Abstract
BACKGROUND: A detailed understanding of the sympathetic innervation of coronary arteries is relevant to facilitate the development of novel treatment approaches.
AIMS: This study aimed to quantitatively examine periarterial innervation in human epicardial coronary arteries.
METHODS: Coronary arteries with adjacent epicardial adipose tissue were excised along the left main coronary artery (LMCA), left anterior descending artery (LAD), left circumflex artery (LCx), and right coronary artery (RCA) from 28 body donors and examined histologically. Immunofluorescence staining was performed to characterise sympathetic nerve fibres.
RESULTS: A total of 42,573 nerve fibres surrounding 100 coronary arteries (LMCA: n=21, LAD: n=27, LCx: n=26, RCA: n=26) were analysed. The nerve fibre diameter decreased along the vessel course (median [interquartile range]): (proximal 46 μm [31-73], middle 38 μm [26-58], distal 31 μm [22-46]; p<0.001), with the largest nerve fibre diameter along the LMCA (50 μm [31-81]), followed by the LAD (42 μm [27-72]; p<0.001). The total nerve fibre density was highest along the RCA (123 nerves/cm² [82-194]). Circumferentially, nerve density was higher in the myocardial tissue area of the coronary arteries (132 nerves/cm² [76-225]) than in the epicardial tissue area (101 nerves/cm² [61-173]; p<0.001). The median lumen-nerve distance was smallest around the LMCA (2.2 mm [1.2-4.1]), followed by the LAD (2.5 mm [1.1-4.5]; p=0.005).
CONCLUSIONS: Human coronary arteries are highly innervated with sympathetic nerve fibres, with significant variation in the distribution and density. Understanding these patterns informs pathophysiological understanding and, potentially, the development of catheter-based approaches for cardiac autonomic modulation.
The autonomic nervous system regulates cardiac function and cardiovascular homeostasis1. Increased sympathetic activity is associated with various cardiovascular diseases, such as heart failure, sudden cardiac death, arrhythmias, vasospastic angina, Takotsubo cardiomyopathy, and atherosclerosis1234. Preganglionic sympathetic nerve fibres arise from the thoracic spinal cord (mostly from Th1-Th5) and then synapse with postganglionic neurons in the paravertebral ganglia (e.g., superior and middle cervical ganglia, stellate ganglion) before reaching the heart25. Modulation of postganglionic nerve fibres has been used to treat refractory atrial or ventricular arrhythmias and refractory coronary spasm, for example, by sympathectomy at the level of the stellate ganglion or thoracic epidural anaesthesia of sympathetic fibres to the heart267.
Macroscopic anatomical studies have shown that postganglionic sympathetic fibres form an anterior and posterior cardiac plexus at the cardiac base in humans. From this plexus originate the left coronary nerve − which runs along the left main coronary artery (LMCA) and then bifurcates into branches running along the left anterior descending artery (LAD) and left circumflex artery (LCx) − and the right coronary nerve − which runs along the right coronary artery (RCA)28. Porcine and canine studies suggest that coronary perivascular innervation consists of a circumferential network of nerve fibres between the media-adventitial layer of the arterial wall910. These fibres run distally parallel to the arteries, with the size and number of nerve fibres declining with decreasing luminal diameter9. However, the distribution and characterisation of nerve fibres surrounding the coronary arteries have not been sufficiently studied in humans. This study aimed to examine coronary innervation and quantitatively analyse the density, distribution, and size of nerve fibres around the major coronary arteries in human body donors. A more detailed knowledge of coronary innervation provides important pathophysiological information and may facilitate the development of autonomic modulation techniques beyond total sympathectomy.
Methods
Full details of materials and methods are available in Supplementary Appendix 1.
The coronary arteries of 28 human body donors were studied to characterise periarterial innervation. All body donors voluntarily consented during their lifetime to body donation at the Anatomical Institute of Saarland University, Homburg, Germany. The ethics committee of the Medical Association of Saarland approved the study (vote number: 162/20).
SAMPLING, PREPARATION AND STAINING
All bodies were fixed in 4% formalin according to Basler’s protocol or in nitrite pickling salt-ethanol-polyethylene glycol (NEP) according to Weigner’s protocol11. A standardised protocol was used to ensure consistency in preparation. Tissue from the coronary arteries was then extracted along the vessel course. Three tissue samples including adjacent epicardial fat tissue were taken from the LAD and RCA (proximal, middle, and distal), two from the LCx (proximal and distal) and one from the LMCA. The landmarks used for excising the arteries are shown in Supplementary Table 1. If the arteries were heavily calcified because of severe atherosclerosis, samples were treated with a decalcifying solution for 24 hours (OSTEOSOFT [Merck]) so that clean sections could be obtained using a tissue microtome. The sections were first stained with haematoxylin and eosin (H&E) to morphologically assess and evaluate the integrity and the basic morphology of the prepared sections. Neuronal tissue and nerve distribution were evaluated using S100 staining (Dako IR 504 [Agilent Technologies]) (Figure 1). For the assessment of efferent sympathetic nerves, tyrosine hydroxylase (TH; anti-TH: ab112 [Abcam]) anti-rabbit fluorescein-5-isothiocyanate (FITC) staining (711-095-152 [Calibre Scientific]) was used (Supplementary Figure 1). All primary antibodies were used in a concentration of 1:100.
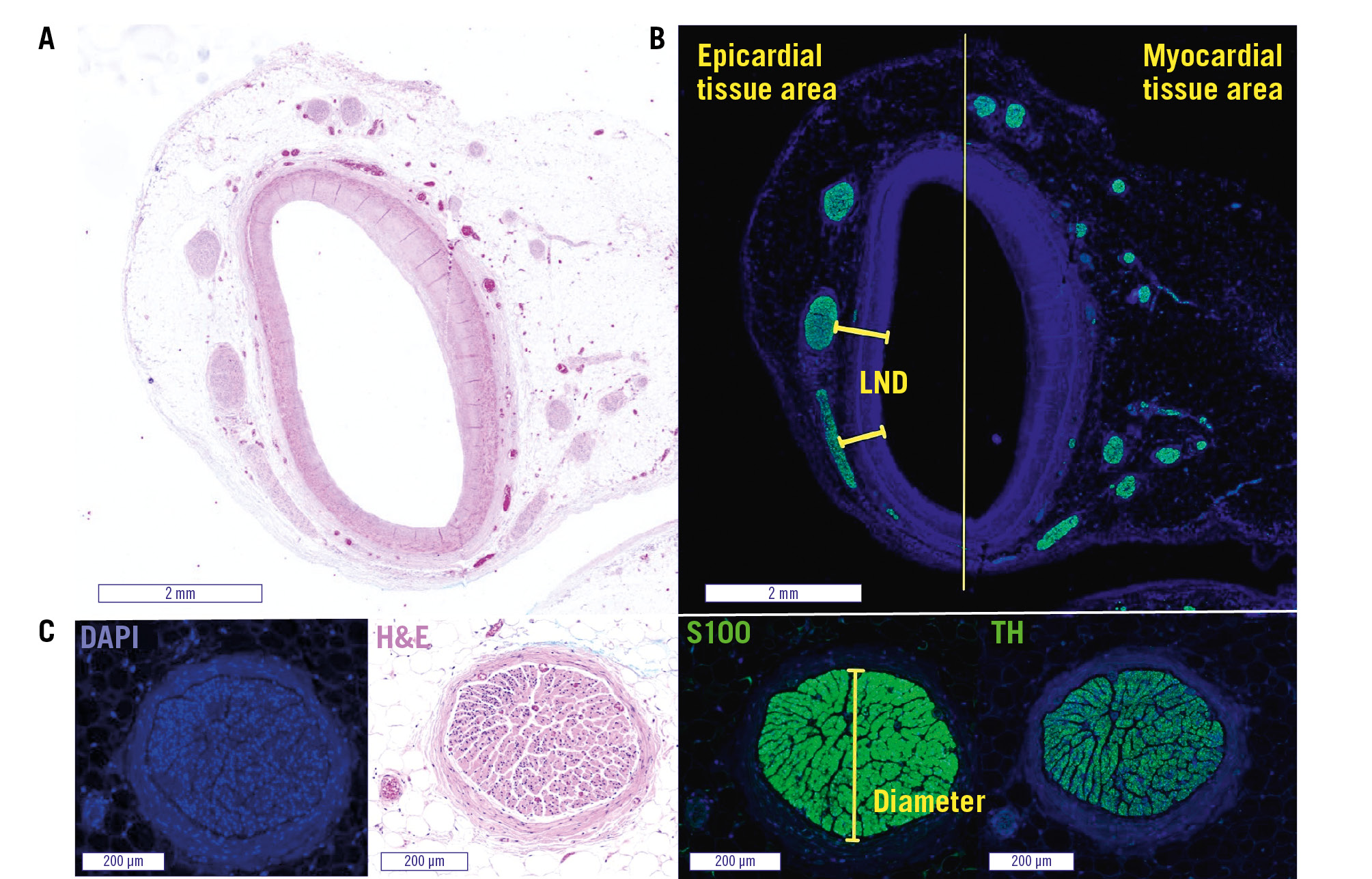
Figure 1. Representative images of the LMCA with periarterial nerves. The left main coronary artery (LMCA) with surrounding tissue in haematoxylin and eosin stain (A) and S100 staining (B). The division into epicardial and myocardial tissue areas and an example of LND measurements are shown in (B). C) shows the same nerve fibre in different stains, with an example of nerve fibre diameter measurement in the S100 staining. DAPI: 4′,6-diamidino-2-phenylindole; H&E: haematoxylin and eosin; LND: lumen-nerve distance; TH: tyrosine hydroxylase
HISTOLOGICAL ANALYSIS
S100 and TH sections were used to analyse the amount of nerve fibres, the smallest nerve fibre diameter, and the shortest distance of nerve fibres to the luminal intima (Figure 1). Nerve fibres were categorised, based on the smallest diameter, as either small (10-49 μm), medium (50-99 μm), large (100-199 μm), or very large (≥200 μm). The lumina and surrounding tissue of the arteries were divided into 2 semicircles and assigned as epicardial and myocardial area according to their orientation. The number of nerve fibres per tissue area was calculated (Supplementary Figure 2A). In addition, anatomical measurements of the coronary arteries were performed in S100 sections to quantify luminal narrowing (Supplementary Figure 2B). Despite the presence of eccentric luminal narrowing in some sections, a positive relationship was found between the shortest distance of nerve fibres to the luminal intima and the shortest distance of nerve fibres to the external elastic lamina (EEL). Therefore, the lumen-nerve distance (LND) measurement was preferred in this study to quantify the distances of periarterial nerve fibres, especially with regard to the development of possible approaches to target these periarterial nerve fibres (Supplementary Figure 3).
STATISTICAL ANALYSIS
Normal distribution was assessed using the Shapiro-Wilk and Kolmogorov-Smirnov tests. Categorical variables are expressed as counts (%) and continuous variables with normal distribution as mean±standard deviation (SD). Median and interquartile range (IQR) are used for variables without normal distribution. The Friedman test was used for comparisons of non-parametric variables between arteries, segments, and tissue areas. A 2-tailed p-value<0.05 was considered statistically significant. SPSS Statistics, version 27 (IBM) was used for statistical analyses.
Results
A total of 100 coronary arteries, including 21 LMCA, 27 LAD, 26 LCx, and 26 RCA from 28 body donors (11 male, 17 female) were analysed. Table 1 summarises the characteristics of the body donors. A total of 546 histological sections were analysed with S100 staining (244 proximal, 123 middle, and 179 distal), and 192 sections were analysed with TH staining. Following S100 and TH staining, 42,573 periarterial nerve fibres were identified and used for analysis. The median lumen diameter of all coronary arteries was 2.9 mm (IQR 2.4-3.6) in the proximal, 2.2 mm (IQR 1.9-2.9) in the middle and 1.7 mm (IQR 1.3-2.2) in the distal segment, respectively (p<0.001). The LMCA had the largest diameter of 3.5 mm (IQR 3.1-5.3), followed by RCA, LCx and LAD with diameters of 2.7 mm (IQR 2.2-3.3), 2.3 mm (IQR 1.7-2.7) and 1.9 mm (IQR 1.5-2.3), respectively (p<0.001). Details on coronary anatomy are shown in Table 1. The number of sections analysed in S100 staining was 59 for the LMCA, 200 for the LAD (proximal n=70, middle n=59, distal n=71), 102 for LCx (proximal n=51, distal n=51), and 185 for the RCA (proximal n=64, middle n=64, distal n=57). Within these sections with S100 staining, a total of 31,009 periarterial nerve fibres were detected and included in the following analyses.
Table 1. Characteristics of body donors, numbers of analysed sections and coronary artery anatomy.
| Characteristics of body donors | All body donors (n=28) | |
|---|---|---|
| Age, years | 78.0±11.3 | |
| Female | 17 (61) | |
| Cardiovascular death according to death certificate | 15 (54) | |
| Analysed sections (S100/TH staining) | Total (546/192) | |
| LMCA | 59/20 | |
| LAD | Proximal | 70/23 |
| Middle | 59/16 | |
| Distal | 71/24 | |
| LCx | Proximal | 51/22 |
| Distal | 51/20 | |
| RCA | Proximal | 64/22 |
| Middle | 64/23 | |
| Distal | 57/22 | |
| Coronary artery anatomy | All coronary arteries (n=100) | |
| External elastic area, mm² | LMCA | 16.1 (11.7-29.5) |
| LAD | 4.5 (2.3-7.6) | |
| LCx | 7.3 (3.4-10.2) | |
| RCA | 9.7 (6.3-14.7) | |
| Internal elastic area, mm² | LMCA | 11.9 (8.8-20.2) |
| LAD | 3.5 (1.5-6.0) | |
| LCx | 5.6 (2.6-8.0) | |
| RCA | 7.3 (4.5-10.9) | |
| Lumen area, mm² | LMCA | 7.7 (6.0-12.9) |
| LAD | 1.8 (0.8-3.1) | |
| LCx | 3.2 (1.5-4.6) | |
| RCA | 4.3 (2.2-6.1) | |
| Lumen diameter, mm | LMCA | 3.5 (3.1-5.3) |
| LAD | 1.9 (1.5-2.3) | |
| LCx | 2.3 (1.7-2.7) | |
| RCA | 2.7 (2.2-3.3) | |
| Coronary artery slides with significant stenosis (>70%)* | LMCA | 11/59 (19) |
| LAD | 28/200 (14) | |
| LCx | 5/102 (5) | |
| RCA | 14/185 (8) | |
| Percentage of stenosis | LMCA | 29.5 (20.7-45.9) |
| LAD | 47.5 (32.9-60.4) | |
| LCx | 43.2 (30.7-54.7) | |
| RCA | 40.3 (30.0-53.9) | |
| Values are mean±standard deviation, n (%) or median (interquartile range). *Significant stenosis was defined as >50% in LMCA. Percentages refer to the total number of S100-stained sections per artery. LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; RCA: right coronary artery; TH: tyrosine hydroxylase | ||
NERVE FIBRE SIZE DECREASED ALONG THE VESSEL COURSE
The median nerve fibre diameter across all coronary arteries and segments was 38 μm (IQR 26-60), with 90% of nerve fibres having a diameter of <103 μm. There was a significant decrease in diameter along the vessel course (proximal 46 μm [IQR 31-73], middle 38 μm [IQR 26-58], distal 31 μm [IQR 22-46]; p<0.001). The nerve fibres with the largest median diameter were found along the LMCA (50 μm [IQR 31-81]), followed by nerve fibres around the LAD (42 μm [IQR 27-72]), RCA (36 μm [IQR 25-54]), and LCx (35 μm [IQR 25-53]; p<0.001). Details and a comparison of quantitative parameters between the coronary arteries are shown in Table 2. Exact p-values for the comparisons can be found in Supplementary Table 2 and Supplementary Table 3. Most nerve fibres (n=19,829; 64%) were small (<50 μm). The remaining nerve fibres were either medium (50-99 μm; n=7,688; 25%) or large (100-199 μm; n=2,757; 9%). Very large nerve fibres (≥200 μm) were rare (n=735; 2%) but most common along the LMCA (Figure 2). Supplementary Table 4 provides details on the size categories of nerve fibres for all coronary arteries and segments. Comparing the median nerve fibre diameters of all coronary arteries by sex, the nerve fibre diameter in males (39 μm [IQR 26-61]) was larger than that in females (38 μm [IQR 26-59]; p<0.001). There was no difference in nerve fibre size in luminal narrowed arteries with <50%, 50-70%, and >70% lumen stenosis. Periarterial nerve fibres located in the epicardial area had a larger diameter (41 μm [IQR 27-65]) than those in the myocardial area (37 μm [IQR 25-56]; p<0.001), across all coronary arteries and segments.
Table 2. Comparison of quantitative parameters between each coronary artery.
| Coronary artery | Proximal segment | Middle segment | Distal segment | Total | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nerve density, n/cm² | Nerve size, µm | LND, mm | Nerve density, n/cm² | Nerve size, µm | LND, mm | Nerve density, n/cm² | Nerve size, µm | LND, mm | Nerve density, n/cm² | Nerve size, µm | LND, mm | |
| LMCA | 122*(92-207) | 50*$(31-81) | 2.19 (1.24-4.14) | - | - | - | - | - | - | 122*(92-207) | 50*$(31-81) | 2.19*$ (1.24-4.14) |
| LAD | 99(67-146) | 51+§†(32-85) | 2.41§† (1.17-4.16) | 77§(55-116) | 43§&(27-73) | 2.69& (1.13-4.85) | 85+§(53-132) | 33+§!(22-53) | 2.31+ (0.95-4.65) | 86§(59-132) | 42+§(27-72) | 2.47+§ (1.09-4.47) |
| LCx | 108(76-160) | 41‡(29-63) | 2.29‡ (1.13-4.38) | - | - | - | 112‡(80-204) | 31‡!(22-45) | 2.82‡! (1.23-5.27) | 109‡(79-166) | 35‡(25-53) | 2.56 (1.17-4.89) |
| RCA | 93€†(70-150) | 44€†(30-66) | 2.66€ (1.49-4.57) | 126(91-202) | 36&(26-53) | 2.72& (1.35-4.54) | 145!(116-259) | 29!(21-43) | 2.33! (1.06-4.27) | 123€(82-194) | 36€(25-54) | 2.58€ (1.30-4.45) |
| Total | 103(75-159) | 46†(31-73) | 2.39† (1.26-4.31) | 102&(69-153) | 38&(26-58) | 2.71 (1.28-4.65) | 112!(73-195) | 31!(22-46) | 2.51! (1.08-4.72) | 106(74-165) | 38(26-60) | 2.50 (1.21-4.52) |
| Values are median (interquartile range). The post hoc analyses of the Friedman test are indicated by superscript symbols. Exact p-values for the comparisons can be found in Supplementary Table 2 and Supplementary Table 3. The number of nerve fibres for each coronary artery can be found in Supplementary Table 5. The following symbols represent statistical significance: *LMCA vs LAD; $LMCA vs LCx; +LAD vs LCx; §LAD vs RCA; ‡LCx vs RCA; €RCA vs LMCA; †proximal vs middle; &middle vs distal; !distal vs proximal. LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; LND: lumen-nerve distance; RCA: right coronary artery | ||||||||||||
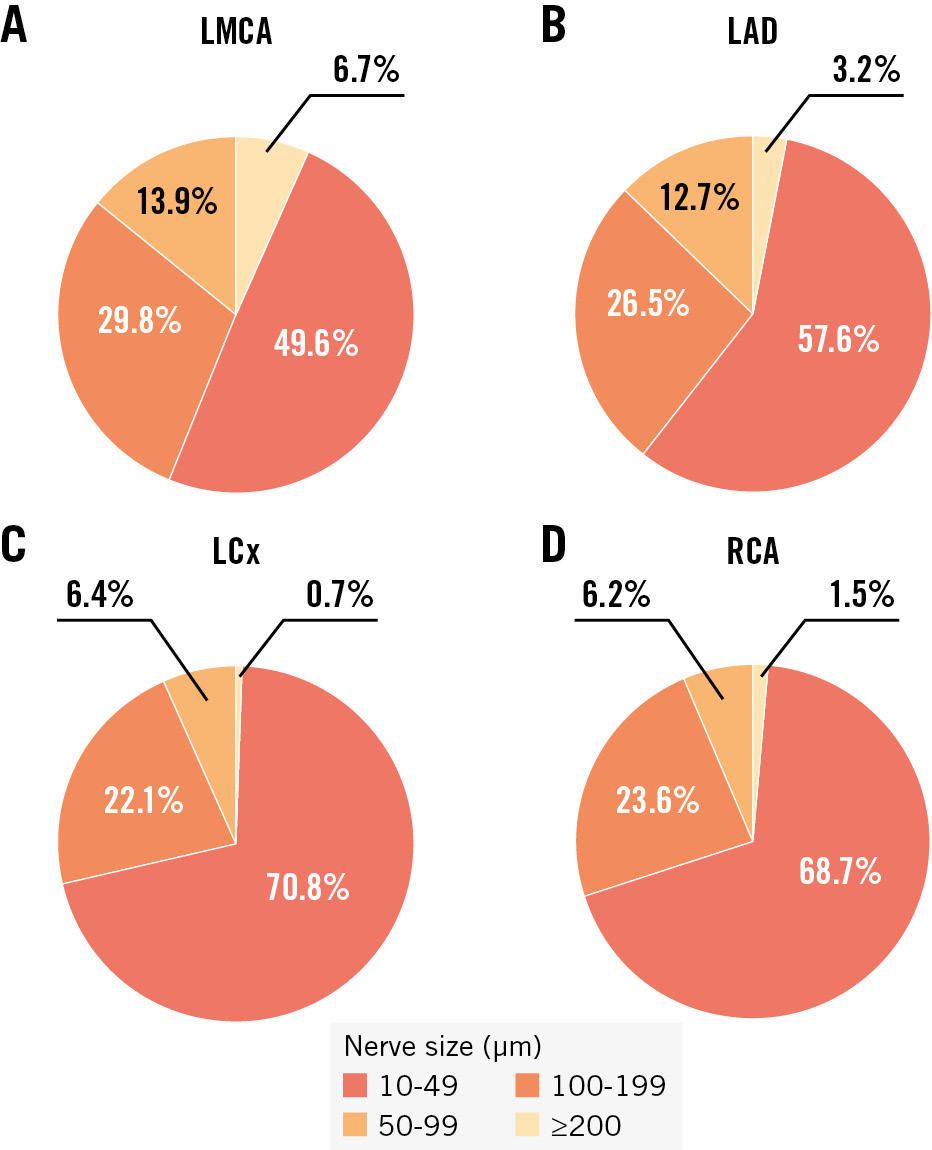
Figure 2. Categories of nerve fibre size per coronary artery. The different colours represent the proportion of each size category of nerve fibres out of the total number of nerves along the coronary artery in the LMCA (A), LAD (B), LCx (C) and RCA (D). LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; RCA: right coronary artery
NERVE FIBRE DENSITY WAS HIGHEST ALONG THE RIGHT CORONARY ARTERY
The median nerve fibre density, calculated as the number of nerve fibres per tissue area, was 106 nerves/cm² (IQR 74-165). Nerve fibre density was higher along the RCA (123 nerves/cm² [IQR 82-194]) than the LMCA (122 nerves/cm² [IQR 92-207]) and the LCx (109 nerves/cm² [IQR 79-166]) (RCA vs LMCA: p=0.05; RCA vs LCx: p<0.001; LMCA vs LCx: p=0.354). The lowest innervation density was found in the LAD, with 86 nerves/cm² (IQR 59-132) (LAD vs RCA: p<0.001; LAD vs LMCA: p=0.046; LAD vs LCx: p=0.285). The distal coronary artery segments were more densely innervated (112 nerves/cm² [IQR 73-195]) than the proximal segments (103 nerves/cm² [IQR 75-159]; p=0.02). The middle segments, which were examined only for the LAD and RCA, had the lowest innervation density in comparison with the other segments (102 nerves/cm² [IQR 69-153]) (Table 2) with a statistical significance in the distal segments (p=0.031) but not the proximal segments (p=0.514). The myocardial area was more densely innervated than the epicardial area (132 nerves/cm² [IQR 76-225] vs 101 nerves/cm² [IQR 61-173]; p<0.001). The median nerve fibre density per tissue area for all coronary arteries and segments is shown in Table 3. The absolute numbers of nerve fibres for all coronary arteries per segment and tissue area are shown in Supplementary Table 5 and Supplementary Table 6.
Interestingly, a significant sex difference in innervation density was found, with a higher density in male body donors of 118 nerves/cm² (IQR 75-214) compared to 97 nerves/cm² (IQR 70-141) in females (p=0.001).
Comparing nerve fibre density in regions with lumen stenosis, regions with lumen stenosis >70% showed a lower nerve fibre density (86 nerves/cm² [IQR 54-127]) compared to regions with lumen stenosis of 50-70%, although this was not significant (101 nerves/cm² [IQR 67-162]; p=0.20). The highest density in coronary arteries with luminal narrowing was found in regions with lumen stenosis <50% (109 nerves/cm² [IQR 77-170]), with a significant difference compared to regions with lumen stenosis >70% (>70% vs <50%: p=0.02; 50-70% vs <50%: p=0.146).
Table 3. Nerve density and lumen-nerve distance according to segment and tissue area.
| Coronary artery | Proximal segment | Middle segment | Distal segment | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Nerve density per tissue area, n/cm² | ||||||||
| Epicardial | Myocardial | Epicardial | Myocardial | Epicardial | Myocardial | Epicardial | Myocardial | |
| LMCA | 126(87-193) | 128(86-228) | - | - | - | - | 126(87-193) | 128(86-228) |
| LAD | 94*(58-179) | 130(79-200) | 72*(52-134) | 87(60-147) | 95(53-150) | 81(48-160) | 89(54-150) | 92(58-168) |
| LCx | 87*(58-163) | 152(91-222) | - | - | 92*(53-242) | 188(113-295) | 89*(57-187) | 160(105-269) |
| RCA | 84*(61-153) | 114(64-185) | 108*(72-171) | 196(103-286) | 132*(93-234) | 179(114-328) | 112*(73-185) | 150(92-263) |
| Total | 98*(62-169) | 130(80-214) | 94*(59-163) | 131(71-213) | 112*(61-206) | 139(77-265) | 101*(61-173) | 132(76-225) |
| Lumen-nerve distance, mm | ||||||||
| Epicardial | Myocardial | Epicardial | Myocardial | Epicardial | Myocardial | Epicardial | Myocardial | |
| LMCA | 1.65*(1.02-3.03) | 2.79(1.54-4.83) | - | - | - | - | 1.65*(1.02-3.03) | 2.79(1.54-4.83) |
| LAD | 2.21*(1.08-3.88) | 2.62(1.30-4.34) | 3.07*(1.15-5.47) | 2.44(1.12-4.22) | 1.87*(0.79-4.18) | 2.61(1.07-4.88) | 2.34*(1.00-4.50) | 2.57(1.17-4.46) |
| LCx | 2.37*(1.18-4.68) | 2.24(1.04-4.14) | - | - | 2.49*(1.07-5.17) | 3.07(1.44-5.39) | 2.42*(1.13-4.93) | 2.66(1.24-4.82) |
| RCA | 2.55*(1.45-4.37) | 2.79(1.54-4.74) | 2.47*(1.28-4.26) | 2.96(1.41-4.80) | 1.83*(0.85-3.81) | 2.68(1.25-4.47) | 2.35*(1.22-4.22) | 2.83(1.40-4.68) |
| Total | 2.18*(1.18-4.06) | 2.62(1.37-4.54) | 2.65*(1.26-4.73) | 2.76(1.31-4.60) | 2.07*(0.90-4.48) | 2.82(1.25-4.87) | 2.26*(1.12-4.32) | 2.71(1.32-4.66) |
| Values are median (interquartile range). The Friedman test was used to test statistical significance. *Statistically significant: epicardial vs myocardial tissue area (p<0.001), respectively. The number of nerve fibres for each coronary artery according to the tissue area can be found in Supplementary Table 6. LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; RCA: right coronary artery | ||||||||
PERIARTERIAL NERVE FIBRES SURROUNDING THE LEFT MAIN CORONARY ARTERY WERE CLOSEST TO THE LUMEN
The median LND across all coronary arteries and segments was 2.50 mm (IQR 1.21-4.52). Nerve fibres along the LMCA had the smallest median distance to the lumen of 2.19 mm (IQR 1.24-4.14), followed by the LAD with a distance of 2.47 mm (IQR 1.09-4.47) and then the LCx with 2.56 mm (IQR 1.17-4.89) (LMCA vs LAD: p=0.05; LMCA vs LCx: p<0.001; LAD vs LCx: p<0.001). Periarterial nerve fibres around the RCA had the greatest distance to the lumen with a mean distance of 2.58 mm (IQR 1.30-4.45) (RCA vs LMCA: p<0.001; RCA vs LAD: p<0.001; RCA vs LCx: p=0.604) (Table 2). The distribution of LND for the coronary arteries is shown in Figure 3. Segmentally, the nerve fibres in the proximal segments had the smallest distance to the lumen of 2.39 mm (IQR 1.26-4.31), followed by the distal segments with a distance of 2.51 mm (IQR 1.08-4.72) and the middle segments with the greatest distance to the lumen of 2.71 mm (IQR 1.28-4.65) (proximal vs middle: p=0.002; middle vs distal: p=0.546; proximal vs distal: p=0.009) (Figure 4). The distribution of periarterial nerve fibres according to the different segments is shown in Supplementary Table 7. Nerve fibres located in the epicardial area were less distant to the arterial lumen than nerve fibres located in the myocardial area (2.26 mm [IQR 1.12-4.32] vs 2.71 mm [IQR 1.32-4.66]; p<0.001) (Table 3 and Supplementary Figure 4).
When comparing the median LND of each coronary artery by sex, there were no significant differences between the LMCA, LAD and RCA. Only the median LND of periarterial nerve fibres around the LCx was lower in males than in females (2.31 mm [IQR 1.16-4.59] vs 2.81 mm [IQR 1.19-5.12]; p<0.001). Considering the mean LND in regions with lumen stenosis, periarterial nerve fibres were less distant in regions with lumen stenosis <50% (2.47 mm [IQR 1.17-4.52]) than in regions with lumen stenosis of 50-70% (2.50 mm [IQR 1.32-4.44]). The greatest median LND in coronary arteries with luminal narrowing was found in regions with lumen stenosis >70% (2.68 mm [IQR 1.22-4.77]; >70% vs <50%: p=0.02; 50-70% vs <50%: p=0.001; >70% vs 50-70%: p<0.001).
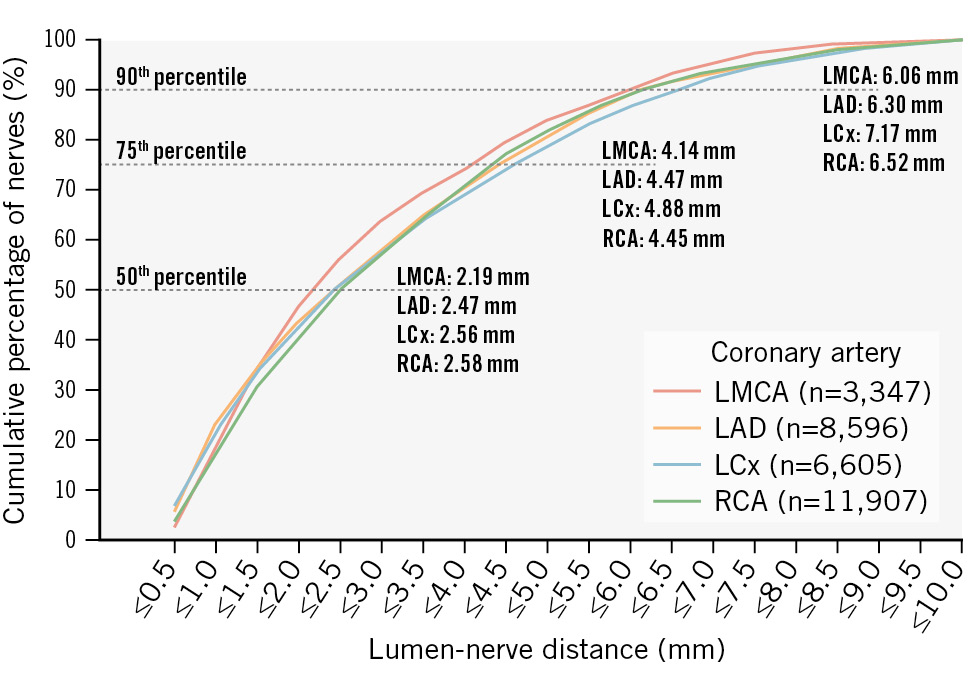
Figure 3. Distribution of periarterial coronary nerve fibres according to lumen-nerve distance in different coronary arteries. Cumulative percentage of lumen-nerve distance (LND) in different coronary arteries. LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; RCA: right coronary artery
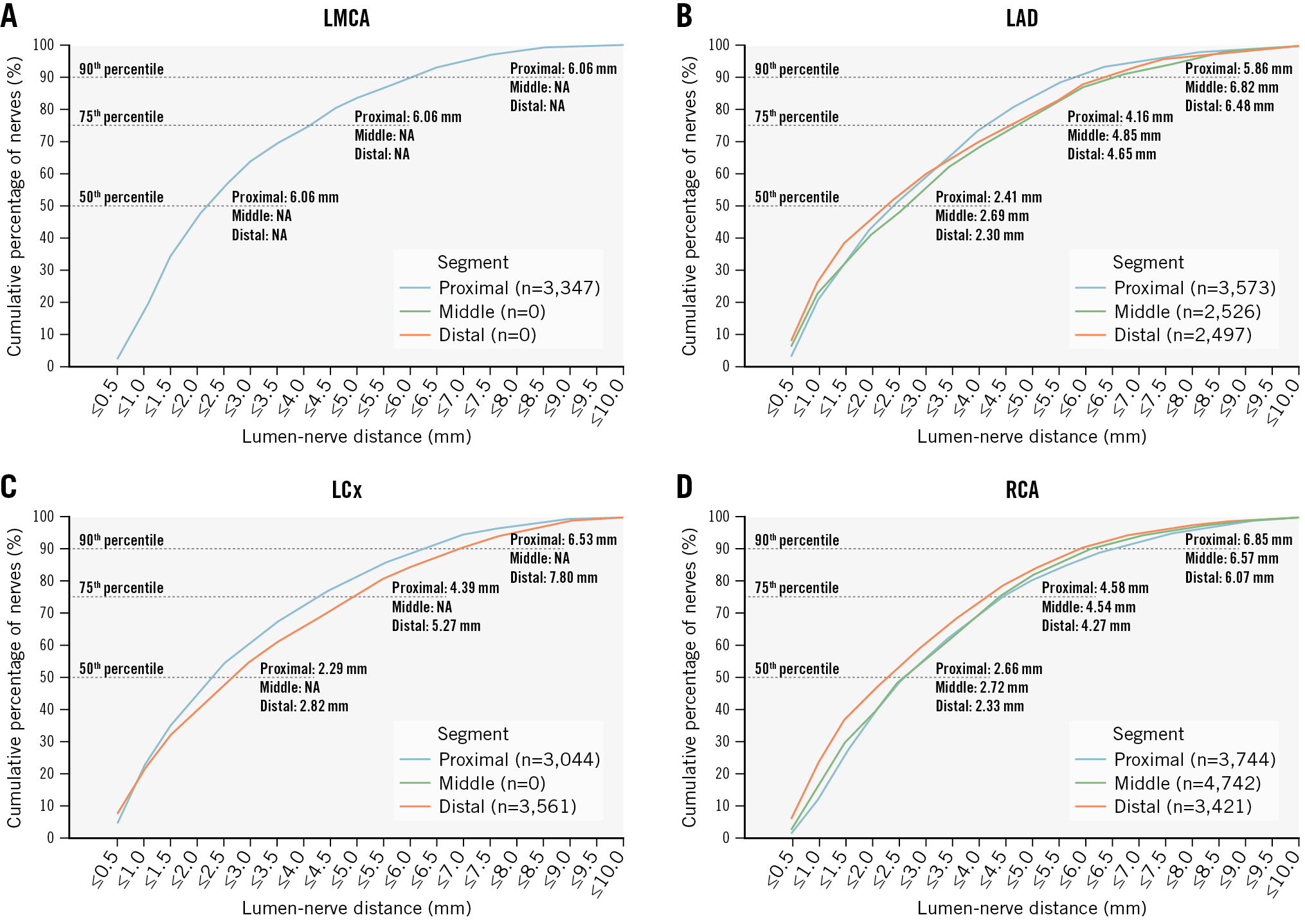
Figure 4. Cumulative percentile of lumen-nerve distance according to segments. The graphs show different coronary arteries and the distribution of lumen-nerve distance (LND) per segment: (A) left main coronary artery (LMCA); (B): left anterior descending artery (LAD); (C) left circumflex artery (LCx); and (D) right coronary artery (RCA). NA: not applicable
ANALYSIS OF TYROSINE HYDROXYLASE-CONTAINING SYMPATHETIC NERVES
A total of 192 TH-stained sections (proximal n=77, middle n=39, and distal n=76), including 11,186 periarterial nerve fibres, were analysed. The number of sections analysed in TH staining was 20 for LMCA, 63 for LAD (proximal n=23, middle n=16, distal n=24), 42 for LCx (proximal n=22, distal n=20), and 67 for RCA (proximal n=22, middle n=23, distal n=22). Nerve fibre size decreased from proximal, middle to distal along the vessel course (46 μm [IQR 31-75], 37 μm [IQR 26-56] and 30 μm [IQR 21-44], respectively; p<0.001). The nerve fibres with the largest median diameter were found along the LMCA (56 μm [IQR 36-100]), followed by nerve fibres around the LAD (39 μm [IQR 26-65]), RCA 36 μm [IQR 25-52] and LCx 35 μm [IQR 24-53]; p<0.001; except RCA vs LCx: p=0.870) (Supplementary Table 8). Periarterial nerve fibres located in the epicardial area had a significantly larger median diameter (40 μm [IQR 27-64]) than nerve fibres located in the myocardial area (36 μm [IQR 25-56]; p<0.001). Comparing the median LND between the coronary arteries, periarterial nerve fibres around the LMCA had the smallest LND (2.17 mm [IQR 1.20-4.06]), followed by the RCA (2.29 mm [IQR 1.14-4.14]), LAD (2.30 mm [IQR 0.98-4.22]) and LCx (2.43 mm [1.05-4.63]; LMCA vs LAD: p=0.018; LMCA vs LCx: p<0.001; LAD vs LCx: p=0.004; RCA vs LMCA: p=0.275; RCA vs LAD: p=0.452; RCA vs LCx: p=0.035) (Supplementary Table 8). The distribution of TH-containing sympathetic nerve fibres according to the different segments is shown in Supplementary Table 9. The nerve fibres located in the epicardial area were closer to the arteries’ lumen than those in the myocardial area (2.01 mm [IQR 0.97-4.00] vs 2.51 mm [IQR 1.21-4.40]; p<0.001) (Supplementary Table 10).
Discussion
To our knowledge, this is the first quantitative analysis of coronary artery innervation in humans. We found that the major coronary arteries are densely innervated with significant differences in the innervation pattern. The largest periarterial nerve fibres and those with the lowest LND were localised around the LMCA, followed by those around the LAD. Nerve fibre size decreased in all coronary arteries from proximal to distal. In the epicardial area, nerve fibres were significantly larger and closer to the arterial lumen, whereas the myocardial area had higher nerve fibre density and greater distance from the arterial lumen (Central illustration). Compared with other coronary arteries, the highest density of nerve fibres was found around the RCA, followed by the LMCA. TH-containing sympathetic nerve fibres showed a similar pattern of innervation in terms of nerve size, density, and distribution, suggesting that most of the nerve fibres analysed in S100 staining were sympathetic nerve fibres.
Previous studies have shown that human coronary arteries are circumferentially supplied with nerves from the cardiac plexus. As most of these histological studies were done at the end of the 20th century or even earlier, limited technical and methodological possibilities did not allow quantitative statements on innervation patterns81213. Herein, nerve fibre size decreased significantly from proximal to distal with a higher nerve fibre density in the distal segments913. In line with our findings, studies investigating the periarterial innervation of other arteries, such as renal arteries, have also found decreasing nerve fibre diameters along the vessel course14. Because these nerve fibres mostly run along the aorta, the proximal parts of the arteries emerging from the aorta have larger nerve fibres than the distal parts. Our results suggest that periarterial nerve fibres ramify along their course, resulting in smaller nerve fibre diameters in the distal segments. This may also explain the greater density of nerve fibres in the distal segments, with large nerve fibres in the proximal segment branching into multiple small nerve fibres along the length of the artery.
Nerve fibre density was lower and median LND was greater in areas of high lumen stenosis (especially in stenoses >70%) than in areas with less stenosis. It is known that after myocardial infarction, sympathetic denervation occurs in the infarcted area and that sympathetic nerve fibres are more vulnerable to ischaemia than cardiomyocytes1516. A possible explanation for this lower nerve fibre density in areas of extensive luminal narrowing is that, because of the vulnerability of the nerve fibres, neurolytic processes occur early because of significant stenosis with associated ischaemia. While nerve fibres close to the arteries may be affected by this phenomenon, nerves with a greater LND may remain unaffected because of their association with other arterial branches, resulting in an increased median LND in highly narrowed coronary arteries. Furthermore, after initial denervation, nerve sprouting signals, such as nerve growth factor, are released to facilitate nerve fibre regrowth. These nerve sprouting signals are not restricted to the infarcted area and induce a generalised increase in cardiac nerve density, which may also result in an increased median LND in highly narrowed coronary arteries17.
In a porcine model, the right side of the heart had a higher nerve fibre density than the left side18. The right coronary artery supplies the cardiac conduction system with blood, which is highly regulated by the autonomic nervous system. It is reasonable to assume that the RCA is the most densely innervated of all the coronary arteries, because it serves as the pathway for periarterial nerves to the conduction system. The highest density was found in the distal segments of the RCA, which may be explained by the fact that they contain the smallest nerve fibres of all coronary arteries and segments. Interestingly, coronary artery spasm, after provocation, was most frequently observed in the RCA192021. This was followed by the LAD and LCx2223. Although the nerve fibre density was higher in the LCx than in the LAD, nerve fibres around the LCx had significantly smaller diameters and a higher proportion of fibres between 10 μm and 50 μm than those around the LAD. This suggests that nerve fibre size, in addition to nerve fibre density, may play a role in the development of spasm. In an autopsy study of patients with coronary artery disease and sudden cardiac death, vasospasms occurred predominantly in the distal RCA, possibly because nerve fibre density is higher in the distal RCA than in the LAD, and the median nerve fibre diameter of the RCA is larger than that of the LCx. Differences between the sexes in terms of innervation density and mean nerve size showed significantly larger nerves and a higher innervation density per tissue area in males when compared with females. One reason for this may be that the epicardial coronary arteries are smaller in females, regardless of body surface area, body mass index, or left ventricular mass2425, resulting in smaller periarterial nerve fibres. Whether this also explains the higher incidence of coronary spasms in males as compared to females remains to be proven20.
Among other factors, coronary spasm seems to play an important role in myocardial ischaemia with no obstructive coronary arteries26. Studies have shown that endoscopic transection of the sympathetic trunk at the level of Th2-Th4 can successfully treat refractory coronary spasm67. In a prospective randomised trial, major adverse cardiac events and all-cause death were significantly reduced at 24 months6. Additionally, radical sympathectomy has been used to treat refractory ventricular arrhythmias in patients with heart failure, long QT syndrome or catecholaminergic polymorphic ventricular tachycardia27. However, the use of radical sympathectomy is limited because of its invasiveness and possible risk of haemothorax and pneumothorax as well as potential long-term safety concerns, as the transection of sympathetic ganglia may also affect postganglionic fibres travelling to other organs. Knowledge of sympathetic nerve distribution along coronary arteries might allow the development of device-based approaches, similar to catheter-based renal denervation28, specifically targeting the left or right coronary arteries.
Coronary artery disease may be another potential clinical application for targeted autonomic modulation of the coronary arteries. The sympathetic nervous system is known to be involved in the progression of atherosclerotic disease29. Recent studies have shown that adventitial segments affected by atherosclerosis develop neuroimmune cardiovascular interfaces and that the sensory and sympathetic nervous system form a structural arterial-brain circuit30. In an atherosclerosis mouse model, coeliac ganglionectomy has been shown to prevent disease progression and stabilise plaque vulnerability30. A promising approach to treating coronary artery disease at its root may be to target the periarterial nerve fibres of coronary arteries and disrupt the interface between the nervous system and the arteries.
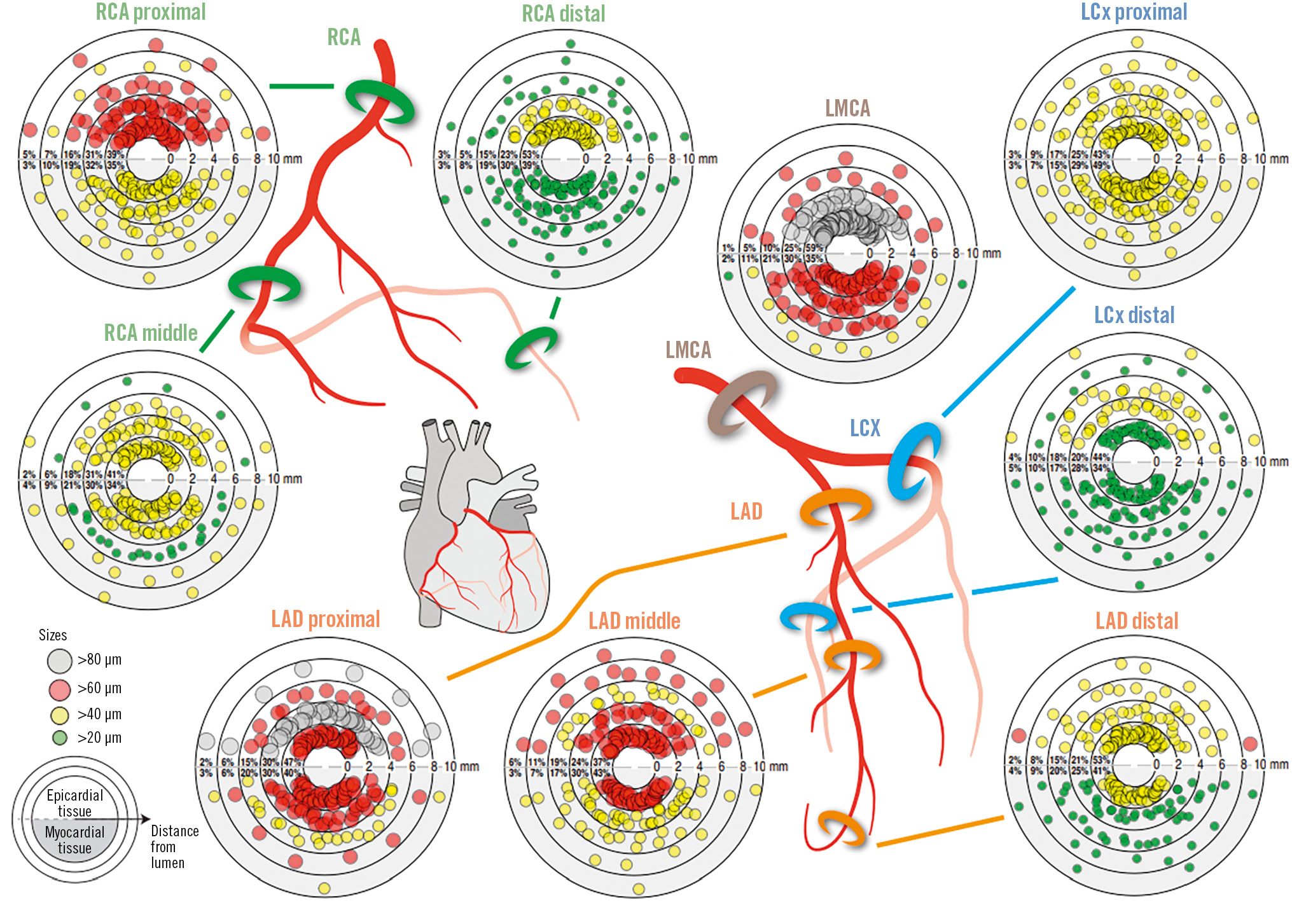
Central illustration. Size and distribution of periarterial nerve fibres in different segments and coronary arteries. Plots show the percentage of nerves in the regions <2, <4, <6, <8, and <10 mm from the coronary lumen and nerve fibres according to the size categories 20-39 µm, 40-59 µm, 60-79 µm and ≥80 µm. To better illustrate the differences in nerve fibre size between the tissue areas, narrower and smaller size categories were chosen, because 84.5% of the nerve fibres measured were smaller than 80 µm. LAD: left anterior descending artery; LCx: left circumflex artery; LMCA: left main coronary artery; RCA: right coronary artery
Limitations
This study included 28 Caucasian body donors from Germany, so the findings may not be fully applicable to other populations or geographical areas. Examination of formalin and NEP-fixed body donors may result in artifacts and tissue shrinkage, which can explain the smaller than expected diameters of the coronary arteries. Because we examined histological sections at 5 mm intervals, we cannot rule out double counting of nerves in subsequent sections. The distribution of nerve fibres can be anticipated and described by sampling along the vessel course, including proximal, middle, and distal points. Nevertheless, because of this selective sampling, variations in perivascular nerve fibre distribution may occur between these points, potentially not fully reflecting the precise anatomical trajectory. However, by standardising the nerve density to the surrounding tissue, we have achieved better comparability between specimens with different tissue proportions.
Conclusions
Nerve fibre size decreased in all coronary arteries from proximal to distal, with the largest periarterial nerves localised around the LMCA and LAD. Nerve fibres were significantly larger and closer to the arterial lumen in the epicardial area, whereas the myocardial area had a higher nerve fibre density and greater distance from the arterial lumen. In segments with coronary artery stenosis, the nerve fibre density was lower in the severely stenosed coronary arteries. Understanding the precise innervation pattern along the coronary arteries will help to optimise existing neuromodulation techniques and, potentially, to develop less invasive catheter-based approaches.
Impact on daily practice
Although cardiac sympathetic modulation has been used to treat refractory coronary spasm and ventricular arrhythmias, periarterial nerve fibres have not been considered in these procedures, because their distribution has not been quantified. Our study shows that nerve fibre density, nerve fibre size, and distance from the lumen vary between coronary arteries and circumferentially, allowing interventional targeting to modulate separate segments of the heart. Further studies are needed to assess the feasibility, efficacy, and safety of such an interventional modulation procedure.
Acknowledgements
Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) Project ID: 322900939 (SFB TRR219-S02). We sincerely thank those who donated their bodies to science for anatomical research. Results from such research can potentially increase humankind’s overall knowledge, which can then improve patient care. Therefore, these donors and their families deserve our highest gratitude. We gratefully acknowledge Julia Weber, Nina Rebmann, and Jeannette Zimolong for their excellent technical support. We thank Armin Schweitzer for their artwork.
Funding
This study was supported by the German Heart Foundation as part of the Kaltenbach Doctoral Fellowship (Project ID K/30/21).
Conflict of interest statement
Until May 2024, L. Lauder received speaker honoraria/consulting fees from AstraZeneca, Medtronic, Pfizer, and ReCor Medical. M. Böhm is supported by the Deutsche Forschungsgemeinschaft (German Research Foundation; TTR219, project number: 32 322900939); and reports personal fees from Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Cytokinetics, Edwards Lifesciences, Medtronic, Novartis, ReCor Medical, Servier, and CSL Vifor during the conduct of the study. F. Mahfoud is supported by Deutsche Gesellschaft für Kardiologie (DGK), Deutsche Forschungsgemeinschaft (SFB TRR219, Project-ID 322900939), and Deutsche Herzstiftung. Saarland University has received scientific support from Ablative Solutions, Medtronic and ReCor Medical. Until May 2024, F. Mahfoud has received speaker honoraria/consulting fees from Ablative Solutions, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Inari, Medtronic, Merck, ReCor Medical, Servier, and Terumo. The other authors have no conflicts of interest to declare relevant to the contents of this paper.
Supplementary data
To read the full content of this article, please download the PDF.

