Cory:
Unlock Your AI Assistant Now!
Accurate balloon-expandable valve (BEV) sizing for transcatheter aortic valve implantation (TAVI) is particularly important considering the high radial force and the adverse consequences of inaccurate sizing. Under- or oversizing can lead to suboptimal results, increasing the risk of paravalvular regurgitation, higher residual gradients which may potentially impact durability, and increasing the risk of annular injury1.
Current practice suggests that the ideal annular area oversizing with BEVs is between 0% and 10%. In intermediate annular sizes, this could be effectively achieved using the current SAPIEN 3 Ultra BEV (Edwards Lifesciences) technology by adding or removing volume from the inflation balloon23.
In this analysis of the multicentre OPERA-TAVI registry, we aimed to compare outcomes of patients receiving the SAPIEN 3 Ultra BEV in optimal or intermediate sizing zones (within or outside the 0-10% area oversizing range, respectively)4.
The registry protocol was approved by the local institutional review board at each participating centre.
Excluding native bicuspid aortic valves (n=38), a total of 723 patients with available native annular area measurements received the SAPIEN 3 Ultra transcatheter heart valve (THV). Among these, 275 patients (38.0%) were within the optimal sizing zones, whereas 448 patients (62.0%) were in the intermediate sizing zones. Most patients in the intermediate zones received a larger THV size.
After propensity score matching (PSM), a total of 223 pairs of patients with similar clinical and anatomical characteristics were compared. The variables included and the specifics of the PSM, as well as statistical analysis, were consistent with those previously used in the registry4. The primary outcomes were device success and early safety as defined by the Valve Academic Research Consortium (VARC)-3 consensus5.
Patients had a median age of 82 years and a low surgical risk profile. About 64% of patients had moderate/severe leaflet calcification, and 8% had moderate/severe left ventricular outflow tract calcification.
At 30 days, neither VARC-3 device success (90.6% vs 91.9%; p=0.74) nor VARC-3 early safety (88.3% vs 85.2%; p=0.40) differed between patients in the optimal or intermediate sizing zones (Central illustration).
Importantly, the rate of annular rupture was similar between groups (1.3% vs 0.4%; p=0.62).
At 30 days, the rates of patient-prosthesis mismatch (42.2% vs 27.5%; p=0.10) and paravalvular regurgitation (moderate/severe: 2.1% vs 0.0%; p=0.10; mild: 20.1% vs 13.5%; p=0.06) were numerically lower for patients in intermediate sizing zones.
These data provide reassurance about the safety and effectiveness of TAVI using the SAPIEN 3 Ultra BEV in intermediate sizing zones.
Recently, a novel BEV with intermediate sizes has been approved in Europe, facilitating more accurate patient-specific BEV sizing6. The possibility to choose an intermediate, balloon-expandable THV size has potential advantages in terms of obtaining the best valve performance with lower leaflet tissue stress. However, the real impact of the introduction of intermediate BEV sizes remains unclear.
The main limitations of this subanalysis were related to the small sample size which implied a statistical underpower and the fact that the registry did not capture the eventual changes in filling volume adopted in each case by TAVI operators. Further, prospective studies with longer follow-up are needed to understand the importance of having dedicated intermediate sizes of BEVs.
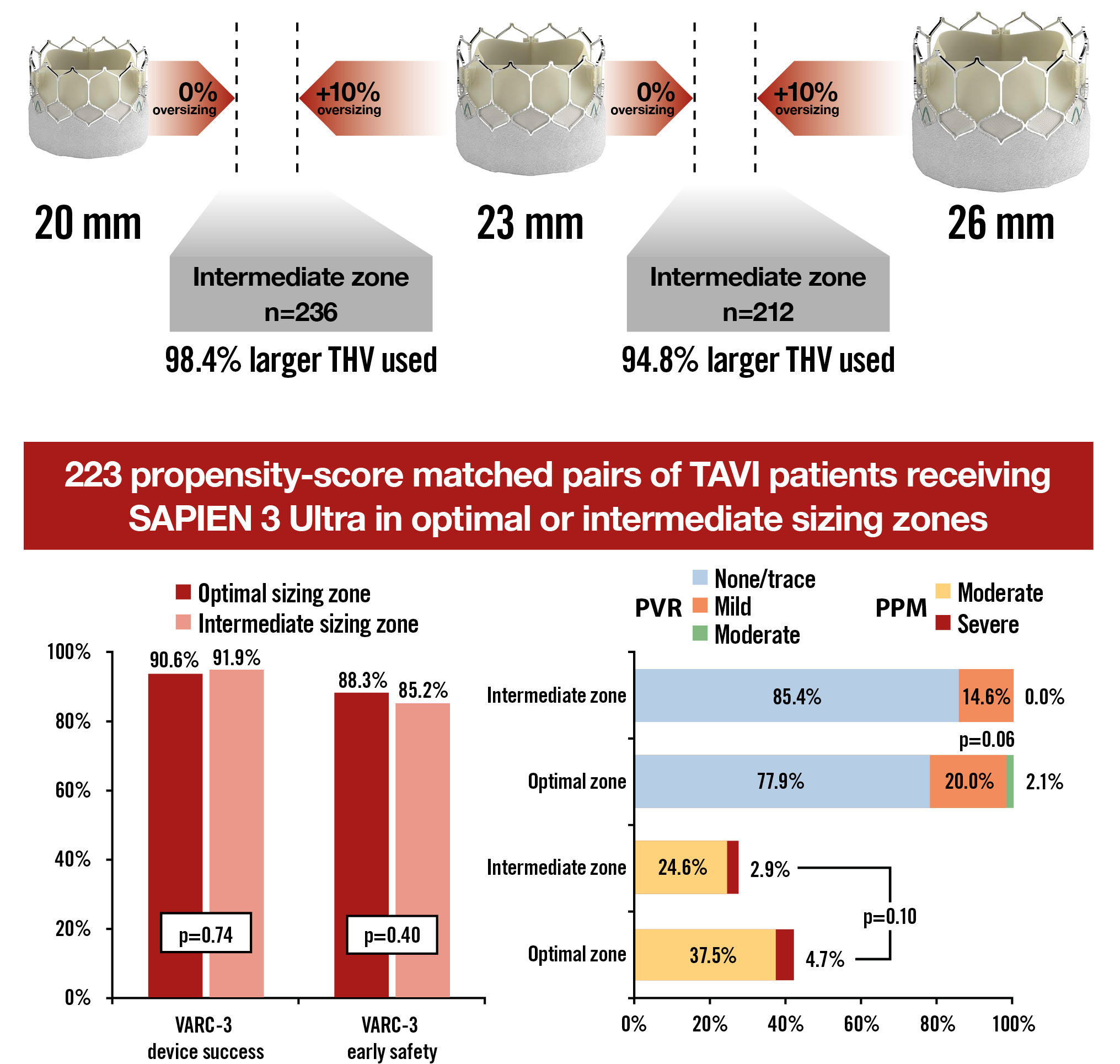
Central illustration. Outcomes of balloon-expandable SAPIEN 3 Ultra transcatheter heart valve in intermediate sizing zones. PPM: patient-prosthesis mismatch; PVR: paravalvular regurgitation; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; VARC: Valve Academic Research Consortium
Guest Editor
This paper was guest edited by Franz-Josef Neumann, MD, PhD; Department of Cardiology and Angiology, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany.
Conflict of interest statement
T. Pilgrim reports research, travel or educational grants to the institution without personal remuneration from Biotronik, Boston Scientific, Edwards Lifesciences, and ATsens; and speaker fees and consultancy fees to the institution from Biotronik, Boston Scientific, Edwards Lifesciences, Abbott, Medtronic, Biosensors, and Highlife. M. Abdel-Wahab has reported that his institution received speaker honoraria and/or consultancy fees on his behalf from Boston Scientific and Medtronic. A. Latib has reported serving on advisory boards or as a consultant for Medtronic, Boston Scientific, Philips, Edwards Lifesciences, and Abbott. D. Mylotte has reported that he is a consultant for Medtronic, Boston Scientific, and MicroPort. M. Barbanti has reported that he is a consultant for Boston Scientific, Edwards Lifesciences, and Medtronic. The other authors have no conflicts of interest relevant to the contents of this paper to declare. The Guest Editor reports consultancy fees from Novartis and Meril Life Sciences; speaker honoraria from Boston Scientific, Amgen, Daiichi Sankyo, and Meril Life Sciences; speaker honoraria paid to his institution from BMS/Pfizer, Daiichi Sankyo, Boston Scientific, Siemens, and Amgen; and research grants paid to his institution from Boston Scientific and Abbott.

