intention-to-treat population was estimated using the Kaplan-Meier method. According to the trial protocol, patients without a BARC Type 2-5 bleeding event between randomisation and 1 year were censored at the time of death, last known contact, or at 365 days, whichever occurred first. Cox proportional hazards models were used to generate hazard ratios (HRs) with 95% confidence intervals (CIs), with formal interaction testing between the main exposure of treatment allocation (placebo vs aspirin) and HIR status (HIR vs non-HIR) to assess effect modification. Univariate Cox proportional hazards regression analyses evaluating the predictors of MACCE occurrence in the study population were conducted to explore the consistency with the HIR parameters reported by the ESC definition. Additionally, a sensitivity analysis compared the treatment effects of ticagrelor monotherapy versus DAPT in patients with and without high thrombotic risk. High thrombotic risk was defined as meeting the criteria for HIR or undergoing complex PCI, based on the definition by Giustino et al15.
A two-sided p-value of <0.05 was considered statistically significant. All analyses were performed using Stata version 16.0 (StataCorp).
Results
Prevalence of clinical and angiographic inclusion criteria in the CCS population
A total of 2,503 CCS patients were randomised in the TWILIGHT trial and included in this analysis. Multivessel CAD (71.7%), LM or proximal LAD involvement (59.8%) and stent length >30 mm (57.0%) were the most prevalent angiographic inclusion criteria, while vascular disease (69.5%), age ≥65 years (56.3%) and diabetes (40.2%) were the most common clinical features (Figure 1, Supplementary Table 2). The most frequent intersection of criteria was the combination of vascular disease, multivessel CAD, LM or proximal LAD involvement and stent length >30 mm (Supplementary Figure 1).
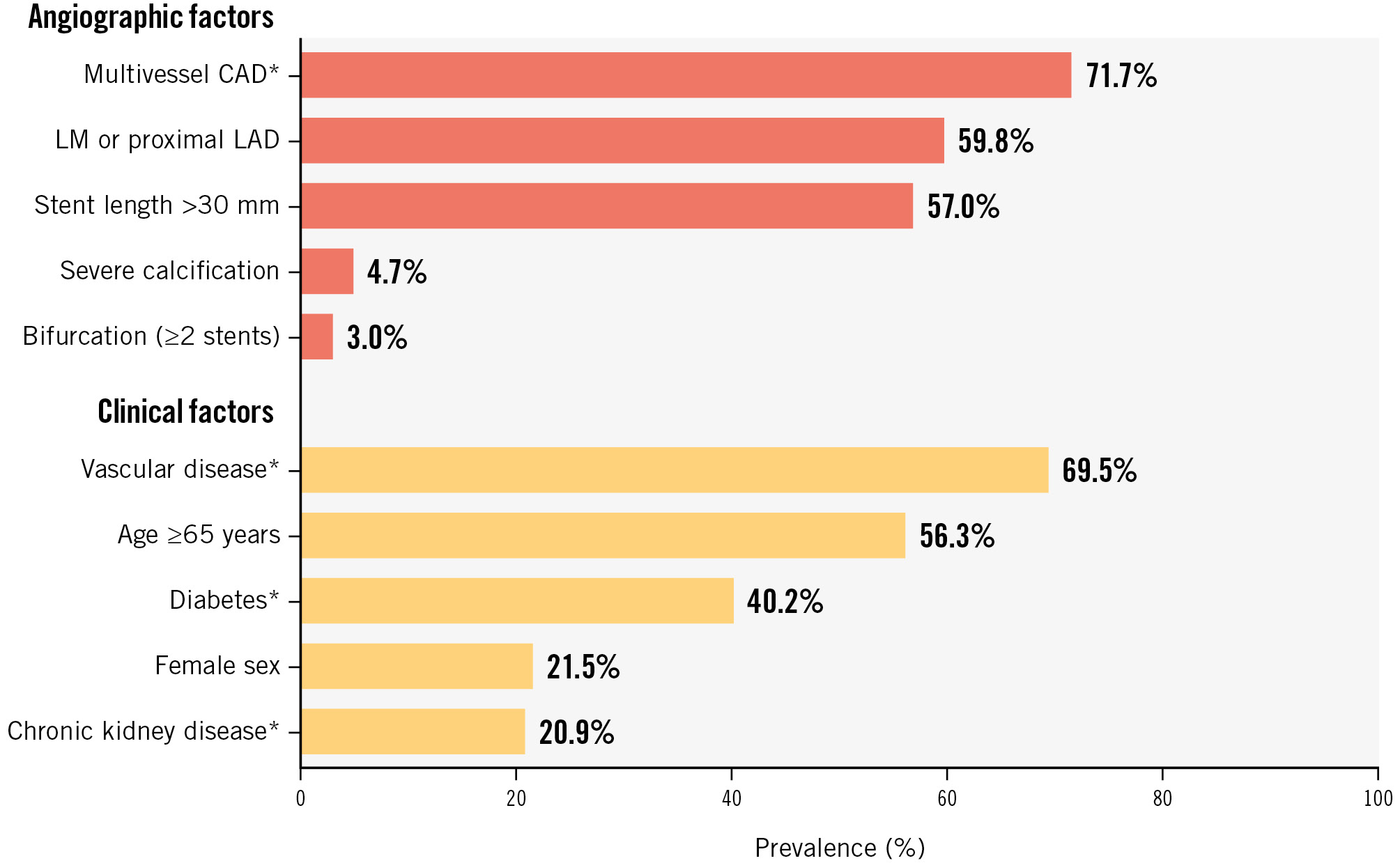
Figure 1. Prevalence of the clinical and angiographic inclusion criteria in the CCS population of the TWILIGHT trial. *Included in the ESC definition of high ischaemic risk. CAD: coronary artery disease; CCS: chronic coronary syndrome; ESC: European Society of Cardiology; LAD: left anterior descending artery; LM: left main
Patient characteristics
The ESC definition classified 50.5% of patients as HIR (N=1,264) and 49.5% as non-HIR (N=1,239) (Central illustration). Table 1 and Table 2 show the baseline clinical and procedural characteristics of the study population. There were no significant differences between treatment arms in the HIR and non-HIR strata. Overall, as compared to non-HIR patients, those classified as HIR were more commonly male, had a higher prevalence of cardiovascular risk factors, and more frequently had a history of atherosclerotic coronary or peripheral artery disease. Consistently, HIR patients were more likely to have multiple vessels and lesions treated during the index PCI.
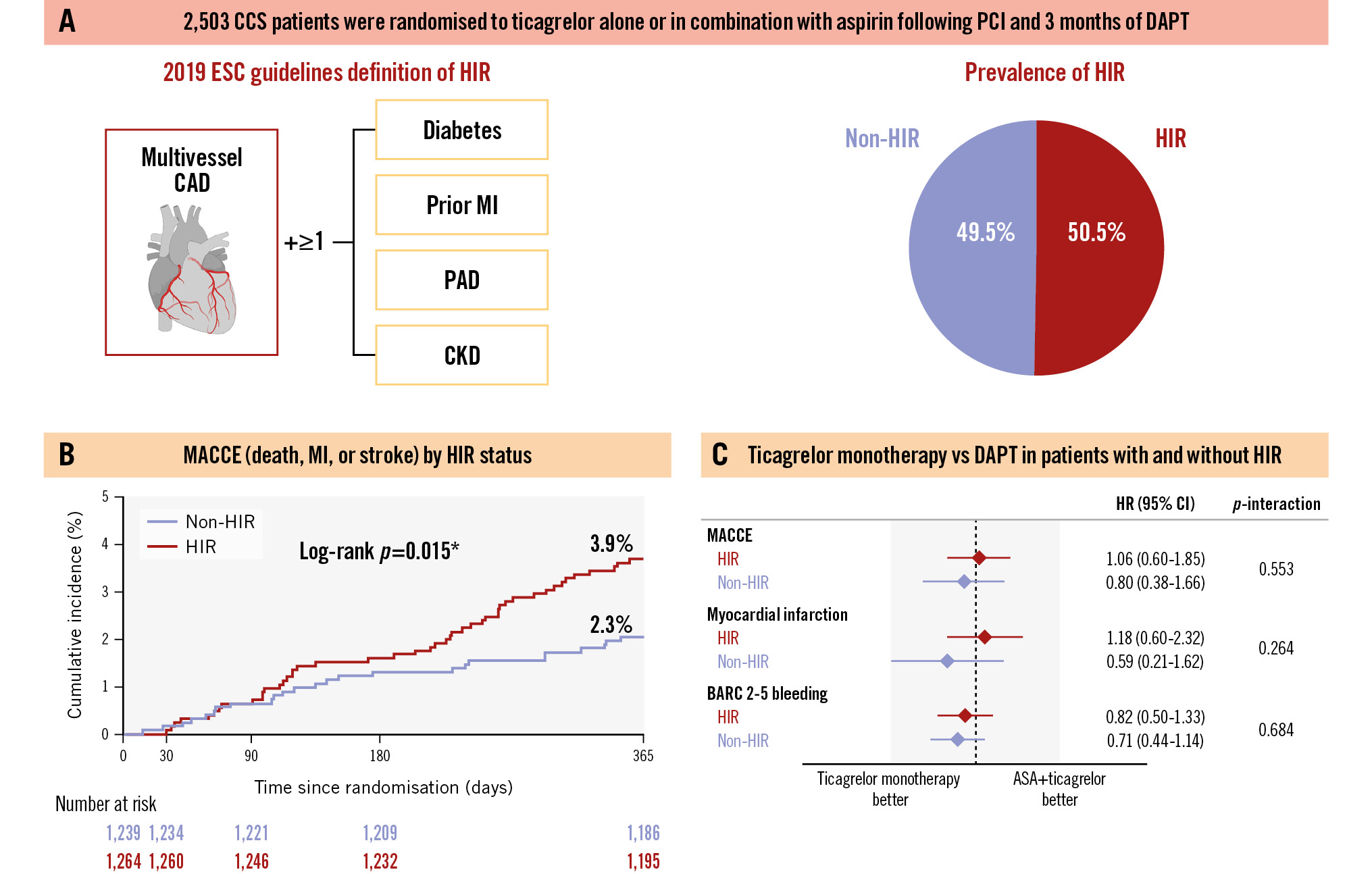
Central illustration. The TWILIGHT-CCS study. The TWILIGHT-CCS study included 2,503 patients with CCS undergoing PCI, randomised to ticagrelor alone or ticagrelor plus aspirin after completing 3 months of ticagrelor-based DAPT. Patients were stratified as HIR or non-HIR using the 2019 ESC guidelines definition, with 50.5% classified as HIR (A). At 1 year after randomisation, HIR patients had a significantly higher incidence of MACCE, including death, myocardial infarction, and stroke, compared to non-HIR patients (B). However, risks of both ischaemic and bleeding events at 1 year were similar between ticagrelor monotherapy and ticagrelor-based DAPT, regardless of HIR status (C). *p-value <0.05. ASA: aspirin; BARC: Bleeding Academic Research Consortium; CAD: coronary artery disease; CCS: chronic coronary syndrome; CI: confidence interval; CKD: chronic kidney disease; DAPT: dual antiplatelet therapy; ESC: European Society of Cardiology; HIR: high ischaemic risk; HR: hazard ratio; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction; PAD: peripheral artery disease; PCI: percutaneous coronary intervention
Table 1. Baseline clinical characteristics
| High ischaemic risk (N=1,264) | Non-high ischaemic risk (N=1,239) | |||||
|---|---|---|---|---|---|---|
| Ticagrelor N=654 (51.7%) | Ticagrelor+ASA N=610 (48.3%) | p-value | Ticagrelor N=627 (50.6%) | Ticagrelor+ASA N=612 (49.4%) | p-value | |
| Age, years | 65.5±9.6 | 65.3±10.1 | 0.751 | 66.1±9.1 | 66.2±9.0 | 0.753 |
| Female sex | 111 (17.0) | 102 (16.7) | 0.905 | 155 (24.7) | 169 (27.6) | 0.247 |
| Enrolling region | 0.614 | 0.613 | ||||
| North America | 336 (51.4) | 300 (49.2) | 265 (42.3) | 271 (44.3) | ||
| Europe | 262 (40.1) | 261 (42.8) | 291 (46.4) | 267 (43.6) | ||
| Asia | 56 (8.6) | 49 (8.0) | 71 (11.3) | 74 (12.1) | ||
| BMI, kg/m2 | 28.4 (25.6-31.6) | 28.1 (25.4-32.0) | 0.794 | 27.6 (24.7-31.0) | 27.9 (25.3-31.5) | 0.260 |
| Current smoker | 98 (15.0) | 109 (17.9) | 0.166 | 100 (15.9) | 91 (14.9) | 0.599 |
| Diabetes | 360 (55.0) | 349 (57.2) | 0.438 | 149 (23.8) | 148 (24.2) | 0.863 |
| Hypercholesterolaemia | 530 (81.0) | 485 (79.5) | 0.494 | 453 (72.2) | 461 (75.3) | 0.218 |
| Hypertension | 547 (83.6) | 519 (85.1) | 0.481 | 498 (79.4) | 478 (78.1) | 0.570 |
| Previous MI | 318 (48.6) | 321 (52.6) | 0.155 | 124 (19.8) | 110 (18.0) | 0.418 |
| Previous PCI | 441 (67.4) | 415 (68.0) | 0.819 | 283 (45.1) | 276 (45.1) | 0.989 |
| Previous CABG | 123 (18.8) | 94 (15.4) | 0.109 | 38 (6.1) | 55 (9.0) | 0.051 |
| Peripheral artery disease | 84 (12.8) | 78 (12.8) | 0.976 | 30 (4.8) | 34 (5.6) | 0.540 |
| Chronic kidney disease | 173 (27.8) | 161 (27.4) | 0.880 | 71 (11.9) | 67 (11.6) | 0.865 |
| Prior bleeding | 7 (1.1) | 8 (1.3) | 0.692 | 3 (0.5) | 7 (1.1) | 0.191 |
| Anaemia | 126 (20.3) | 119 (20.4) | 0.958 | 114 (19.2) | 94 (16.3) | 0.189 |
| LVEF, % | 55.2±9.3 | 53.1±9.3 | 0.034 | 56.1±9.0 | 54.9±9.3 | 0.224 |
| Data are n (%), mean±standard deviation, or median (interquartile range). ASA: aspirin; BMI: body mass index; CABG: coronary artery bypass grafting; LVEF: left ventricular ejection fraction; MI: myocardial infarction; PCI: percutaneous coronary intervention | ||||||
Table 2. Baseline procedural characteristics.
| High ischaemic risk (N=1,264) | Non-high ischaemic risk (N=1,239) | |||||
|---|---|---|---|---|---|---|
| Ticagrelor N=654 (51.7%) | Ticagrelor+ASA N=610 (48.3%) | p-value | Ticagrelor N=627 (50.6%) | Ticagrelor+ASA N=612 (49.4%) | p-value | |
| Radial access | 415 (63.5) | 384 (63.0) | 0.852 | 441 (70.3) | 415 (67.8) | 0.336 |
| Multivessel CAD | 654 (100) | 610 (100) | - | 209 (33.3) | 192 (31.4) | 0.461 |
| Target vessel | ||||||
| Left main | 32 (4.9) | 44 (7.2) | 0.083 | 17 (2.7) | 23 (3.8) | 0.297 |
| Left anterior descending | 300 (45.9) | 276 (45.2) | 0.823 | 382 (60.9) | 365 (59.6) | 0.644 |
| Left circumflex | 265 (40.5) | 250 (41.0) | 0.867 | 143 (22.8) | 125 (20.4) | 0.309 |
| Right coronary artery | 270 (41.3) | 264 (43.3) | 0.473 | 178 (28.4) | 198 (32.4) | 0.129 |
| Venous bypass graft | 22 (3.4) | 15 (2.5) | 0.340 | 3 (0.5) | 7 (1.1) | 0.191 |
| No. of vessels treated | 0.328 | 0.784 | ||||
| 1 | 456 (69.7) | 411 (67.4) | 546 (87.1) | 526 (85.9) | ||
| 2 | 183 (28.0) | 177 (29.0) | 70 (11.2) | 76 (12.4) | ||
| ≥3 | 15 (2.3) | 22 (3.6) | 11 (1.8) | 10 (1.6) | ||
| No. of lesions treated | 0.519 | 0.340 | ||||
| 1 | 341 (52.1) | 333 (54.6) | 419 (66.8) | 386 (63.1) | ||
| 2 | 241 (36.9) | 206 (33.8) | 155 (24.7) | 173 (28.3) | ||
| ≥3 | 72 (11.0) | 71 (11.6) | 53 (8.5) | 53 (8.7) | ||
| Moderate/severe calcification | 114 (17.4) | 111 (18.2) | 0.722 | 112 (17.9) | 99 (16.2) | 0.430 |
| Bifurcation | 72 (11.0) | 67 (11.0) | 0.988 | 77 (12.3) | 70 (11.4) | 0.646 |
| Chronic total occlusion | 51 (7.8) | 38 (6.2) | 0.276 | 43 (6.9) | 43 (7.0) | 0.907 |
| Total stent length, mm | 33.0 (20.0-51.0) | 32.0 (19.0-52.0) | 0.565 | 34.0 (23.0-51.0) | 36.0 (23.0-52.0) | 0.698 |
| Data are n (%) or median (interquartile range). ASA: aspirin; CAD: coronary artery disease | ||||||
Prevalence and clinical impact of a high ischaemic risk status
At 12 months after randomisation, HIR patients had a higher cumulative incidence of MACCE as compared with non-HIR patients (3.9% vs 2.3%; p=0.015). Conversely, the incidence of BARC Type 2-5 bleeding was not significantly different between the two groups (5.1% vs 5.7%; p=0.455) (Supplementary Figure 2).
Clinical outcomes according to randomised treatment allocation in patients with and without HIR
The effect of ticagrelor monotherapy versus DAPT on MACCE was similar in HIR (4.0% vs 3.8%, HR 1.06, 95% CI: 0.60-1.85) and non-HIR patients (2.1% vs 2.6%, HR 0.80, 95% CI: 0.38-1.66; pinteraction=0.553) (Table 3, Figure 2, Central illustration). Similarly, BARC Type 2-5 bleeding rates were not significantly different between the ticagrelor monotherapy and DAPT arms, regardless of HIR status (HIR: 4.7% vs 5.7%, HR 0.82, 95% CI: 0.50-1.33; non-HIR: 4.9% vs 6.7%, HR 0.71, 95% CI: 0.44-1.14; pinteraction=0.684) (Table 3, Figure 2, Central illustration). No significant interactions between HIR status and the effect of randomised antiplatelet strategy were detected for any of the exploratory secondary endpoints (Table 3).
Such results were consistent when stratifying patients into high (N=1,565) versus non-high thrombotic risk (N=938) (Supplementary Table 3).
Table 3. Adverse events at 1 year, stratified by randomised treatment allocation, in patients with and without high ischaemic risk.
| High ischaemic risk (N=1,264) |
Non-high ischaemic risk (N=1,239) |
Interactionp-value‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcomes | Ticagrelor mono therapy (N=654) | Ticagrelor+ASA (N=610) | Hazard ratio (95% CI) | p-value | Ticagrelor mono therapy (N=627) | Ticagrelor+ASA (N=612) | Hazard ratio (95% CI) | p-value | |
| MACCE | 26(4.0) | 23 (3.8) |
1.06 (0.60-1.85) |
0.851 | 13 (2.1) |
16 (2.6) |
0.80 (0.38-1.66) |
0.545 | 0.553 |
| Cardiovascular death, MI or ischaemic stroke | 25 (3.9) |
21 (3.5) |
1.11 (0.62-1.98) |
0.727 | 11 (1.8) |
14 (2.3) |
0.77 (0.35-1.70) |
0.520 | 0.469 |
| All-cause death | 6 (0.9) |
8 (1.3) |
0.70 (0.24-2.02) |
0.510 | 6 (1.0) |
6 (1.0) |
0.98 (0.32-3.03) |
0.969 | 0.672 |
| Cardiovascular death | 5 (0.8) |
6 (1.0) |
0.78 (0.24-2.55) |
0.680 | 4 (0.6) |
4 (0.7) |
0.99 (0.25-3.94) |
0.983 | 0.799 |
| MI | 19 (3.0) |
15 (2.5) |
1.18 (0.60-2.32) |
0.634 | 6 (1.0) |
10 (1.6) |
0.59 (0.21-1.62) |
0.305 | 0.264 |
| Ischaemic stroke | 3 (0.5) |
2 (0.3) |
1.40 (0.23-8.39) |
0.711 | 2 (0.3) |
0 (0.0) |
N/A | N/A | 0.992 |
| Stent thrombosis | 6 (0.9) |
3 (0.5) |
1.87 (0.47-7.48) |
0.376 | 0 (0.0) |
3 (0.5) |
N/A | N/A | 0.989 |
| BARC 2, 3 or 5 bleeding | 30 (4.7) |
34 (5.7) |
0.82 (0.50-1.33) |
0.419 | 30 (4.9) |
41 (6.7) |
0.71 (0.44-1.14) |
0.153 | 0.684 |
| BARC 3 or 5 bleeding | 10 (1.6) |
13 (2.2) |
0.71 (0.31-1.63) |
0.421 | 7 (1.1) |
7 (1.2) |
0.99 (0.35-2.82) |
0.982 | 0.631 |
| TIMI major bleeding | 2 (0.3) |
8 (1.3) |
0.23 (0.05-1.09) |
0.065 | 3 (0.5) |
3 (0.5) |
0.99 (0.20-4.89) |
0.987 | 0.203 |
| GUSTO moderate or severe bleeding | 7 (1.1) |
8 (1.3) |
0.81 (0.29-2.24) |
0.689 | 6 (1.0) |
5 (0.8) |
1.18 (0.36-3.88) |
0.780 | 0.636 |
| ISTH major bleeding | 11 (1.7) |
14 (2.3) |
0.73 (0.33-1.60) |
0.430 | 8 (1.3) |
7 (1.2) |
1.13 (0.41-3.12) |
0.813 | 0.502 |
| Data are number of events (%), unless otherwise indicated. The percentages mentioned above represent Kaplan-Meier rates at 12 months after the index procedure. ‡P-value is obtained from the interaction test between HIR status and randomised treatment allocation. ASA: aspirin; BARC: Bleeding Academic Research Consortium; CI: confidence interval; GUSTO: Global Utilisation of Streptokinase and Tissue plasminogen activator for Occluded arteries; ISTH: International Society on Thrombosis and Haemostasis; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction; N/A: not applicable; TIMI: Thrombolysis in Myocardial Infarction | |||||||||
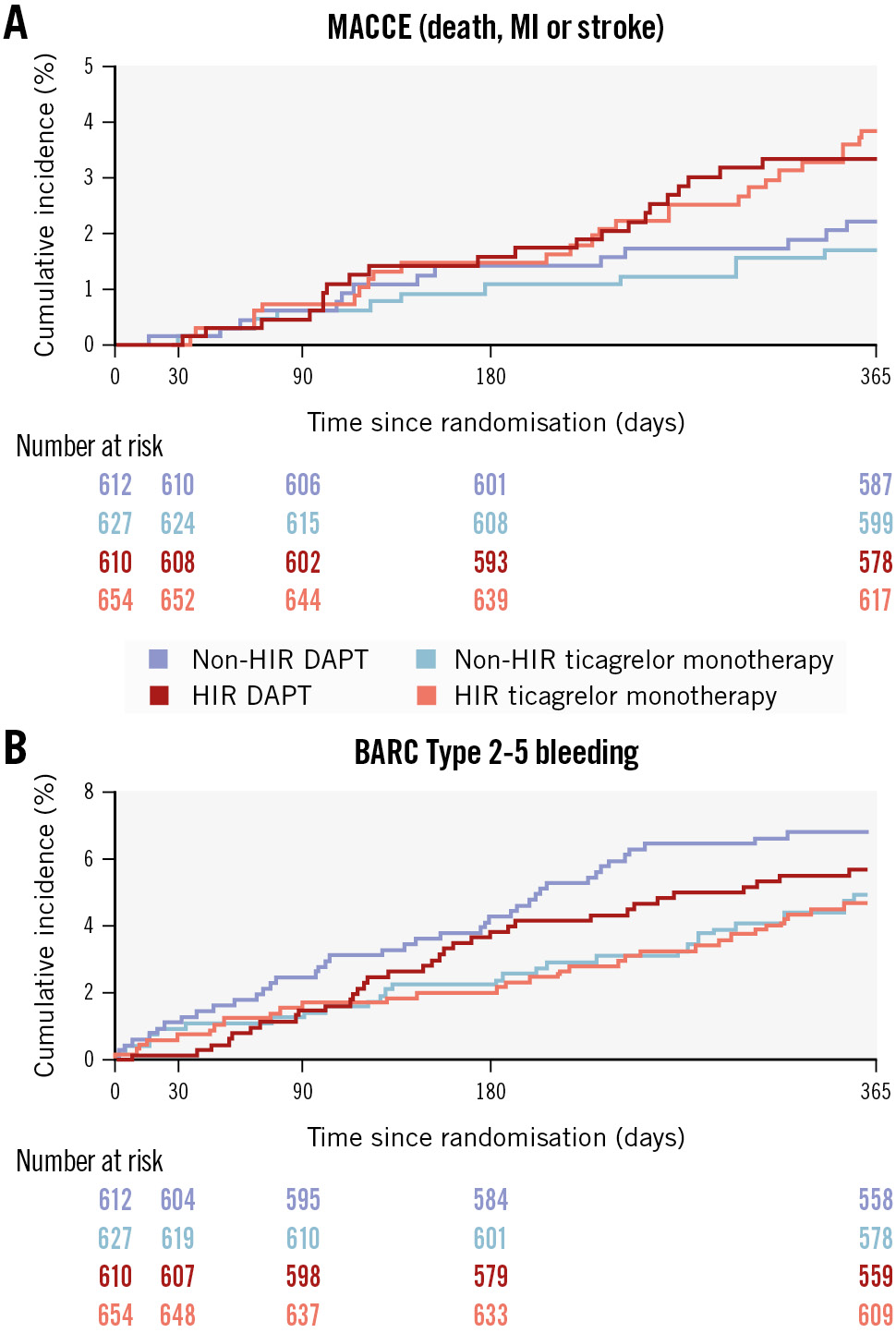
Figure 2. Cumulative incidence of MACCE and BARC Type 2-5 bleeding by randomised treatment allocation and HIR status. The Kaplan-Meier curves show the event rates for MACCE (A) and BARC 2-5 bleeding (B) at 1 year after randomisation in HIR (red/pink curves) and non-HIR (blue/light blue curves) patients treated with ticagrelor monotherapy (light blue/pink lines) or ticagrelor-based DAPT (blue/red lines). BARC: Bleeding Academic Research Consortium; DAPT: dual antiplatelet therapy; HIR: high ischaemic risk; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction
Clinical and angiographic predictors of MACCE
Increased risk of MACCE was observed for vascular disease (HR 1.37, 95% CI: 1.02-1.85; p=0.038), diabetes mellitus (HR 1.37, 95% CI: 1.06-1.77; p=0.018) and LM or proximal LAD involvement (HR 1.38, 95% CI: 1.05-1.81; p=0.021) (Supplementary Table 4). Patients with CKD had a borderline significant elevated risk of MACCE (HR 1.34, 95% CI: 0.99-1.81; p=0.057). All other clinical and angiographic features were not significantly associated with MACCE (Supplementary Table 4).
Discussion
The main findings of this analysis, which included CCS patients undergoing PCI and randomised in the TWILIGHT trial, are the following:
• The 2019 ESC definition classified approximately half of the patients as HIR and effectively identified those at higher risk of MACCE. Factors associated with an increased risk of MACCE in the TWILIGHT CCS population were vascular disease, diabetes, and LM or proximal LAD involvement, aligning with the criteria outlined in the ESC definition.
• Ticagrelor monotherapy, following a 3-month course of DAPT, was not associated with an increased risk of MACCE compared with standard ticagrelor-based DAPT, regardless of HIR status.
Patients with CCS undergoing PCI are generally perceived to be at lower ischaemic risk compared to those treated for MI. However, a significant proportion of CCS patients undergoing PCI present with clinical and/or angiographic complexity, which contributes to a substantial risk of recurrent ischaemic events16. Notably, diabetic patients, who account for up to 40% of patients undergoing PCI for CCS, have double the risk of cardiac death or MI in the first years after PCI compared with non-diabetic patients1718. Similarly, CKD heightens susceptibility to ischaemic events following stent implantation, which is due not only to associated comorbidities but also to factors such as inflammation, vascular calcifications, and endothelial dysfunction19. Additionally, patients with extensive atherosclerosis or prior atherosclerotic cardiovascular events are more likely to experience recurrent cardiac events20.
In 2019, the ESC guidelines on CCS introduced a multiparametric definition of HIR to identify patients who might benefit most from prolonged DAPT and/or potent P2Y12 inhibitors8. This was largely based on findings from the PEGASUS-TIMI 54 trial, which demonstrated a significant reduction in ischaemic events with prolonged ticagrelor-based DAPT compared with aspirin monotherapy in patients with prior MI, with consistent benefits observed in those with multivessel CAD621. However, prolonged DAPT with aspirin and ticagrelor was also associated with an increased risk of Thrombolysis in Myocardial Infarction (TIMI) major bleeding, potentially offsetting the ischaemic protection6. Furthermore, the time from PCI to randomisation in the PEGASUS-TIMI 54 trial ranged from 1 to 3 years, making its findings less generalisable for decision-making immediately post-PCI.
The HIR criteria defined by the 2019 ESC guidelines were consistent with the main correlates of MACCE in the present study, including LM or proximal LAD involvement, diabetes, vascular disease (including PAD and prior MI), and CKD. The specific design of the TWILIGHT trial – wherein high-risk patients were treated with ticagrelor-based DAPT for 3 months after PCI and subsequently randomised only if they remained free from adverse events – explains the high prevalence of HIR (50.5%) and the relatively low MACCE rate (3%) observed in the CCS population13. Indeed, treating CCS patients with ticagrelor was not recommended by practice guidelines at the time the trial was conducted, but the TWILIGHT inclusion criteria were in line with the subsequent 2019 ESC guidelines recommendation for ticagrelor-based DAPT in CCS (only in case of high-risk elective stenting)8. Nonetheless, patients who met the HIR definition experienced an almost twofold higher risk of MACCE, highlighting the critical importance of risk stratification at the time of PCI in CCS patients.
Notably, the strategy of ticagrelor monotherapy was associated with a similar risk of MACCE compared with 12 months of ticagrelor-based DAPT even in patients classified as HIR. On the other hand, we did not observe a significant bleeding risk reduction in either the HIR or non-HIR groups. This result was consistent with a previous substudy and is potentially attributable to the imbalance in baseline characteristics between CCS and ACS patients (e.g., older age and lower prevalence of female sex and Asian race in the CCS cohort), the higher rate of ticagrelor discontinuation among CCS patients, and, most importantly, the lack of statistical power in the CCS subgroup13. However, this finding was recently corroborated by a large individual patient-data meta-analysis showing no benefit of ticagrelor monotherapy over DAPT in terms of BARC 2-5 bleeding in approximately 6,000 CCS patients (4.5% vs 4.8%, HR 0.92, 95% CI: 0.72-1.17; p=0.478), while a significant reduction in bleeding was observed in the ACS setting22.
The potential benefits of a DAPT regimen with potent P2Y12 inhibitors over clopidogrel in CCS patients remain a topic of debate. The ALPHEUS trial demonstrated no significant reduction in PCI-related MI and higher rates of minor bleeding at 30 days with ticagrelor compared to clopidogrel23. Similarly, the Intensified Loading With Prasugrel Versus Standard Loading with Clopidogrel in Invasive-treated Patients With Biomarker-Negative Angina Pectoris (SASSICAIA) trial found no additional benefit with a prasugrel loading strategy compared to standard clopidogrel loading24. In contrast, the PRASugrel For Japanese PatIenTs with Coronary Artery Diseases Undergoing Elective PCI (PRASFIT-Elective) study, which evaluated major cardiovascular events up to 1 year as the primary endpoint, demonstrated the superiority of prasugrel over clopidogrel in Japanese patients undergoing elective PCI25. More recently, promising data supporting the use of ticagrelor monotherapy over clopidogrel-based DAPT or clopidogrel monotherapy have been reported. An individual patient-data meta-analysis of six randomised clinical trials (RCTs) showed that clopidogrel monotherapy significantly reduced the risk of bleeding as compared to standard DAPT with either clopidogrel or ticagrelor, but at the cost of a 37% higher risk of death and MI, while ticagrelor monotherapy reduced bleeding without increasing ischaemic events26. Similarly, a network meta-analysis evaluating different antithrombotic strategies within the first year after PCI found that clopidogrel-based DAPT was associated with a higher risk of all-cause and cardiovascular mortality compared with ticagrelor monotherapy27. When our results are considered in light of these recent meta-analyses, it appears that short DAPT followed by ticagrelor monotherapy represents a safe and effective antithrombotic regimen for patients with CCS and HIR undergoing PCI. However, a tailored risk assessment that incorporates both ischaemic and bleeding risk scores is essential to identify patients most likely to benefit from this strategy28.
Finally, important considerations in broadening ticagrelor use to CCS patients are the associated costs and the limited approval for reimbursement in certain countries. Ticagrelor-based DAPT has been shown to be cost-effective in high-risk CCS patients2930, and it is reasonable to speculate that the same might apply to ticagrelor monotherapy, particularly with the increasing availability of generic formulations. Nevertheless, dedicated cost-effectiveness analyses are warranted.
Limitations
Firstly, the results of this post hoc analysis of an RCT should be regarded as hypothesis-generating, necessitating further dedicated studies for confirmation. Secondly, given the inclusion criteria of the TWILIGHT trial, the study focused on a selected high-risk population, potentially excluding patients with lower ischaemic and bleeding risks. As a result, it is likely that the proportion of HIR patients in our study may overestimate the prevalence of HIR in the general CCS population. Similarly, the absence of a comparator arm treated with clopidogrel, which is the most commonly used P2Y12 inhibitor in patients with CCS, is an inherent limitation of the present analysis. Thirdly, the relatively small sample size in the CCS group, along with the low absolute number of events, may have increased the risk of type II error. Fourthly, while this substudy considered MACCE as the main outcome of interest, the primary endpoint of the TWILIGHT trial was BARC Type 2-5 bleeding. Fifthly, as with the main trial, these results may not be generalisable to patients who do not complete an initial 3-month DAPT run-in phase. Finally, the most updated HIR definition from the 2024 ESC guidelines on CCS, which include slightly different angiographic risk criteria, were not available at the time this analysis was designed9.
Conclusions
In patients with CCS undergoing PCI who were randomised in the TWILIGHT trial, the ESC definition of HIR identified those at heightened risk of MACCE at 1 year. Short-term DAPT followed by ticagrelor monotherapy resulted in a similar risk of ischaemic and bleeding events compared to standard ticagrelor-based DAPT in both HIR and non-HIR patients. Although hypothesis-generating, because of the small sample size and post hoc design of the analysis, these findings support the potential expansion of current guideline indications for ticagrelor monotherapy in CCS patients undergoing PCI.
Impact on daily practice
A consistent proportion of patients undergoing percutaneous coronary intervention (PCI) for chronic coronary syndrome (CCS) have a high risk of recurrent ischaemic events, for which guidelines recommend a prolonged dual antiplatelet therapy (DAPT) course and/or potent P2Y12 inhibition; despite being effective, such strategies might increase bleeding and worsen patients’ prognosis. In this analysis, we focused on CCS patients enrolled in the TWILIGHT trial and applied the European Society of Cardiology guidelines definition to identify those at high ischaemic risk (HIR). As compared to 12-month ticagrelor-based DAPT, ticagrelor monotherapy after a 3-month DAPT course was associated with a similar incidence of major adverse cardiac and cerebrovascular events and bleeding at 1 year regardless of the HIR status, thereby supporting its use in patients with CCS and HIR undergoing PCI.
Funding
This work was supported by an investigator-initiated grant from AstraZeneca.
Conflict of interest statement
U. Baber has received honoraria from AstraZeneca and Boston Scientific. D.J. Angiolillo has received consulting fees or honoraria from Abbott, Amgen, AstraZeneca, Bayer, Biosensors, Boehringer Ingelheim, Bristol-Myers Squibb, Chiesi, CSL Behring, Daiichi Sankyo, Eli Lilly, Faraday, Haemonetics, Janssen, Merck, Novartis, Novo Nordisk, PhaseBio, PLx Pharma, Pfizer, Sanofi, and Vectura, outside the present work; and also declares that his institution has received research grants from Amgen, AstraZeneca, Bayer, Biosensors, CeloNova, CSL Behring, Daiichi Sankyo, Eisai, Eli Lilly, Faraday, Gilead, Janssen, Matsutani Chemical Industry Co., Merck, Novartis, Osprey Medical, RenalGuard Solutions, and the Scott R. MacKenzie Foundation. D.J. Cohen received research grant support (to institution) from Edwards Lifesciences, Abbott, Boston Scientific, Medtronic, CathWorks, Philips, Zoll Medical, and iRhythm; and consulting income from Edwards Lifesciences, Abbott, and Elixir Medical. J. Escaned has received consulting and lecture fees from Abbott, Philips, Boston Scientific, and Medtronic; and has received lecture fees from Abiomed, Terumo, and Biosensors. C.M. Gibson received research support from Johnson & Johnson and CSL Behring; he also receives consulting support from AstraZeneca, BMS, CSL Behring, Johnson & Johnson, Janssen, and Bayer. K. Huber has received lecture fees from AstraZeneca and Bayer. A. Kastrati is an inventor in a patent application related to drug-eluting stent technology; he also serves in the Data and Safety Monitoring Board of the TARGET IV trial sponsored by the Cardiovascular Research Foundation in New York, USA. R. Mehran reports institutional research payments from Abbott, Alleviant Medical, Beth Israel Deaconess Medical Center, Concept Medical, CPC Clinical Research, Cordis, Elixir Medical, Faraday Pharmaceuticals, Idorsia Pharmaceuticals, Janssen, MedAlliance, Mediasphere Medical, Medtronic, Novartis, Protembis GmbH, RM Global BioAccess Fund Management, and Sanofi US Services, Inc.; personal fees from Elixir Medical, IQVIA, Medtronic, Medscape/WebMD Global, and Novo Nordisk; has equity <1% in Elixir Medical, Stel, and ControlRad (spouse); and no fees from SCAI (Women in Innovations Committee Member), Faculty Cardiovascular Research Foundation (CRF), and Women as One (Founding Director); and honorarium from AMA - JAMA Cardiology (Associate Editor), and ACC (BOT Member, SC Member CTR Program). P.G. Steg received research grants from Amarin, Bayer, Sanofi, and Servier; and speaking or consulting fees from Amarin, Amgen, AstraZeneca, Bayer/Janssen, Bristol-Myers Squibb, Idorsia, MyoKardia, Novartis, Novo Nordisk, PhaseBio, Pfizer, Regeneron, Sanofi, and Servier. V. Kunadian has received personal fees/honoraria from Bayer, AstraZeneca, Abbott, Amgen, and Daiichi Sankyo. S.R. Mehta has received grant support from and has served on an executive committee and as site investigator for AstraZeneca. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

