Abstract
Background: Whether saline-induced hyperaemia captures exercise-induced coronary flow regulation remains unknown.
Aims: Through this study, we aimed to describe absolute coronary flow (Q) and microvascular resistance (Rμ) adaptation during exercise in participants with angina with non-obstructive coronary artery disease (ANOCA) and to explore the correlations between saline- and exercise-derived coronary flow reserve (CFR) and microvascular resistance reserve (MRR).
Methods: Rμ, Q, CFR and MRR were assessed in the left anterior descending artery using continuous thermodilution with saline infusion at 10 mL/min (rest), 20 mL/min (hyperaemia) and finally at a 10 mL/min infusion rate during stress testing with a dedicated supine cycling ergometer. An incremental workload of 30 watts every two minutes was applied. A saline-derived CFR (CFRsaline) cutoff <2.5 was used to identify coronary microvascular dysfunction (CMD).
Results: CFRsaline-defined CMD was observed in 53.3% of the participants (16/30). While cycling, these patients less of an ability to increase Q (7 [interquartile range [IQR] 30.5-103.0] vs 21 [IQR 5.8-45.0] mL/min/30 watts; p=0.01) due to a smaller decrease of Rμ (109 {IQR 32-286} vs 202 [IQR 102-379] Wood units [WU]/30 watts; p<0.01) as compared with the group with normal CFRsaline. In the overall population, CFRsaline and exercise-derived CFR (CFRexercise) were 2.70±0.90 and 2.85±1.54, respectively, with an agreement classification of 83.3%. A good correlation between saline and exercise techniques for both CFR (r=0.73; p<0.0001) and MRR (r=0.76; p<0.0001) was observed. Among participants with normal CFRsaline, 28.7% (4/14) had an impaired CFRexercise <2.5 at the peak of exercise due to a moderate and late decrease of Rμ.
Conclusions: Saline-induced hyperaemia provided a valid surrogate for exercise physiology independently of the absolute level of CFR and MRR, although exercise provided more granularity to evaluate adaptation among participants with exercise-related CMD.
Angina with non-obstructive coronary artery disease (ANOCA) affects up to 50% of patients with chronic coronary syndrome undergoing coronary angiography12. ANOCA can result from various mechanisms that underlie the insufficient increase in coronary blood flow from rest to stress, such as coronary microvascular dysfunction (CMD) in two-thirds of cases34. Despite being associated with adverse outcomes256 and impaired quality of life78, patients with ANOCA exhibit a wide range of symptoms and signs that are frequently misdiagnosed as non-cardiac conditions, resulting in underdiagnosis and inadequate treatment9.
According to the European Society of Cardiology (ESC), the investigation of CMD should be considered (Class IIa recommendation) for a comprehensive diagnostic evaluation of patients with suspected ANOCA, assessing coronary flow reserve (CFR)10. Recent developments have introduced a new invasive method based on continuous thermodilution which enables direct11, accurate12, safe13, operator-independent and reproducible1415 assessment of absolute coronary blood flow (Q; mL/min) and microvascular resistance (Rμ; Wood units [WU]). Baseline absolute Q and Rμ are assessed using an infusion of saline at 10 mL/min in the left anterior descending artery (LAD) through a dedicated catheter, while hyperaemia is provoked by a saline infusion at 20 mL/min, triggering adenosine triphosphate release1617. The concept of microvascular resistance reserve (MRR) was then introduced as a specific index for the microvasculature, independent of autoregulation and myocardial mass18.
It is unknown whether this saline-induced hyperaemia (SIH) captures the complex systemic, metabolic, mechanical and neurohormonal mechanisms leading to Q regulation during exercise. Our objectives were to describe absolute Q and Rμ adaptation during exercise in participants with ANOCA and to explore correlations between saline- and exercise-derived CFR and MRR.
Methods
Study design and eligibility
The present study included outpatients who were suspected of having ANOCA based on the criteria of the European Association of Percutaneous Cardiovascular Interventions (EAPCI) expert consensus19. Suspicion of microvascular angina was defined as symptoms of myocardial ischaemia (i.e., rest or effort angina, exertional dyspnoea) and/or evidence of myocardial ischaemia (i.e., presence of reversible defect, abnormality on a functional imaging test) and the absence of obstructive coronary artery disease (CAD) on coronary angiography (i.e., <50% coronary epicardial lumen diameter reduction or fractional flow reserve [FFR]>0.80). Exclusion criteria were the inability to perform the cycling exercise, radial artery puncture not being feasible, recent acute coronary syndrome (<6 months), associated cardiomyopathy, impaired left ventricular systolic function (<50%), valvular heart disease (more than mild, ≥2/4), atrial fibrillation, presence of a pacemaker or defibrillator, severe renal failure (estimated glomerular filtration rate <30 mL/min/m2) and pregnancy. Participants were asked to discontinue coffee, nicotine and antianginal medications including beta blockers, calcium channel blockers and nitrates 48 hours before angiography.
This study was approved by the local ethics committee (CER-PINOCA) of Sorbonne University. It was supported and driven by the ACTION Study Group. All individuals provided oral and written informed consent before enrolment.
Clinical data collection
Smoking status was recorded as active (current smoker or cessation <3 months) or not. Family history of CAD was defined as any coronary event that occurred in first-degree relatives of the individual before 55 years of age in males and before 65 years in females. Dyslipidaemia was defined as known but untreated dyslipidaemia, according to the ESC guidelines, or treatment with lipid-lowering medications20. Individuals were qualified as diabetic if they were previously taking antidiabetic drugs, if their fasting glucose level was greater than 1.26 g/L on two blood samplings, or if glycated haemoglobin was greater than 6.5%. Hypertension was identified as an average blood pressure of three consecutive readings greater than 140/90 mmHg during a previous hospitalisation or visit or if individuals had previously been taking antihypertensive drugs. The presence of chronic inflammatory or immunosuppressive disease, such as human immunodeficiency virus infection, viral hepatitis or any other chronic inflammatory disease including cancer, was recorded. Baseline clinical evaluation included a physical examination, a resting electrocardiogram (ECG), and a blood sampling including complete blood count, serum electrolytes, creatinine, and low-density lipoprotein cholesterol.
Coronary angiography protocol
Coronary angiography was performed via the right radial artery. A 6 Fr guiding catheter was advanced in the left coronary ostium. First, absolute Q and Rμ were measured at rest and during hyperaemia using continuous thermodilution with intracoronary saline infusion. The LAD was instrumented with a pressure/temperature sensor-tipped guidewire (St Jude/Abbott) and an over-the-wire coronary infusion catheter (RayFlow [Hexacath]) positioned in the proximal segment and connected to an infusion pump (MEDRAD [Bayer HealthCare]). Heart rate, ECG, symptoms and haemodynamic data were recorded at baseline and during infusion of saline at room temperature at 10 mL/min for 1 minute (resting data) and 20 mL/min for 2 minutes (hyperaemic data), respectively. Distal coronary pressure (Pd) and distal blood temperature (T) were recorded simultaneously during continuous saline infusion by the temperature/pressure wire positioned in the distal part of the coronary artery, at least 60 mm beyond the catheter tip. After achievement of a steady distal temperature, the temperature/pressure wire was pulled back into the infusion microcatheter so that the temperature of the infused saline could be measured.
The same set of measurements were then recorded during exercise on a dedicated supine bicycle ergometer (ERG 911 BP/X-RAY [Schiller]) attached to the catheter laboratory table at an initial workload of 30 watts and increased by 30 watts every 2 minutes (Moving image 1). At each step, the pressure/temperature sensor-tipped guidewire was advanced to the distal part of the coronary artery and then pulled back into the infusion microcatheter 30 seconds before completion of the step. Haemodynamic and temperature data were recorded simultaneously during continuous saline infusion at 10 mL/min.
Using this approach, absolute Rμ, Q and CFR were assessed at baseline, after saline infusion and at each step of exercise (Figure 1). CMD was defined as a saline-derived CFR (CFRsaline) <2.5. Data analysis was blinded to this classification.
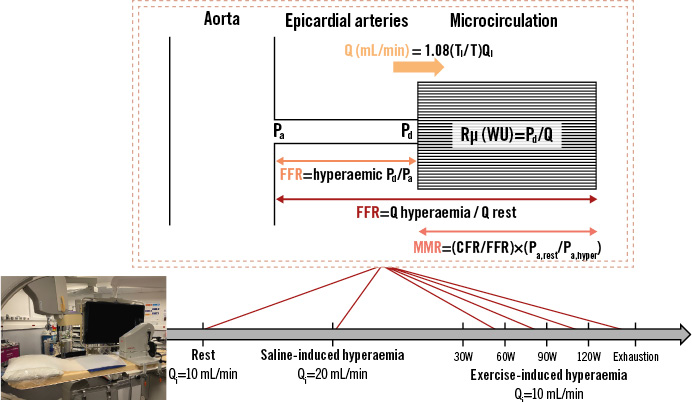
Figure 1. Coronary physiological assessment using saline- and exercise-induced hyperaemia. Absolute microvascular resistance (Rµ) and blood flow (Q) were assessed at rest (continuous saline infusion rate [Qi]=10 mL/min), after saline-induced hyperaemia (continuous Qi=20 mL/min) and at each step of exercise (continuous Qi=10 mL/min). Fractional flow reserve (FFR) and coronary flow reserve (CFR) were assessed after saline-induced hyperaemia and at each step of exercise, until exhaustion. The temperature of the infused saline (Ti) was recorded, as well as the the distal blood temperature after complete mixing (T). MRR: microvascular resistance reserve; Pa: aortic pressure; Pd: distal coronary pressure; W: watts; WU: Wood unit
Physiological measurements
Absolute Rμ and absolute Q were calculated offline using the CoroFlow platform (Coroventis). Absolute Q was calculated at each step using the infusion rate of saline (Qi), temperature of the infused saline (Ti) and T after complete mixing, both expressed as a difference to normal blood temperature (Q=1.08[Ti/T]Qi). When describing the absolute variation of blood flow, we normalised the coronary flow to aortic pressure (Pa) using the following formula: measured Q (mL/min) x 100 (mmHg)/measured Pa (mmHg). Absolute Q was expressed in mL/min. Absolute Rμ was calculated for each condition as distal coronary pressure divided by blood flow (Rμ=Pd/Q), expressed in mmHg/(L/min) or WU. CFR was calculated as the ratio of hyperaemic blood flow to baseline resting blood flow for both modalities of hyperaemia induction (saline infusion and exercise). Physiological CFR (CFRexercise) was calculated as the ratio of exercise peak to baseline resting coronary blood flow. FFR was calculated as the ratio of distal coronary pressure to aortic pressure during hyperaemia (FFR=Pd,hyper/Pa,hyper). MRR was calculated as the ratio of CFR to FFR, corrected for driving pressures (MRR=[CFR/FFR]x[Pa,rest/Pa,hyper]). Rate pressure product (RPP) was calculated by multiplying the maximum heart rate by the peak systolic blood pressure.
The maximal theoretical exercise capacity (expressed as metabolic equivalents of tasks [METs]) was calculated for each patient using the following formulae: METs=18–(0.15 x age) for males, and METs=14.7–(0.13 x age) for females, with METs=watts x 0.07921. The percentage of theoretical workload achieved was calculated as the workload effectively achieved divided by the theoretical workload.
Statistical analysis
Continuous normal variables are reported as mean±standard deviation (SD) and non-normal data are reported as median (interquartile range [IQR]). The categorical variables are reported as frequency and percentage per modality. Continuous variables were compared using a paired or unpaired Student’s t-test or the Mann-Whitney U test, as appropriate. Categorical variables were compared using the chi-square or Fisher’s exact tests. The agreement between CFR measurements with saline infusion and exercise-induced hyperaemia was assessed using Pearson’s r correlation and Bland-Altman analysis. A minimal sample size of 29 participants was required to detect a hypothesised Spearman coefficient correlation r=0.5 between CFRsaline and CFRexercise using an 80% power and 5% significance level test (α=0.05). A p-value<0.05 was considered statistically significant for all statistical tests without adjustment for multiplicity. Statistical analysis was performed using R statistical software, version 4.1.0 (R Foundation for Statistical Computing) and Prism, version 9 (GraphPad Software).
Results
Clinical characteristics
Of the 30 participants who completed the full protocol, 16 had CFRsaline-defined CMD. Invasive physiological assessment was performed after a median of 3 (IQR 2-4) previous exams, including non-invasive ischaemia tests, computed coronary tomography angiography and invasive coronary angiography. The median time delay between symptom onset and physiological assessment was 18 (IQR 4-38) months. Participants with CMD were more likely to be female with a high prevalence of dyslipidaemia, arterial hypertension, and chronic inflammatory disease. Participants’ symptoms are presented in Table 1. Baseline characteristics including non-invasive stress testing and medication are summarised in Supplementary Table 1. The study flowchart is presented in Supplementary Figure 1.
Table 1. Clinical characteristics of the study population.
| Total N=30 | Normal CFR N=14 | Microvascular dysfunction N=16 | |
|---|---|---|---|
| Age, years | 56.3±12.7 | 53.5±13.0 | 58.8±12.2 |
| Female | 15 (50.0) | 4 (28.6) | 11 (68.8) |
| Active smoker | 5 (16.7) | 2 (14.3) | 3 (18.8) |
| Dyslipidaemia | 21 (70.0) | 10 (71.4) | 11 (68.8) |
| Family history of CAD | 5 (16.7) | 4 (28.6) | 1 (6.3) |
| Arterial hypertension | 19 (63.3) | 7 (50.0) | 12 (75.0) |
| Diabetes | 8 (26.7) | 5 (35.7) | 3 (18.8) |
| Chronic inflammatory disease | 10 (33.3) | 5 (35.7) | 5 (31.3) |
| Previous PCI | 7 (23.3) | 4 (28.6) | 3 (18.8) |
| LVEF, % | 65.0±6.75 | 65.9±7.1 | 64.0±6.6 |
| Body mass index, kg/m2 |
26.4±4.88 | 26.3±5.2 | 26.5±4.7 |
| LDL-C, g/L | 0.96±0.35 | 0.88±0.39 | 1.02±0.32 |
| Estimated glomerular filtration rate, mL/min | 86.8±17.6 | 87.3±20.3 | 86.3±15.6 |
| Symptoms | |||
| Angina | |||
| Typical | 11 (36.7) | 3 (21.4) | 8 (50.0) |
| Atypical | 17 (56.7) | 11 (78.6) | 6 (37.5) |
| CCS angina grade | |||
| II | 8 (26.7) | 3 (21.4) | 5 (31.3) |
| III | 2 (10.0) | 0 (0.0) | 2 (12.5) |
| IV | 1 (3.3) | 0 (0.0) | 1 (6.3) |
| Extrathoracic pain | 7 (23.3) | 2 (14.3) | 5 (31.3) |
| Dyspnoea | 17 (56.7) | 8 (57.1) | 9 (56.3) |
| NYHA Class | |||
| II | 14 (46.7) | 7 (50.0) | 7 (43.8) |
| III | 3 (1.0) | 1 (7.1) | 2 (12.5) |
| Syncope | 2 (6.7) | 0 (0.0) | 2 (12.5) |
| Palpitations | 5 (16.7) | 2 (14.3) | 3 (18.8) |
| Data are presented as n (%) or mean±SD. CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; CFR: coronary flow reserve; LDL-C: low-density lipoprotein cholesterol; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; SD: standard deviation | |||
Saline-induced hyperaemia
As expected, CFRsaline was lower in participants with CMD (2.02±0.37 vs 3.49±0.62; p<0.001), as was MRR (2.20±0.39 vs 3.91±0.85; p<0.001). While FFR and resting and hyperaemic RPP did not differ according to CMD status, participants with CMD had a smaller decrease in Rμ (547±282 vs 935±178 WU; p<0.001) during hyperaemia (Table 2).
Table 2. Haemodynamic responses to saline- versus exercise-induced hyperaemia.
| Resting state | Saline-induced hyperaemia | Exercise-induced hyperaemia at peak of exercise | ||||
|---|---|---|---|---|---|---|
| CMD | No CMD | CMD | No CMD | CMD | No CMD | |
| N=16 | N=14 | N=16 | N=14 | N=16 | N=14 | |
| HR, bpm | 74.6±15.1 | 67.9±10.9 | 71.3±13.7 | 66.4±10.8 | 105±16.4 | 112±14.7 |
| SBP, mmHg | 135±20.7 | 129±16.0 | 142±22.0 | 132±15.9 | 170±18.3 | 160±21.4 |
| RPP, bpm∙mmHg | 10,126±2,693 | 8,695±1,466 | 10,227±2,878 | 8,800±2,043 | 18,000±3,954 | 17,948±3,816 |
| FFR | - | - | 0.89±0.06 | 0.88±0.05 | 0.92±0.06 | 0.92±0.05 |
| Q, mL/min | 108±40 | 76±16 | 212±71 | 265±65 | 210±102 | 291±107 |
| Rµ, WU | 1,051±418 | 1,305±237 | 504±176 | 370 ±106 | 699±362 | 460±248 |
| Pd, mmHg | 100±13 | 97±12) | 96±16 | 93±19 | 115±18 | 110±20 |
| CFR | - | - | 2.02±0.37 | 3.49±0.62 | 1.90±0.45 | 3.94±1.64 |
| MRR | - | - | 2.20±0.39 | 3.91±0.85 | 1.78±0.47 | 3.68±1.37 |
| Data are presented as mean±SD. CFR: coronary flow reserve; CMD: coronary microvascular dysfunction; FFR: fractional flow reserve; HR: heart rate; MRR: microvascular resistance reserve; Pd: distal coronary pressure; Q: coronary blood flow; RPP: rate pressure product; Rµ: microvascular resistance; SBP: systolic blood pressure; SD: standard deviation; WU: Wood units | ||||||
Exercise-induced hyperaemia
CFRexercise was 2-fold lower among participants with CMD (1.90±0.45 vs 3.94±1.64; p<0.001), as was true for MRR (1.78±0.47 vs 3.68±1.37; p<0.001). The mean duration of exercise testing was 5.47±2.10 minutes and did not differ according to CMD status (5.00±2.31 minutes vs 6.00±1.75 minutes). RPP at the exercise peak as well as heart rate, workload percentages, and charge did not differ between groups (Table 2). Participants with CMD had a smaller decrease in Rμ (109 [IQR 32-286] vs 202 [IQR 102-379] WU per 30 watts; p<0.01) and coronary flow increase (7 [IQR 30.5-103.0] vs 21 [IQR 5.8-45.0] mL/min per 30 watts; p=0.01) during exercise versus those without CFRsaline-defined CMD (Figure 2). When normalised to Pa, a lower coronary flow increase (7.8 [IQR 5.4-32.2] vs 25.4 [IQR 6.2-56.9] mL/min per 30 watts) was still observed during exercise among patients with CFRsaline-defined CMD. Examples of the tracings are shown in Supplementary Figure 2. Infused saline and distal blood temperatures during exercise are shown in Supplementary Table 2 and Supplementary Figure 3. No complications were observed during the procedures.
Table 2. Haemodynamic responses to saline- versus exercise-induced hyperaemia.
| Resting state | Saline-induced hyperaemia | Exercise-induced hyperaemia at peak of exercise | ||||
|---|---|---|---|---|---|---|
| CMD | No CMD | CMD | No CMD | CMD | No CMD | |
| N=16 | N=14 | N=16 | N=14 | N=16 | N=14 | |
| HR, bpm | 74.6±15.1 | 67.9±10.9 | 71.3±13.7 | 66.4±10.8 | 105±16.4 | 112±14.7 |
| SBP, mmHg | 135±20.7 | 129±16.0 | 142±22.0 | 132±15.9 | 170±18.3 | 160±21.4 |
| RPP, bpm∙mmHg | 10,126±2,693 | 8,695±1,466 | 10,227±2,878 | 8,800±2,043 | 18,000±3,954 | 17,948±3,816 |
| FFR | - | - | 0.89±0.06 | 0.88±0.05 | 0.92±0.06 | 0.92±0.05 |
| Q, mL/min | 108±40 | 76±16 | 212±71 | 265±65 | 210±102 | 291±107 |
| Rµ, WU | 1,051±418 | 1,305±237 | 504±176 | 370 ±106 | 699±362 | 460±248 |
| Pd, mmHg | 100±13 | 97±12) | 96±16 | 93±19 | 115±18 | 110±20 |
| CFR | - | - | 2.02±0.37 | 3.49±0.62 | 1.90±0.45 | 3.94±1.64 |
| MRR | - | - | 2.20±0.39 | 3.91±0.85 | 1.78±0.47 | 3.68±1.37 |
| Data are presented as mean±SD. CFR: coronary flow reserve; CMD: coronary microvascular dysfunction; FFR: fractional flow reserve; HR: heart rate; MRR: microvascular resistance reserve; Pd: distal coronary pressure; Q: coronary blood flow; RPP: rate pressure product; Rµ: microvascular resistance; SBP: systolic blood pressure; SD: standard deviation; WU: Wood units | ||||||
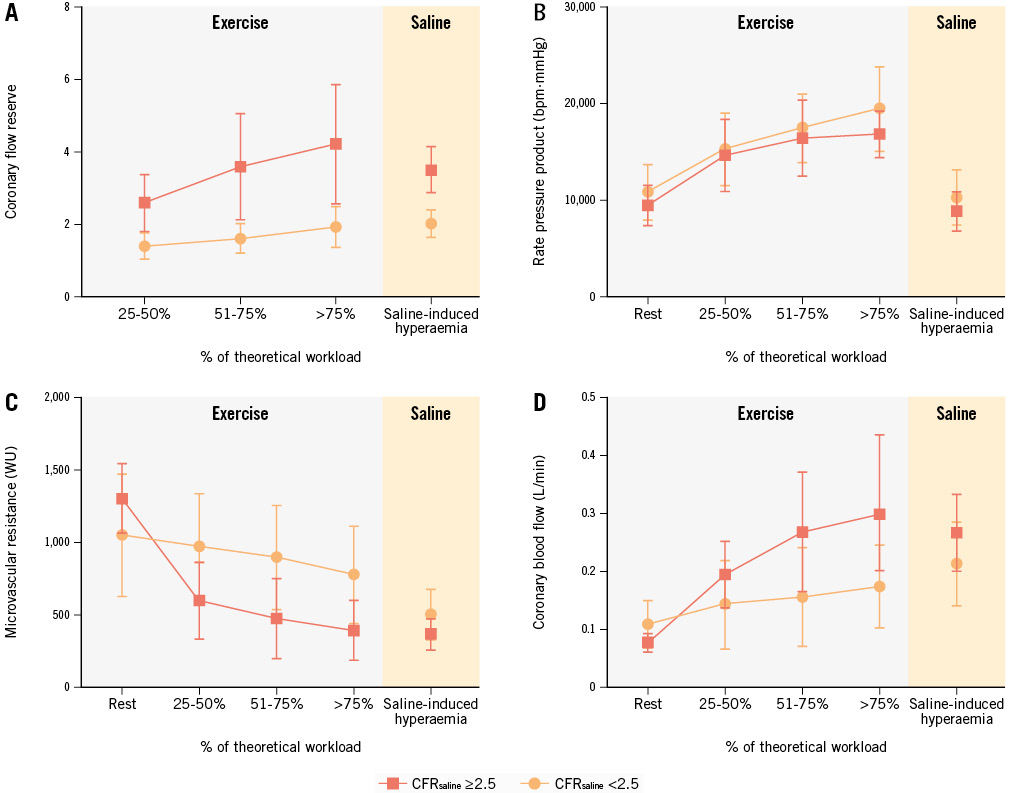
Figure 2. Microvascular haemodynamic adaptation during physiological stress. Coronary flow reserve (A), rate pressure product (B), microvascular resistance (C) and absolute coronary flow (D) are represented according to the percentage of the theoretical workload achieved by patients. The grey area denotes physical exercise and the orange area denotes saline-induced hyperaemia. WU: Wood unit
Saline- versus exercise-induced hyperaemia
CFRexercise and CFRsaline did not differ within the study population (2.70±0.90 vs 2.85±1.54; p=0.46) and were well correlated (Spearman coefficient r=0.73, 95% confidence interval: 0.49-0.86; p<0.0001) with a mean bias of −0.15 (limits of agreement: −2.28 to 1.98) (Central illustration). Classification agreement of CFR between saline- and exercise-induced hyperaemia was 83.3%, with 15 participants exhibiting both abnormal CFRsaline and CFRexercise, and 10 participants showing normal values for both measures. Saline-derived MRR (MRRsaline) and exercise-derived MRR (MRRexercise) were also well correlated (Spearman coefficient r=0.76, 95% confidence interval: 0.57-0.88; p<0.0001) with a mean bias of 0.33 (limits of agreement: −1.39 to 2.05) (Central illustration).
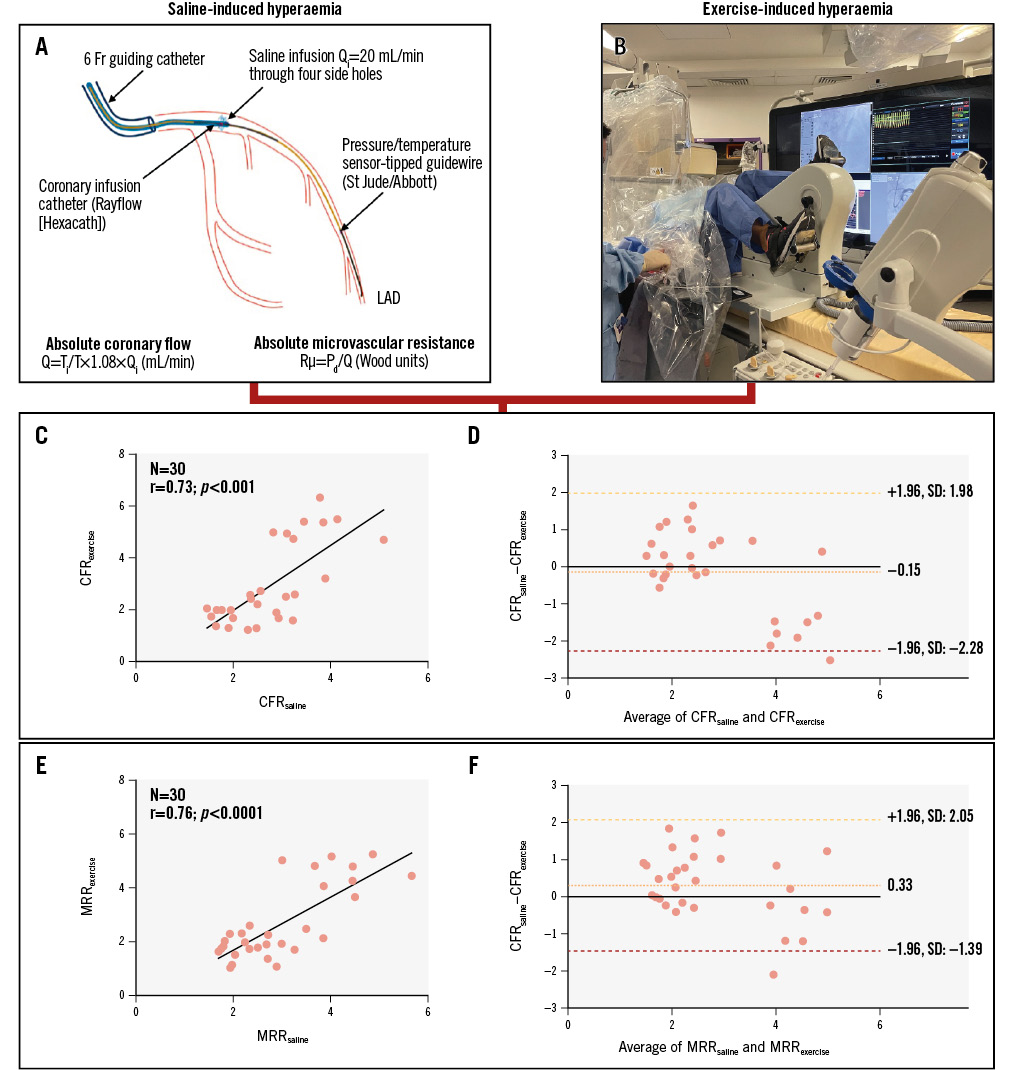
Central illustration. Agreement between CFR as induced by saline infusion at 20 mL/min (CFRsaline) and by physiological exercise test (CFRexercise). A) Procedure for saline-induced hyperaemia. B) Exercise-induced hyperaemia setup. C) Plots of the individual values of CFRsaline versus CFRexercise with their corresponding Bland-Altman plot (D). E) Plots of the individual values of MRRsaline versus MRRexercise with their corresponding Bland-Altman plot (F). CFR: coronary flow reserve; Fr: French; LAD: left anterior descending artery; MRRexercise: exercise-derived microvascular resistance reserve; MRRsaline: saline-derived MRR; Pd: distal coronary pressure; Q: absolute coronary flow; Qi: infusion rate of saline; Rµ: absolute microvascular resistance; SD: standard deviation; T: distal blood temperature after complete mixing with saline; Ti: temperature of the infused saline
Discordant CASES: impaired CFRexercise with normal CFRsaline
The haemodynamic profile of the four participants who had CFRsaline ≥2.5 but CFRexercise <2.5 is shown in Figure 3. They exercised for 77.5±20.6% of their theoretical workload, i.e., 105±17.3 watts. The mean MRRsaline and Rμ were 3.41±0.36 and 417±134 WU, respectively, in this subgroup. While Rμ decreased by 916±296 WU after saline-induced hyperaemia, these four participants had a moderate and late decrease of their Rμ during exercise, with a plateau up to 75% of their theoretical workload. Rμ decreased by 463±167 WU from baseline up to 50% of their theoretical workload, by 496±223 WU up to 75% of their theoretical workload and by 670±288 WU beyond 75% of their theoretical workload. Three of the four patients displayed ischaemia on non-invasive stress testing (Supplementary Table 3). Clinical characteristics, symptoms, and medication details for these patients are also provided in Supplementary Table 3.
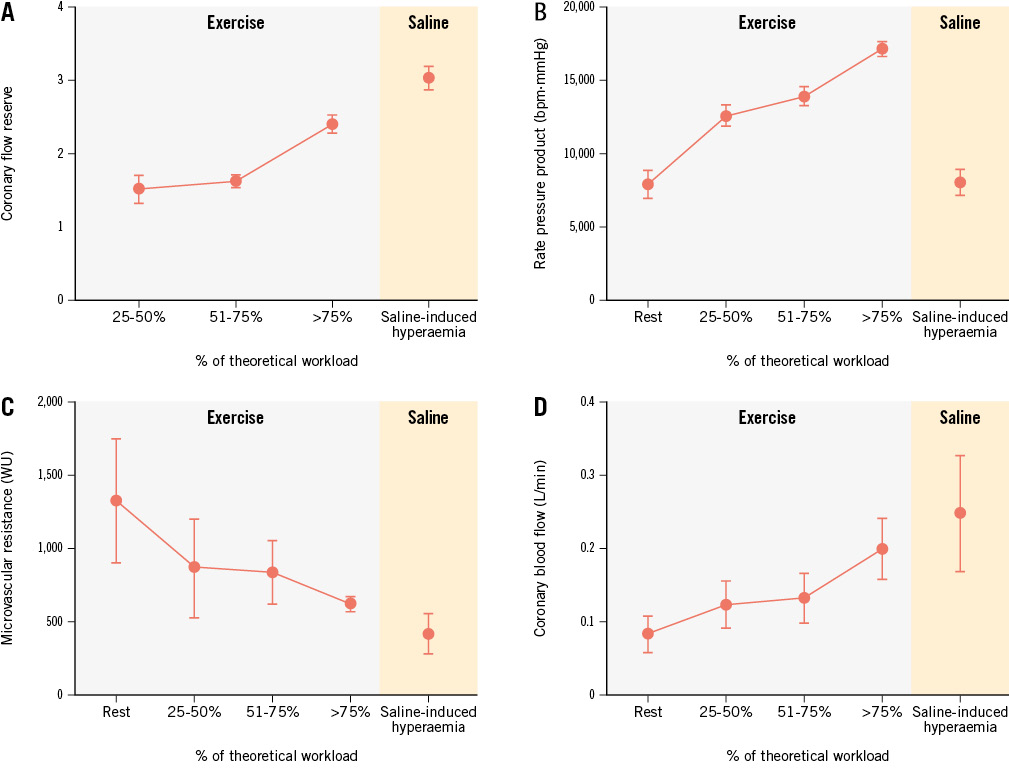
Figure 3. Haemodynamic profiles of patients with CFRsaline ≥2.5 and CFRexercise <2.5. Coronary flow reserve (A), rate pressure product (B), microvascular resistance (C) and absolute coronary flow (D) are represented according to the percentage of the theoretical workload achieved by patients. The grey area denotes physical exercise and the orange area denotes saline-induced hyperaemia. CFRexercise: exercise-induced coronary flow reserve; CFRsaline: saline-induced coronary flow reserve; WU: Wood unit
Discussion
This study provides a comprehensive description of the relationships between absolute coronary blood flow and Rμ adaptations during exercise in humans suspected of having ANOCA. The head-to-head comparison between saline-induced hyperaemia and a physiological exercise stress test is unique. The principal observations are the following: (1) CMD is characterised by lower coronary flow augmentation during physical exercise as well as during saline-induced hyperaemia, due to the inefficiency in reducing microvascular resistance; (2) coronary haemodynamic responses were similar between SIH and exercise stress; (3) CFRsaline was well correlated with CFRexercise; (4) MRRsaline was well correlated with MRRexercise; (5) monitoring of CFR and Rμ during exercise unmasked exercise-related CMD previously labelled as normal by SIH – these individuals had a moderate and late decrease of Rμ during exercise, with a plateau up to 75% of their theoretical workload.
During exercise, coronary microvascular tone involves a complex interplay between vasoactive influences, as well as neurohormonal, endothelial, and metabolic factors, leading to enhanced coronary blood flow as a consequence of reduced Rμ22. The present study demonstrates that the exercise-mediated response of Rμ in participants with CMD was characterised by a plateau instead of a progressive reduction across the different stages of the cycling exercise. The lack of subsequent increase in coronary blood flow and oxygen delivery may lead to symptomatic myocardial ischaemia in patients with ANOCA. Although Rμ appears as a potential, interesting approach for identifying CMD, it may be biased by patient-specific factors including myocardial mass2324. In addition, the established cutoff warrants further investigation for interpatient evaluation. The microvascular resistance reserve index, which is specific to the microcirculation, has been proposed to overcome this limitation with rule-out and rule-in cutoffs of <2.1 and >2.7, respectively25. MRRexercise and MRRsaline were well correlated in our study population. However, MRR’s predictive value and clinical relevance should be investigated in larger studies. Additional information will be furnished through the ongoing multicentre Euro-CRAFT Registry (ClinicalTrials.gov: NCT05805462) which will assess clinical outcomes after a 1-year follow-up period. With more precise upcoming diagnostic and prognostic cutoffs, MRR should be used as a gold standard in conjunction with measurements of absolute microvascular resistance to assess microvascular dysfunction.
CFR is a relative measure of resting and hyperaemic absolute coronary flow and is well suited for interpatient evaluation. A continuous increase of coronary flow proportional to cycling workload was observed in participants with normal microvascular function as opposed to those with CMD in whom CFR remained <2.5 across all stages of exercise. Saline infusion at a rate of 20 mL/min in the LAD appeared to provide a hyperaemic state close to physiological adaptation to exercise. We observed an 83.3% classification agreement between CFRsaline and CFRexercise, with a good correlation between the two methods. Participants with and without CFRsaline-defined CMD achieved similar cycling workload percentages (65.2±28.8% vs 71.3±18.5%; p=0.77). Microvascular haemodynamic adaptations at such a workload are correlated with those of the maximal vasodilation reached by SIH. This finding highlights that a relatively low level of exercise might be sufficient and effective as compared with SIH for the diagnosis of microvascular dysfunction. This would have practical implications and potentially make exercise more applicable to routine use. The mechanisms underlying SIH are multifaceted and involve the release of vasoactive compounds, such as adenosine triphosphate mediated by haemolysis, and shear stress26. Conversely, previous studies have shown that adenosine is associated with a larger augmentation in coronary blood flow and a greater reduction in myocardial resistance, leading to an overestimation of CFR122728. Similarly, Ryan et al observed only a moderate correlation in the evaluation of epicardial disease using adenosine-derived FFR compared to exercise. Along with our observations, these findings could support continuous thermodilution as a better physiological test to evaluate coronary circulation and microcirculation compared with the use of pharmacological agents such as adenosine29. Measurements of CFR and Rμ seem to be more reliable and reproducible using saline-induced hyperaemia, with smaller standard deviations as compared to exercise. This may be related to the multiple systemic, metabolic, mechanical and neurohormonal mechanisms that can be implicated at variable individual levels during exercise as compared with the localised SIH.
Physiological exercise brought additional information compared with SIH. We were able to describe a specific pattern with normal CFRsaline and impaired CFRexercise during physical exercise despite a similar workload among the four patients. These patients were characterised by a slow and delayed adaptation of Rμ to exercise with a minimal increase in coronary flow during the early stages of exercise, up to 75% of the theoretical workload. This finding suggests that by simulating and jumping straight to maximal hyperaemia with continuous saline infusion, we may be missing the nuances involved in what happens to these patients during physiological exercise. Indeed, by using only the SIH method, 29% (4/14) of patients would have been misdiagnosed as not having CMD, when in fact, their microvascular response to exercise was not normal. This exercise-related CMD likely reflects an imbalance between endogenous vasodilatory and vasoconstrictive factors, impeding sufficient vasodilation at the initiation and early stages of exercise. The comprehensiveness of the observed discordance in participants with normal endothelial-independent function, as indicated by a normal CFRsaline but impaired CFRexercise, could have been enhanced by incorporating endothelial-dependent vasodilation testing with acetylcholine infusion. These aspects should be considered for future research to ensure a more thorough evaluation of vascular function. These findings shed light on potential mechanisms underlying myocardial ischaemia and symptom development in patients with CMD and support the implementation of this method for investigating the coronary microcirculation in clinical practice. In addition to replicating the results of a physiological haemodynamic adaptation, the continuous thermodilution method has several advantages. It is less operator dependent and avoids the use of specific pharmacological microvascular vasodilators when assessing absolute maximal coronary flow and absolute minimal Rμ, making it easy to implement in the cath lab. Exercise testing could be used to assess patients with a high pretest probability for CMD and a CFRsaline derived from SIH that is in the grey zone.
In our study, half of the participants had a confirmed diagnosis of CMD following a median time of 18 (IQR 4-38) months after symptoms onset and a median of 3 (IQR 2-4) previous exams, and these patients should benefit from tailored management30 including upfront beta-blocker therapy with long-acting nitrate derivatives when symptom control is inadequate19. The randomised trial WARRIOR is currently assessing the potential of treatment with aspirin, statins, and angiotensin-converting enzyme inhibitors for this indication (ClinicalTrials.gov: NCT03417388). Further research is needed to correlate the measurements derived from intracoronary continuous thermodilution with patients’ symptoms and prognoses and to use a tailored treatment approach targeting various aspects of microcirculatory and metabolic adaptations.
Limitations
This study was restricted to participants capable of exercising during the catheterisation procedure. The small number of this heterogeneous group of patients with different potential pathophysiological forms calls for caution in the interpretation of the results. Given that the overall study population had symptoms, a CFR threshold of 2.5 instead of 2.0 ensured that the microvascular function of the group “without microvascular dysfunction” was truly normal. A 2.0 threshold may have had enhanced specificity at the cost of sensitivity. Exercise-derived haemodynamic data have been correlated with saline-derived data but not with imaging-demonstrated ischaemia. However, according to the expert consensus document on ischaemia with non-obstructive coronary arteries (INOCA), signs of ischaemia may be present but are not necessary for diagnosis19. To overcome the variability of Rμ, which depends on the mass of the myocardium related to the artery under study, measurements were only performed in the LAD, which is the most representative of the whole myocardial mass, and the majority of validation data were gathered from studies involving the LAD1323. Moreover, intergroup comparisons were also made using MRR, which is independent of myocardial mass. Intracoronary continuous thermodilution has never been evaluated during exercise-induced hyperaemia. However, measurements were taken using the stable signal of distal blood temperature. Saline infusion provides greater reproducibility for calculating CFR, MRR and Rμ, while the reproducibility of exercise-derived data remains unexplored. The findings presented in this study have not been adjusted for type 2 error.
Conclusions
Participants with CMD displayed compromised coronary flow augmentation during physical exercise as well as during saline-induced hyperaemia, due to a smaller decrease of microvascular resistance. Saline-induced hyperaemia provided a valid surrogate for exercise physiology independently of the absolute levels of CFR and MRR, although exercise provided more granularity to evaluate adaptation among participants with exercise-related CMD.
Impact on daily practice
Participants with coronary microvascular dysfunction displayed compromised coronary flow (Q) augmentation during physical exercise as well as during saline-induced hyperaemia, due to a smaller decrease in microvascular resistance (Rμ). The newly introduced continuous thermodilution method, which enables the direct quantification of absolute Q and Rμ through saline infusion, provides a valid surrogate for exercise physiology. Evaluation of patients with angina with non-obstructive coronary artery disease using this precise, reproducible and accurate method could enhance the assessment of microvascular function, although exercise seems to provide more granularity to evaluate participants with exercise-defined microvascular dysfunction.
Acknowledgements
The authors would like to acknowledge the contributions of the Pitié-Salpetrière cath lab PT-CHIPS to this study.
Funding
ACTION Study Group.
Conflict of interest statement
M. Zeitouni received research grants and honorarium from Bayer, BMS-Pfizer, la Fédération Française de Cardiologie, Servier, AstraZeneca, Novo Nordisk, and Abbott. J. Silvain received research grants and honorarium from AstraZeneca, Bayer Healthcare SAS, Abbott Medical France SAS, Biotronik, Boehringer Ingelheim France, CSL Behring, Gilead Science, and Sanofi-Aventis France; and has been a stockholder of PharmaSeeds, Terumo France SAS, and Zoll. G. Montalescot received research grants and honorarium from Abbott, Amgen, AstraZeneca, Ascendia, Bayer, Bristol-Myers Squibb, Boehringer Ingelheim, Boston Scientific, CeleCor, CSL Behring, Idorsia, Lilly, Novartis, Novo Nordisk, Opalia, Pfizer, Quantum Genomics, Sanofi, and Terumo. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Cycling during thermodilution using a supine bicycle ergometer (ERG 911 BP/XRAY) attached to the catheter laboratory table.

