Transcatheter edge-to-edge repair (TEER) using the MitraClip device (Abbott) has been a significant advancement in the management of patients with severe mitral regurgitation (MR) who are considered high risk for conventional surgery1. Despite its widespread acceptance and growing application, the long-term benefits and patient selection criteria continue to be explored and debated within the clinical community234.
This single-centre retrospective study represents a comprehensive review of long-term outcomes with TEER using MitraClip, performed at the cardiac surgery department of San Raffaele University Hospital in Milan, Italy. It included a cohort of 150 consecutive patients with severe MR (≥3+) on echocardiographic assessment, treated between October 2008 and January 2013 for both primary (PMR) and secondary MR (SMR) (Supplementary Table 1), who were followed for 10 years after the procedure. Before the intervention, all patients were evaluated by a dedicated Heart Team.
EVEREST eligibility criteria5 were used as a reference, but patients outside them were also included. SMR patients were retrospectively assessed for COAPT eligibility6. All patients received first-generation MitraClip devices.
The cohort primarily consisted of males (78%), and the median age was 73.2 years. A significant portion (107 patients, 71.3%) suffered from SMR due to left ventricular dysfunction, as indicated by a median left ventricular ejection fraction (LVEF) of 26% (about 30% of them had an LVEF <20%, and 16.5% of patients were COAPT-like). This subset of patients typically presents a more complex challenge due to the underlying cardiac pathology contributing to the MR.
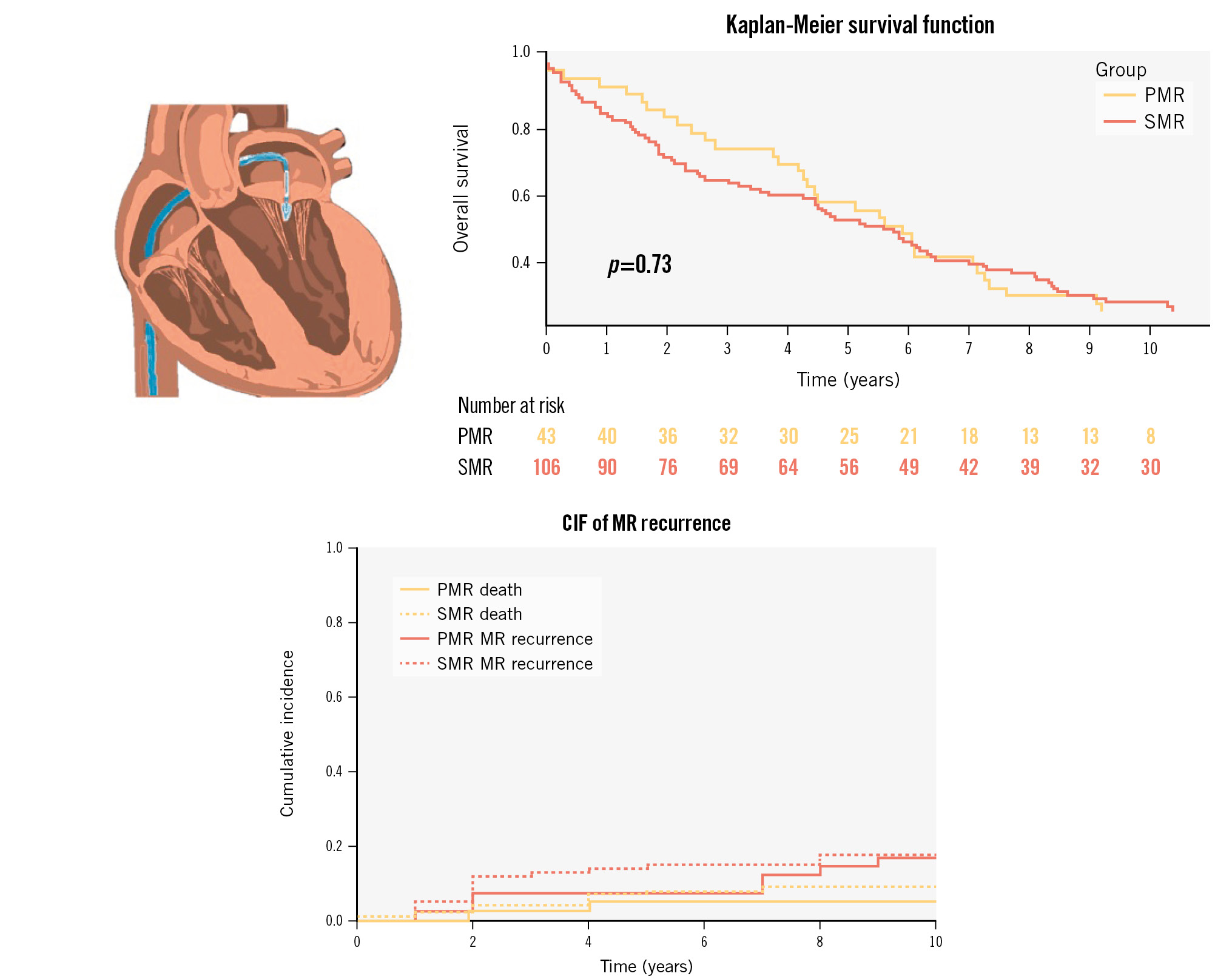
The procedural success was measured by the degree of MR reduction achieved immediately post-implantation. Initial results were promising, with most patients experiencing a significant decrease in MR severity. However, residual MR greater than 3+ was still observed in 11.3% of the patients at the time of discharge (Supplementary Table 2). Over the course of follow-up, comprehensive echocardiographic data were acquired with a high retention rate (96% of patients, median time 4.6 [1.4-5.9] years). The median follow-up duration was 5.7 years, during which a substantial number of patients (119) passed away, with almost half of these deaths (48.7%) attributable to cardiovascular causes. Heart failure-related rehospitalisation was 51.1±5.1%, and 12 patients underwent further procedures during the follow-up period (Supplementary Figure 1, Supplementary Figure 2, Supplementary Table 3, Central illustration). The long-term durability of the repair was assessed, and the cumulative incidence function (CIF) of MR recurrence, with death as the competing risk, was 49±21.5% for SMR and 23.8±23.8% for PMR at 1 year, and 21.5±4% for SMR and 19±6% for PMR at the 10-year benchmark (Supplementary Figure 3, Central illustration).
Survival analysis showed that only 25.3% (38/150 patients) of the initial cohort were still alive after 10 years. Survivors, compared to non-survivors, tended to be younger (age: 66.3±10.9 years vs 73.5±10.0 years; p<0.01), had a lower incidence of atrial fibrillation (21.1% vs 46.4%; p<0.01), better kidney function (estimated glomerular filtration rate: 58.7 [37.3-100.7] ml/min vs 46.1 [34.4-65.7] ml/min; p=0.01), and lower predicted mortality rates according to the Seattle Heart Failure Model (SHFM). Notably, these patients also demonstrated a higher procedural success rate, with a significant reduction in MR observed immediately post-procedure (residual MR 0-1: 89.5% vs 59.8%; p=0.004). There were no differences in secondary MR aetiology distribution (78.9% vs 68.8%; p=0.23), LVEF (32±14 vs 38±18; p=0.06), or EVEREST-like patients (50.0% vs 44.6%; p=0.57) (Supplementary Table 4, Supplementary Table 5).
A logistic regression analysis was performed to identify factors independently associated with increased mortality risk. Age (odds ratio [OR] 1.07, 95% confidence interval [CI]: 1.02-1.13; p=0.006) and the presence of significant residual MR ≥2+ (OR 8.72, 95% CI: 2.08-36.61; p=0.003) were the most potent predictors of poor outcomes, highlighting the importance of achieving optimal MR reduction during the initial procedure (Supplementary Table 6). Residual MR 2+ was confirmed to be associated with 10-year mortality after removing patients with acute residual MR 3+ and 4+ (OR 17, 95% CI: 2.3-131.4; p=0.006 for 2+ vs 0/1+). A COAPT-like profile was not associated with survival benefit in the SMR subgroup. In the SMR subgroup, predicted 5-year SHFM versus observed mortality was 49.5% versus 29.9%; p<0.001. At 10 years post-MitraClip, 79% of living patients were in New York Heart Association Class I-II.
This study provides valuable insights into the long-term outcomes with TEER using MitraClip, marking it, to the best of our knowledge, as the longest follow-up reported to date234. The findings underscore the effectiveness of the procedure in a select group of patients while also emphasising the critical nature of patient selection and procedural success in determining long-term survival and quality of life7.
The major limitations of the study are its retrospective single-centre nature, the small number of high-risk patients treated and eligible for 10-year follow-up, and the first experience of our operators in using the first-generation MitraClip device, which is now outdated.
In conclusion, TEER with MitraClip offers a treatment option for patients with severe MR who are not candidates for surgery. While the procedure has shown promising results, the ongoing challenge remains in refining patient selection and improving techniques to ensure the best possible outcomes. As technology advances and more data become available, these insights will continue to shape the future of MR management, ensuring that patients receive the most effective and personalised care possible.
Central illustration. Ten-year outcomes of patients treated with 1st-generation MitraClip between 2008 and 2013 for primary and secondary MR. CIF: cumulative incidence function; MR: mitral regurgitation; PMR: primary MR; SMR: secondary MR
Conflict of interest statement
F. Maisano received grants and/or institutional research support from Abbott, Medtronic, Edwards Lifesciences, Biotronik, Boston Scientific, NVT, Terumo, Venus Medtech, and Pie Medical Imaging; he received consulting fees, personal and institutional honoraria from Abbott, Medtronic, Edwards Lifesciences, Xeltis, Venus Medtech, Occlufit, Simulands, Mtex, and Squadra; he has received royalty income/IP rights from Edwards Lifesciences; and he received stock from Magenta Medical, Transseptal Solutions, and 4Tech. P. Denti received speaker honoraria from Abbott and Edwards Lifesciences; has been a consultant for HVR, Approxima, InnovHeart, and Pi-Cardia. N. Buzzatti has been a consultant for InnovHeart. F. Ancona received speaker honoraria from Abbott. M. De Bonis received speaker honoraria from Medtronic and Edwards Lifesciences; and is co-founder and CMO of Startric. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

