Abstract
BACKGROUND: Although femoropopliteal-specific stents have durable patency, stent thrombosis (ST) may occur, which can lead to acute limb ischaemia (ALI).
AIMS: We aimed to investigate the clinical features and outcomes of ALI caused by femoropopliteal ST in patients with lower extremity artery disease.
METHODS: This multicentre retrospective study included 499 patients with ALI − of whom 108 patients had ALI caused by femoropopliteal ST (ST-ALI) and 391 patients had ALI caused by other aetiologies (de novo ALI) − who underwent treatment between September 2011 and March 2023. Clinical features and outcomes were compared between the two groups. The primary outcome measure was 12-month amputation-free survival; factors associated with amputation or death were investigated using multivariate Cox proportional hazards regression analysis.
RESULTS: Patients with ST-ALI were significantly more likely to exhibit conventional atherosclerotic risk factors, including diabetes mellitus (63% vs 26%) and haemodialysis (51% vs 10%) compared to patients with de novo ALI, whereas patients with de novo ALI were older (80 years vs 74 years) and more likely to have atrial fibrillation (49% vs 18%) than patients with ST-ALI. The 12-month amputation-free survival rate was significantly lower in the ST-ALI group than that in the de novo ALI group (51% vs 76%; p<0.001). Multivariate analysis revealed that ST-ALI, older age, haemodialysis, atrial fibrillation, the presence of a wound, peak C-reactive protein level, and non-ambulatory status all have an independent, positive association with death or major amputation.
CONCLUSIONS: The current study revealed that patients with ST-ALI had worse clinical outcomes than those with de novo ALI, highlighting the need to maximise ST prevention.
Rapid progress in endovascular therapy (EVT) has led to the extension of its use even for complex lesions of the femoropopliteal (FP) artery, which are the primary manifestation of lower extremity artery disease (LEAD)1234. Although EVT using drug-coated balloons is currently regarded as the standard approach for the management of these lesions, stent-based EVT is necessary for complex FP diseases, such as diffuse, calcified, or total occlusion lesions1. However, the use of FP stents in EVT increases the risk of acute stent thrombosis (ST), which occurs in 3-10% of cases and can ultimately result in acute limb ischaemia (ALI)5.
ALI is an incapacitating and potentially fatal vascular emergency67. Its primary causes include in situ thrombosis, arterial embolisation, and bypass graft thrombosis, and ALI with these aetiologies has been associated with high rates of limb loss and mortality68910. However, few studies have focused on ALI caused by FP-ST (ST-ALI), and its clinical outcomes are yet to be fully elucidated. The present study aimed to reveal the clinical features and outcomes of ST-ALI, compared with ALI of other aetiologies.
Methods
STUDY POPULATION
This multicentre, retrospective, observational study was conducted at 8 cardiovascular and vascular surgical centres across Japan. The study included 499 patients with ALI − of whom 108 had ST-ALI and 391 had ALI caused by other factors, including in situ thrombosis, arterial embolisation, and bypass graft thrombosis in the FP arterial lesions (de novo ALI) − treated at these centres between September 2011 and March 2023. This study was performed in accordance with the Declaration of Helsinki, and each centre obtained ethical approval for the study from the relevant institutional review board. As the study was observational, with no intervention or invasiveness and without the use of human biological specimens, it was exempt from the requirement to obtain written informed consent from patients, in accordance with the Ethical Guidelines for Medical and Health Research Involving Human Subjects in Japan. Instead, relevant study information was made publicly available, and individuals were given the opportunity to refuse the inclusion of their data.
ACUTE LIMB ISCHAEMIA TREATMENT
Treatment strategies for ALI included conservative therapy, primary major amputation, open surgical revascularisation (OSR), and EVT; the strategy was selected at the discretion of the physician based on patient status, the severity of ischaemia, and anatomical features. Major amputation was performed in cases of irreversible limb ischaemia (Rutherford classification stage III). EVT included catheter thrombectomy, catheter-directed thrombolysis, pharmacomechanical thrombolysis, or a combination of these, followed by angioplasty or stent implantation. OSR included direct thrombectomy, thrombectomy using a Fogarty catheter (Edwards Lifesciences), endarterectomy, and primary bypass with autovein or prosthetic grafts. Timing of the revascularisation procedure depended mainly on the severity of ischaemia on presentation. Mild degrees of ischaemia allowed more time for a diagnostic workup and patient optimisation.
OUTCOME MEASURES
After treatment of ALI, all patients were regularly followed up at the outpatient clinic and underwent a postinterventional examination including clinical symptoms, haemodynamic changes in ankle-brachial index, and an evaluation of restenosis via Duplex ultrasonography. The primary outcome measure was amputation-free survival (AFS), and the secondary endpoints were major adverse limb events (MALE) and occlusion at 12 months.
DEFINITIONS
AFS was defined as the time until major amputation of the index limb and/or death from any cause, whichever occurred first. MALE were defined as a composite of major amputation and major reintervention (new bypass graft, jump/interposition graft revision, or thrombectomy/thrombolysis), and occlusion was defined as reocclusion of the treated artery11. ALI severity was determined according to the Rutherford classification proposed by the Society for Vascular Surgery/International Society for Cardiovascular Surgery: stage I − the limb is not immediately threatened; stage IIa − the limb is marginally threatened; stage IIb − the limb is immediately threatened; and stage III − the limb is irreversibly threatened or non-viable12. Major amputation was defined as above-the-ankle amputation. The degree of calcification was graded based on arterial wall calcium deposits detected during fluoroscopy according to the Peripheral Arterial Calcium Scoring Scale13.
STATISTICAL ANALYSIS
Data on baseline characteristics are presented as frequencies and percentages for discrete variables and as median (interquartile range) for continuous variables. Crude comparisons in baseline characteristics between patients with ST-ALI and de novo ALI were performed using the Student’s t-test for continuous variables and the chi-squared test for categorical variables. Cumulative incidence rates were assessed using the Kaplan-Meier method, and the rates are reported as estimate±standard error. Differences between the groups were assessed using the log-rank test when necessary. Factors associated with amputation or death were analysed in patients with Rutherford classification stage I or II ALI, because Rutherford classification stage III ALI is generally treated with primary amputation. The association of AFS with baseline clinical and procedural variables was first analysed using univariate Cox proportional hazards regression analysis. Next, multivariate Cox proportional hazards regression analysis was performed to adjust for confounders and identify risk factors for amputation or death. Clinical factors that were known to affect outcomes and that achieved p<0.05 in the univariate analysis were entered into the multivariate Cox regression analysis. The interaction effect of baseline characteristics on the association between ALI aetiology and amputation or mortality risk was also analysed using a Cox proportional hazards regression model. A p-value<0.05 was considered to indicate statistical significance. Statistical analyses were performed using SPSS software, version 24.0J (IBM).
Results
BASELINE PATIENT CHARACTERISTICS AND CLINICAL FEATURES
Baseline patient, clinical, and procedural characteristics are summarised in Table 1 and Table 2. The prevalence of conventional atherosclerotic risk factors, including hypertension (82% vs 71%; p=0.020), dyslipidaemia (52% vs 32%; p<0.001), diabetes mellitus (63% vs 26%; p<0.001), haemodialysis (51% vs 10%; p<0.001), and coronary artery disease (57% vs 24%; p<0.001), was significantly higher in the ST-ALI group than in the de novo ALI group, whereas patients in the de novo ALI group were older (80 years vs 74 years; p<0.001) and had a higher prevalence of atrial fibrillation (49% vs 18%; p<0.001) than those in the ST-ALI group. The onset-to-admission time (ST-ALI group: 18 hours; de novo ALI group: 12 hours; p=0.277) did not differ significantly between the two groups, whereas the ST-ALI group had a lower peak creatine phosphokinase (CPK) level (656 U/L vs 1,200 U/L; p=0.005) and a less severe Rutherford classification (stage I: 41% vs 16%; stage II: 56% vs 73%; stage III: 3% vs 11%; p<0.001) than the de novo ALI group. In terms of the procedures performed, primary surgical bypass therapy (7% vs 1%; p=0.017), primary amputation (8% vs 2%; p=0.024), and surgical thrombectomy (50% vs 12%; p<0.001) were more frequently carried out in the de novo ALI group than in the ST-ALI group, whereas catheter thrombectomy (73% vs 39%; p<0.001) and local thrombolysis (41% vs 15%; p<0.001) were more frequently performed in the ST-ALI group than in the de novo ALI group. The lesion details of the initial EVT in the ST-ALI group are summarised in Table 3. Chronic total occlusion accounted for 49% (n=53) and the popliteal artery was involved for 58% (n=63) of lesions before the initial EVT in the ST-ALI group.
Table 1. Baseline patient characteristics.
| Patient characteristics | ||||
|---|---|---|---|---|
| Variable | Total N=499 |
ST-ALI N=108 |
De novo ALI N=391 |
p-value |
| Age, years | 78 [69, 85] | 74 [67, 80] | 80 [70, 87] | <0.001 |
| Male sex | 297 (60) | 72 (67) | 225 (58) | 0.087 |
| Ambulatory status | 337 (68) | 78 (72) | 259 (66) | 0.240 |
| Hypertension | 368 (74) | 89 (82) | 279 (71) | 0.020 |
| Dyslipidaemia | 182 (36) | 56 (52) | 126 (32) | <0.001 |
| Diabetes | 169 (34) | 68 (63) | 101 (26) | <0.001 |
| Chronic kidney disease | 169 (34) | 69 (64) | 100 (26) | <0.001 |
| Haemodialysis | 93 (19) | 55 (51) | 38 (10) | <0.001 |
| Current smoker | 79 (16) | 12 (11) | 67 (17) | 0.129 |
| Stroke | 113 (23) | 22 (20) | 91 (23) | 0.523 |
| Coronary artery disease | 155 (31) | 62 (57) | 93 (24) | <0.001 |
| Atrial fibrillation | 211 (42) | 19 (18) | 192 (49) | <0.001 |
| Number of antithrombotic drugs | 1 [0, 2] | 2 [1, 2] | 0 [0, 1] | <0.001 |
| Aetiology of de novo ALI | ||||
| Embolism | 235 (60) | |||
| Pre-existing PAD | 129 (33) | |||
| In situ thrombosis | 102 (26) | |||
| Graft failure | 27 (7) | |||
| Other | 27 (7) | |||
| Thrombosed stent of ST-ALI | ||||
| BMS | 44 (41) | |||
| DCS | 12 (11) | |||
| DES | 17 (16) | |||
| SG | 27 (25) | |||
| IWS | 5 (5) | |||
| Unknown | 3 (2) | |||
| Data are presented as median [interquartile range] or number (%). ALI: acute limb ischaemia; BMS: bare metal stent; DCS: drug-coated stent; de novo ALI: ALI caused by other aetiologies; DES: drug-eluting stent; IWS: interwoven stent; PAD: peripheral arterial disease; SG: stent graft; ST: stent thrombosis; ST-ALI: ALI caused by femoropopliteal ST | ||||
Table 2. Baseline clinical, and procedural characteristics.
| Clinical characteristics | ||||
|---|---|---|---|---|
| Variable | Total N=499 |
ST-ALI N=108 |
De novo ALI N=391 |
p-value |
| Onset-to-admission time, hrs | 12 [5, 60] | 18 [6, 48] | 12 [4.4, 72] | 0.277 |
| Peak CPK, U/L | 1,148 [225, 4,380] | 656 [119, 3,357] | 1,200 [282, 4,762] | 0.005 |
| Peak CRP, mg/dL | 7.8 [2.5, 16.0] | 6.7 [1.2, 16.3] | 7.8 [2.8, 15.9] | 0.153 |
| Presence of wound | 54 (11) | 24 (22) | 30 (8) | <0.001 |
| CIA or EIA thrombus | 101 (20) | 4 (4) | 97 (25) | <0.001 |
| Rutherford classification | <0.001 | |||
| I | 104 (21) | 44 (41) | 60 (16) | |
| IIa | 187 (38) | 38 (35) | 149 (38) | |
| IIb | 161 (32) | 23 (21) | 138 (35) | |
| III | 47 (9) | 3 (3) | 44 (11) | |
| Procedural characteristics | ||||
| Variable | Total N=499 |
ST-ALI N=108 |
De novo ALI N=391 |
p-value |
| Primary bypass | 28 (6) | 1 (1) | 27 (7) | 0.017 |
| Primary amputation | 33 (7) | 2 (2) | 31 (8) | 0.024 |
| Conservative therapy | 10 (2) | 1 (1) | 9 (2) | 0.366 |
| Surgical thrombectomy | 209 (42) | 13 (12) | 197 (50) | <0.001 |
| Catheter thrombectomy | 233 (47) | 79 (73) | 154 (39) | <0.001 |
| Local thrombolysis | 102 (20) | 44 (41) | 58 (15) | <0.001 |
| Additional stenting | 155 (31) | 45 (42) | 110 (28) | 0.007 |
| Data are presented as median [interquartile range] or number (%). ALI: acute limb ischaemia; CIA: common iliac artery; CPK: creatine phosphokinase; CRP: C-reactive protein; de novo ALI: ALI caused by other aetiologies; EIA: external iliac artery; ST: stent thrombosis; ST-ALI: ALI caused by femoropopliteal ST | ||||
Table 3. Baseline characteristics at initial treatment in the ST-ALI group.
| Lesion characteristics | |
|---|---|
| Variables | N=108 |
| Angiographical data missing | 4 (4) |
| Rutherford category | |
| 2 | 10 (9) |
| 3 | 32 (30) |
| 4 | 13 (12) |
| 5 | 47 (44) |
| 6 | 6 (5) |
| TASC II classification | |
| A | 3 (3) |
| B | 11 (10) |
| C | 56 (52) |
| D | 34 (31) |
| Chronic total occlusion | 53 (49) |
| In-stent restenosis | 12 (11) |
| Ostial lesion | 35 (32) |
| Popliteal artery involvement | 63 (58) |
| Below-the-knee vessel run-off | |
| 0 | 10 (9) |
| 1 | 56 (52) |
| 2 | 26 (24) |
| 3 | 12 (11) |
| Lesion length, cm | 25 [15, 30] |
| PACSS classification | |
| 0 | 35 (33) |
| 1 | 11 (10) |
| 2 | 10 (9) |
| 3 | 11 (10) |
| 4 | 37 (34) |
| Data are presented as number (%) or median [interquartile range]. ALI: acute limb ischaemia; PACSS: Peripheral Arterial Calcium Scoring System; ST: stent thrombosis; ST-ALI: ALI caused by femoropopliteal ST; TASC: TransAtlantic Inter-Society Consensus | |
CLINICAL OUTCOMES OF ST-ALI VERSUS DE NOVO ALI
The 12-month AFS was significantly lower in the ST-ALI group than that in the de novo ALI group (51.3±5.1% vs 75.0±2.5%; p<0.001) (Central illustration). Figure 1 shows that the 12-month rates of freedom from MALE and occlusion were also significantly lower in the ST-ALI group than those in the de novo ALI group (46.5±5.6% vs 85.4±2.2%; p<0.001 and 50.0±5.5% vs 86.6±2.2%; p<0.001, respectively). As shown in Supplementary Table 1 and Supplementary Figure 1, the ST-ALI group also had lower rates of freedom from death (62.3±5.0% vs 78.4±2.4%; p=0.004), freedom from major amputation (78.6±4.9% vs 94.6±1.4%; p<0.001), and freedom from thrombectomy/thrombolysis (57.6±5.8% vs 91.4±1.8%; p<0.001) than the de novo ALI group.
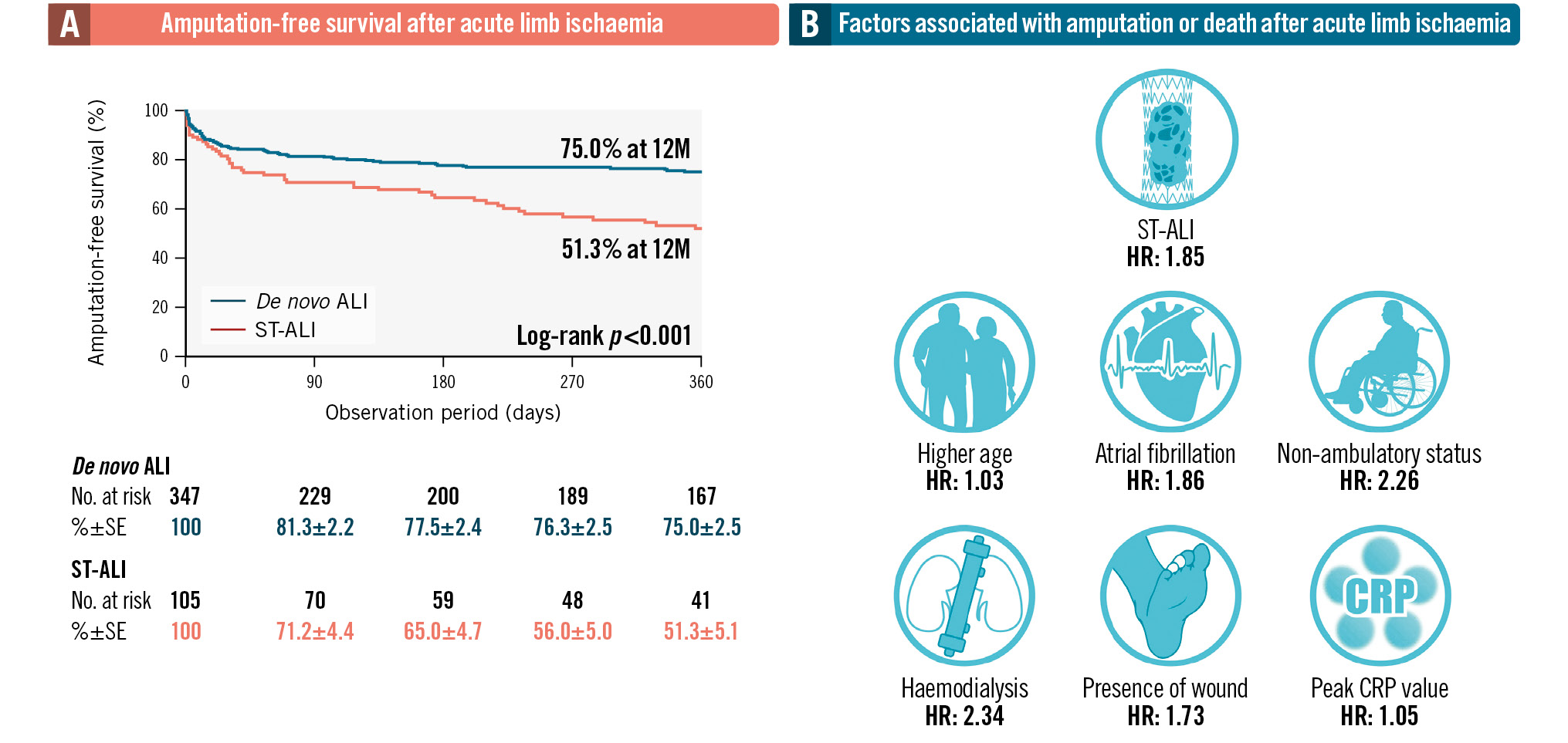
Central illustration. Amputation-free survival rate and prognostic factors after acute limb ischaemia. A) The 12-month AFS was significantly lower in the ST-ALI group than in the de novo ALI group (51.3±5.1% vs 75.0±2.5%; p<0.001), indicating better outcomes for patients with de novo ALI than for those with ST-ALI. B) ST-ALI as well as older age, haemodialysis, atrial fibrillation, the presence of wound, peak CRP level, and non-ambulatory status remained significantly associated with an increased risk of death or amputation. ALI: acute limb ischaemia; CRP: C-reactive protein; de novo ALI: ALI caused by other aetiologies; HR: hazard ratio; SE: standard error; ST: stent thrombosis; ST-ALI: ALI caused by femoropopliteal ST
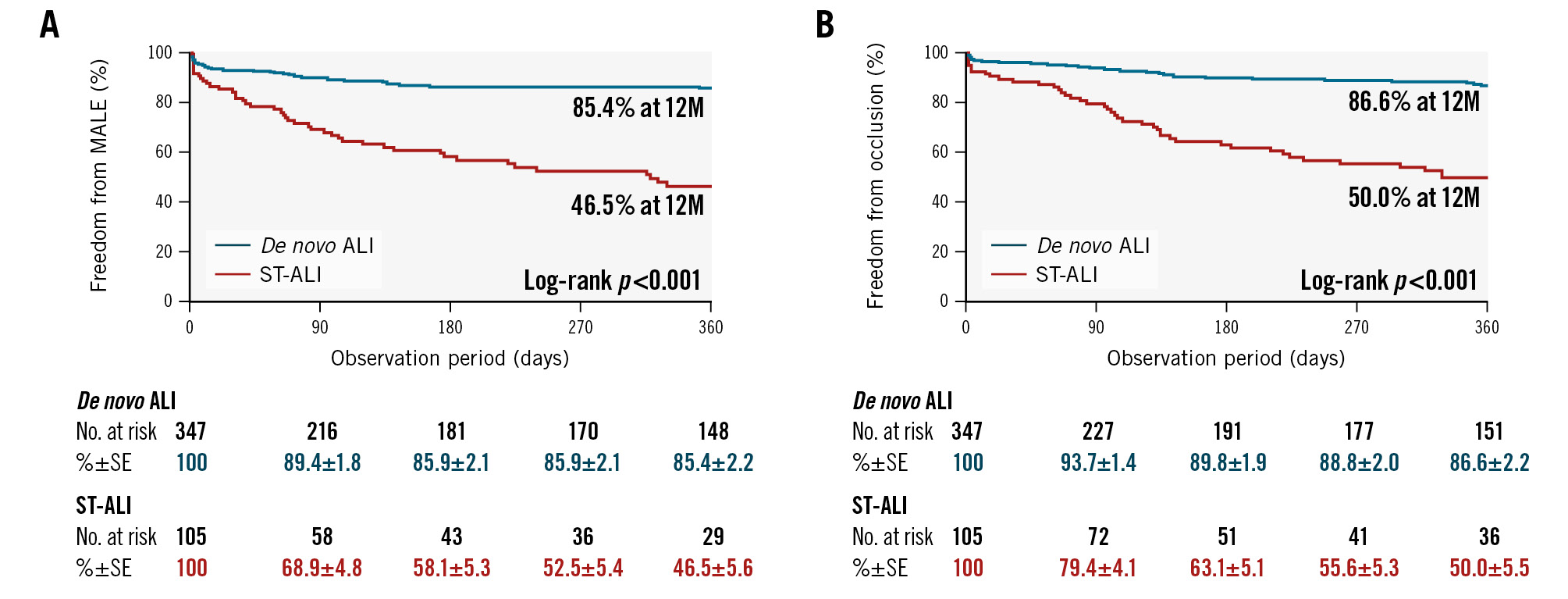
Figure 1. Twelve-month rates of MALE and occlusion. The incidence of MALE and occlusion of the treated artery were measured over a 12-month period following treatment for ALI and compared between patients with ST-ALI and those with de novo ALI. The 12-month rates of freedom from MALE (A) and occlusion (B) were significantly lower in the ST-ALI group than those in the de novo ALI group (46.5±5.6% vs 85.4±2.2%; p<0.001, and 50.0±5.5% vs 86.6±2.2%; p<0.001, respectively), indicating better outcomes for patients with de novo ALI than for those with ST-ALI. ALI: acute limb ischaemia; de novo ALI: ALI caused by other aetiologies; MALE: major adverse limb events; ST: stent thrombosis; ST-ALI: ALI caused by femoropopliteal ST
FACTORS ASSOCIATED WITH DEATH OR MAJOR AMPUTATION IN PATIENTS WITH RUTHERFORD CLASSIFICATION STAGE I OR II ACUTE LIMB ISCHAEMIA
The baseline characteristics significantly associated with death and major amputation were older age (hazard ratio [HR] 1.04 per 1-year increase, 95% confidence interval [CI]: 1.02-1.06; p<0.001), female sex (HR 1.69, 95% CI: 1.25-2.30; p<0.001), non-ambulatory status (HR 4.19, 95% CI: 2.93-5.99; p<0.001), haemodialysis (HR 2.39, 95% CI: 1.66-3.46; p<0.001), current smoking (HR 0.24, 95% CI: 0.11-0.50; p<0.001), coronary artery disease (HR 1.51, 95% CI: 1.06-2.16; p=0.024), atrial fibrillation (HR 1.49, 95% CI: 1.04-2.11; p=0.028), ST-ALI (HR 2.06, 95% CI: 1.44-2.96; p<0.001), peak C-reactive protein (CRP) level (HR 1.06 per 1 mg/dL increase, 95% CI: 1.04-1.07; p<0.001), and the presence of a wound (HR 2.63, 95% CI: 1.71-4.05; p<0.001). After multivariate analysis, ST-ALI (HR 1.85, 95% CI: 1.14-2.99; p=0.012) as well as older age (HR 1.03 per 1-year increase, 95% CI: 1.01-1.05; p=0.023), haemodialysis (HR 2.34, 95% CI: 1.45-3.79; p<0.001), atrial fibrillation (HR 1.86, 95% CI: 1.25-2.77; p=0.002), the presence of a wound (HR 1.73, 95% CI: 1.08-2.76; p=0.022), peak CRP level (HR 1.05 per 1 mg/dL increase, 95% CI: 1.03-1.07; p<0.001), and non-ambulatory status (HR 2.26, 95% CI: 1.49-3.43; p<0.001) remained significantly associated with an increased risk of death or amputation (Table 4). Subgroup analyses showed that no baseline characteristics had any interaction effect on the association between ST-ALI and the primary outcome (Figure 2).
Table 4. Univariate and multivariate analyses to identify factors associated with death or amputation.
| Variable | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Hazard ratio [95% CI] | p-value | Hazard ratio [95% CI] | p-value | |
| Age | 1.04 [1.02-1.06] | <0.001 | 1.03 [1.01-1.05] | 0.023 |
| Female sex | 1.69 [1.25-2.30] | <0.001 | 1.24 [0.84-1.82] | 0.277 |
| Non-ambulatory status | 4.19 [2.93-5.99] | <0.001 | 2.26 [1.49-3.43] | <0.001 |
| Hypertension | 0.92 [0.62-1.38] | 0.692 | ||
| Dyslipidaemia | 0.89 [0.62-1.28] | 0.526 | ||
| Diabetes mellitus | 1.28 [0.89-1.82] | 0.183 | ||
| Haemodialysis | 2.39 [1.66-3.46] | <0.001 | 2.34 [1.45-3.79] | <0.001 |
| Current smoker | 0.235 [0.11-0.50] | <0.001 | 0.54 [0.24-1.20] | 0.130 |
| Coronary artery disease | 1.51 [1.06-2.16] | 0.024 | 1.13 [0.73-1.73] | 0.590 |
| Cerebral infarction | 1.18 [0.78-1.78] | 0.446 | ||
| Atrial fibrillation | 1.49 [1.04-2.11] | 0.028 | 1.86 [1.25-2.77] | 0.002 |
| Number of antithrombotic drugs | 1.12 [0.93-1.36] | 0.227 | ||
| ST-ALI | 2.06 [1.44-2.96] | <0.001 | 1.85 [1.14-2.99] | 0.012 |
| Onset to admission | 0.99 [0.99-1.01] | 0.775 | ||
| Peak CPK | 1.00 [0.99-1.00] | 0.143 | ||
| Peak CRP | 1.06 [1.04-1.07] | <0.001 | 1.05 [1.03-1.07] | <0.001 |
| Presence of a wound | 2.63 [1.71-4.05] | <0.001 | 1.73 [1.08-2.76] | 0.022 |
| CIA or EIA thrombus | 0.91 [0.57-1.47] | 0.708 | ||
| Rutherford classification | 1.36 [0.87-2.12] | 0.181 | ||
| Pre-existing PAD | 1.36 [0.87-1.99] | 0.198 | ||
| Primary bypass | 0.59 [0.22-1.60] | 0.301 | ||
| Conservative therapy | 1.03 [0.26-4.17] | 0.966 | ||
| Surgical thrombectomy | 0.75 [0.52-1.09] | 0.129 | ||
| Catheter thrombectomy | 1.01 [0.71-1.44] | 0.951 | ||
| Local thrombolysis | 1.00 [0.66-1.52] | 0.988 | ||
| Additional stenting | 1.32 [0.92-1.89] | 0.134 | ||
| ALI: acute limb ischaemia; CI: confidence interval; CIA: common iliac artery; CPK: creatine phosphokinase; CRP: C-reactive protein; EIA: external iliac artery; PAD: peripheral arterial disease; ST: stent thrombosis | ||||
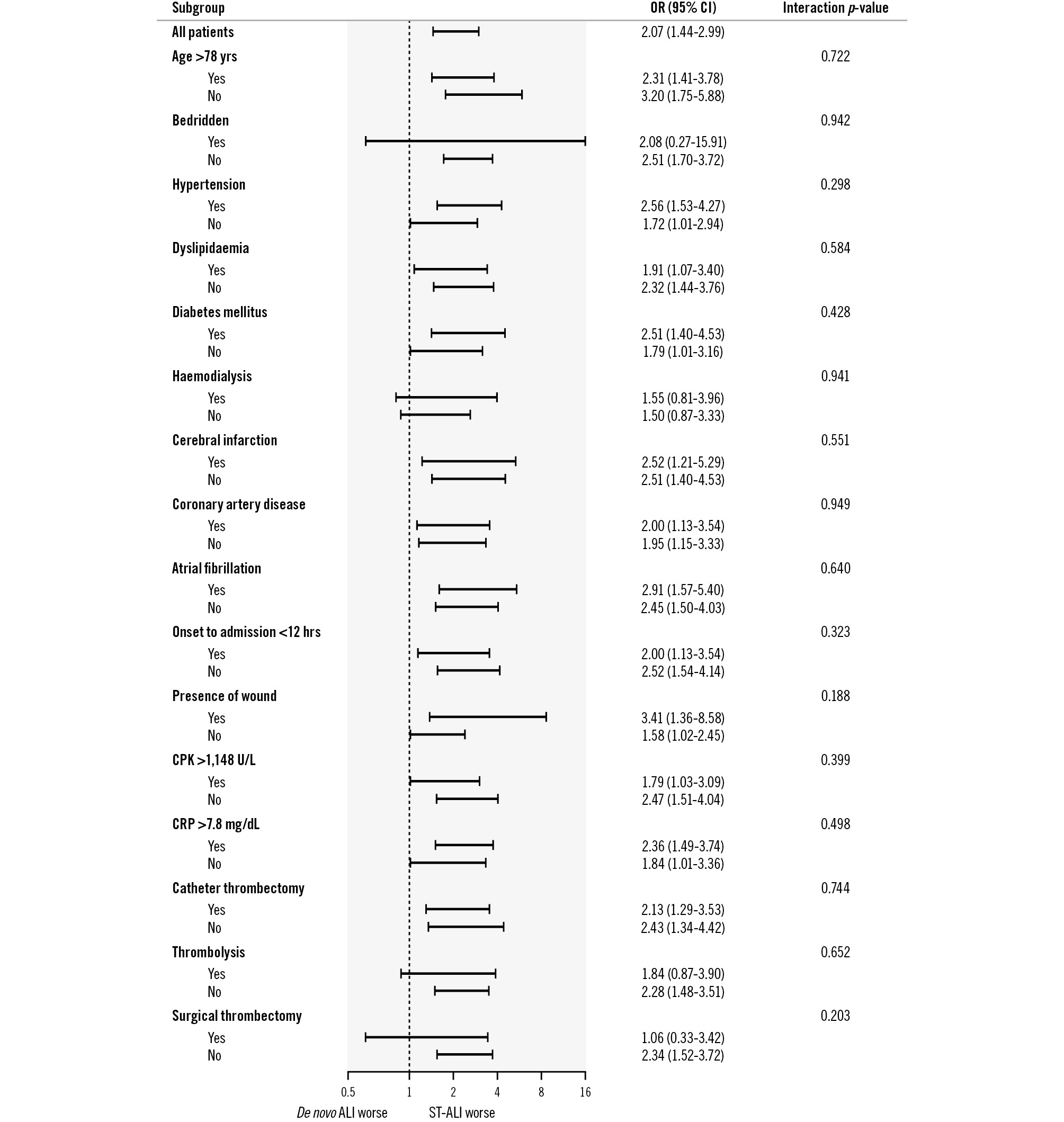
Figure 2. Interaction effect on the association of the aetiology of ALI with amputation or mortality risk. The interaction effect of baseline characteristics on the association between ALI aetiology and amputation or mortality risk was analysed using a Cox proportional hazards regression model. Interactions for baseline characteristics between patients with ST-ALI and those with de novo ALI were not observed in the AFS. AFS: amputation-free survival; ALI: acute limb ischaemia; CI: confidence interval; CPK: creatine phosphokinase; CRP: C-reactive protein; de novo ALI: ALI caused by other aetiologies; OR: odds ratio; ST: stent thrombosis; ST-ALI: ALI caused by femoropopliteal ST
Discussion
SUMMARY OF THE CURRENT STUDY
Previous studies have shown that patients with ST-ALI experience a higher rate of MALE and a composite of mortality and major amputation compared with those without ST514. However, no studies have compared the clinical features and outcomes of patients with ST-ALI to those of patients with ALI with other aetiologies. The current study highlighted that patients with ST-ALI were more likely to exhibit conventional atherosclerotic risk factors than patients with de novo ALI and presented with a less severe Rutherford classification and lower CPK values. Patients with ST-ALI had a significantly lower rate of 12-month AFS than patients with de novo ALI, even after adjusting for baseline characteristics.
CLINICAL FEATURES OF ACUTE LIMB ISCHAEMIA CAUSED BY FEMOROPOPLITEAL STENT THROMBOSIS
All patients presenting with ST-ALI were previously treated for LEAD, and therefore, it is reasonable that they exhibited more atherosclerotic risk factors. In contrast, the aetiology of de novo ALI includes not only in situ thrombosis and graft failure of LEAD but also cardiac embolisation, aortic dissection or embolisation, popliteal artery aneurysm, hypercoagulability associated with chemotherapy or cancer, and iatrogenic causes1. Therefore, patients with de novo ALI are less likely to exhibit atherosclerotic risk factors, are older, and have a higher prevalence of atrial fibrillation.
In terms of limb characteristics, the lesser severity of symptoms in patients with ST-ALI may be attributable to the development of collateral circulation in patients with LEAD. A greater degree of collateral circulation in the FP artery is reported to be associated with better lower-extremity functional performance, as assessed by the 6-minute walk and 4-metre walking velocity tests15. In ST-ALI, limbs exposed to chronic ischaemia may develop collateral circulation, resulting in milder symptoms and lower CPK values compared to those in patients with de novo ALI.
Regarding treatment strategy, Taha et al reported that OSR was more commonly performed than EVT for the treatment of ALI caused by cardiac embolism and prosthetic graft failure9. This was consistent with our findings, in which OSR, including primary bypass and surgical thrombectomy, was more frequently conducted in the de novo ALI group, whereas EVT, such as catheter thrombectomy and local thrombolysis, was performed more often in the ST-ALI group. Although the safety and efficacy of an EVT-first approach to ALI have recently been reported, treatment strategies are often dependent on the aetiologies of ALI (e.g., surgical thrombectomy would be preferred for cardiac embolism and prosthetic graft failure, and thrombolysis would be preferred for small vessel emboli, vein bypass graft, and stent thrombosis)9161718.
PROGNOSIS OF PATIENTS WITH ST-ALI
Another important finding of this study was that the clinical outcomes of patients with ST-ALI were significantly worse than those of patients with de novo ALI. One reason for the lower AFS rate in patients with ST-ALI may be the different baseline characteristics, such as the higher prevalence of atherosclerotic risk factors and the presence of wounds. This is supported by the fact that the Kaplan-Meier curves began to separate after the first month. However, even after adjusting for these confounding factors in the multivariate analysis, ST-ALI remained an independent risk factor for death or amputation. This may be because ST-ALI has a higher recurrence rate than de novo ALI, worsening the prognosis. The 12-month freedom from occlusion rates in the native artery and bypass graft have been reported as 87% and 60%, respectively19, which is consistent with the findings of the current study, where the 12-month freedom from occlusion rate was 86.6% in de novo ALI, which included a relatively high proportion of patients with native artery ALI. In contrast, the recurrence rate of ST-ALI was greater, consequently leading to a lower AFS rate and higher incidence of MALE. Another reason for the poorer prognosis of patients with ST-ALI may be the greater anatomical complexity of ST-ALI, such as the higher proportion of popliteal artery involvement (Table 3). Although angiography was not performed before or after treatment in some patients who underwent OSR, making it difficult to directly compare the anatomical complexity, the anatomical findings at the initial treatment in ST-ALI patients revealed that popliteal stenting, which may lead to worse limb prognosis, accounted for 58% of cases. Interventions in a diseased popliteal artery interrupt the collateral circulation to the lower extremities, and popliteal artery lesions have been reported to be a risk factor for ALI after EVT using a stent5.
Several factors other than ST-ALI − such as age, haemodialysis, atrial fibrillation, non-ambulatory status, the presence of a wound, and peak CRP value − were also risk factors for amputation or death. These findings are consistent with the results of previous studies20212223.
PREVENTION OF ST-ALI
This study demonstrates that patients with ST-ALI have significantly worse clinical outcomes than those with de novo ALI, highlighting the need to prevent ST occurrence. Previously, haemodialysis and lesions involving the popliteal artery were the predictors for acute thrombotic occlusion5. Therefore, selection of a non-stenting strategy would be considered for patients at high risk of ST-ALI. One option within this approach is drug-coated balloon-based EVT. EVT with drug-coated balloons have demonstrated durable patency and a lower thrombosis risk than stent-based EVT5. Therefore, whenever the outcome of drug-coated balloon angioplasty is deemed acceptable, deferred stenting should be considered. Another alternative treatment strategy is surgical bypass, as reported in the BEST-CLI trial, particularly for critical limb-threatening ischaemia patients with a well-functioning saphenous vein graft24.
In addition, implementing optimal medical therapy after revascularisation that may mitigate the occurrence of stent thrombosis is also crucial. Dual pathway inhibitor therapy, which combines an antiplatelet agent and a direct oral anticoagulant, has recently emerged as a promising approach to reduce cardiovascular and lower extremity vascular events, including ALI after EVT25. Further investigation is required to determine the optimal postprocedural antithrombotic regimen for reducing the incidence of ST-ALI.
Limitations
This study has certain limitations. First, although our study represents one of the largest series to investigate ST-ALI, its retrospective nature means that some data, including the antithrombotic therapy following treatment for ALI, were missing. The type, dose and duration of the postprocedural antithrombotic drug, especially whether a low-dose oral anticoagulant was used, would have been interesting to know, but these were not available. In addition, some anatomical information before and/or after treatment for ALI was not available, because many patients treated with OSR did not undergo angiography.
Second, de novo ALI affects a heterogeneous population, and the aetiology and background characteristics of ALI differ, resulting in variations in characteristics and risk factors. In addition, this study included only de novo ALI in FP arterial lesions for comparison with FP-ST, which is clinically challenging. However, the patient characteristics and aetiology of de novo ALI in the current study were similar to those in the previously reported comprehensive national registry of ALI, and the clinical outcomes of de novo ALI in the current study were comparable to those of previous studies that included similar patient and clinical characteristics, supporting the generalisability of the current study6789.
Third, there may be interfacility variations in treatment strategies and technical success rates. Although this multicentre registry included both high-volume cardiovascular and vascular surgical centres, the first-line treatment strategy and its detailed procedure steps may have differed between hospitals, and these differences may have affected patient prognosis. However, recent studies have revealed comparable results between OSR and EVT, and the multivariate analysis of the current study showed that the treatment approach did not affect AFS9171819. Future studies to determine the optimal treatment strategy are needed.
Finally, the precise aetiology of ALI was clinically challenging to distinguish. There may be stent thrombosis cases where thrombotic stent occlusion, due to embolism to the stented segment with superimposed thrombosis, impaired run-in and run-off (e.g., in-stent restenosis or poor below-the-knee vessel run-off).
Conclusions
Patients with ST-ALI were associated with a lower 12-month AFS rate than those with de novo ALI, highlighting the need to maximise ST prevention.
Impact on daily practice
The 12-month amputation-free survival rate was lower in patients with acute limb ischaemia caused by femoropopliteal stent thrombosis (ST-ALI) compared to those with acute limb ischaemia with other aetiologies. ST-ALI is a life-threatening event, and further investigation is required to determine the risk factors for this condition after femoropopliteal stenting. A non-stenting strategy, including surgical bypass or drug-coated balloon-based endovascular therapy, and implementing optimal medical therapy after revascularisation should be considered for patients at high risk of ST-ALI.
Acknowledgements
The authors thank Shunsuke Kojima and Tomofumi Tsukizawa for their excellent assistance with data collection.
Conflict of interest statement
O. Iida received remuneration from Boston Scientific Japan, W. L. Gore & Associates G.K., BD, and Terumo. M. Takahara has received an endowed chair funded by AstraZeneca K.K., Mitsubishi Tanabe Pharma Corp., MSD K.K., Nippon Boehringer Ingelheim Co., Ltd, Ono Pharmaceutical Co., Ltd, and Taisho Toyama Pharmaceutical Co., Ltd. T. Yamaoka received remuneration from Boston Scientific Japan, W. L. Gore & Associates, Japan Lifeline, and Kaneka Medix. T. Nakama is a consultant for BD, Boston Scientific, Cook Medical, Cordis/Cardinal Health, Kaneka Medix, Nipro, and OrbusNeich. K. Tobita is a consultant for W. L. Gore & Associates and Boston Scientific; and has received presentation fees from Medtronic, Kaneka Medix, Terumo, and Medicon. T. Mano received a research grant from Abbott Vascular Japan. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

