Cory:
Unlock Your AI Assistant Now!
Inflammation is a key determinant for the development and progression of atherosclerotic cardiovascular disease (ASCVD), strongly contributing to the so-called “residual cardiovascular (CV) risk”. A large number of clinical trials investigating different agents were conducted before colchicine emerged as the first anti-inflammatory drug to be recommended by guidelines for the management of acute (ACS) and chronic coronary syndromes (CCS). However, despite a strong mechanistic rationale and supporting clinical data, colchicine has never received a Class I recommendation. Recently, a large randomised clinical trial (RCT) in patients who had myocardial infarction (MI) has yielded neutral results, which are not consistent with previous findings. This discrepancy has raised concerns about the true clinical benefit of colchicine in patients with ASCVD, particularly in light of heterogeneity across trials and potential safety concerns. Whether current guideline recommendations are entirely justified or the benefits of colchicine have been overestimated remains a matter of debate.
Pros
Kevin R. Bainey, MD, MSc, FRCPC, FACC
In patients presenting with an acute coronary syndrome, evidence-based secondary prevention pharmacotherapeutic agents have reduced cardiovascular death or MI. Yet, despite these efforts, recurrent events still occur. Inflammation is now seen as a key contributor to the development of ASCVD and susceptibility of vulnerable plaque rupture. Chronic activation of the NOD-, LRR-, and pyrin domain-containing protein 3 (NLRP3) inflammasome pathway leads to the production of interleukin (IL)-1 and IL-6 cytokines resulting in elevated plasma levels of C-reactive proteins (CRP) – a biomarker of inflammation1. However, it is important to understand that early in acute MI there are protective benefits and healing from inflammation with activation of T cells, natural killer cells, and macrophages upregulated by IL-2 and IL-10 cytokines. This makes it challenging to find therapeutic targets to help balance “the good, bad, and the ugly” stages of inflammation in acute MI2.
Colchicine has been deemed a potential therapeutic target in the NLRP3 inflammasome pathway. Used for other inflammatory rheumatological conditions, colchicine has been tested in two ASCVD randomised trials. The Colchicine Cardiovascular Outcomes Trial (COLCOT) enrolled 4,745 acute MI patients to colchicine versus placebo and found a 23% relative risk reduction in the primary composite of death from CV causes, resuscitated cardiac arrest, MI, stroke, or urgent hospitalisation for angina leading to coronary revascularisation in favour of colchicine over a median of 22.6 months. However, there was no reduction in CV death or recurrent MI – these results were driven mainly by a reduction in urgent hospitalisation for angina leading to revascularisation (softer endpoint)3. The Low Dose Colchicine for secondary prevention of cardiovascular disease (LoDoCo2) Trial randomised 5,522 stable ischaemic heart disease (SIHD) patients to colchicine versus placebo and found a 31% relative risk reduction in the primary composite of CV death, spontaneous (non-procedural) MI, ischaemic stroke, or ischaemia-driven coronary revascularisation in favour of colchicine over a median of 28.6 months. There was a reduction in CV death or MI (hard endpoint; hazard ratio [HR] 0.71, 95% confidence interval [CI]: 0.55-0.92) – but an alarming signal towards an increase in non-CV death4. As seen in Figure 1, the 2021 European Society of Cardiology (ESC) Guidelines on cardiovascular disease prevention in clinical practice quickly listed colchicine as a Class IIb Level of Evidence (LoE) A (high quality of randomised evidence) recommendation for secondary prevention of ASCVD5. The 2023 ACC/AHA Guidelines on chronic coronary disease list it as a Class IIb, LoE B-R (moderate quality of randomised evidence) for secondary prevention6. The 2024 ESC Guidelines for chronic coronary syndromes lists it as a Class IIa, LoE A indication7. Recently, the 2025 ACC/AHA Guidelines for the management of patients with acute coronary syndromes recommended the use of chronic colchicine as a reasonable therapy to reduce the risk of major adverse cardiac events (Class IIb, LoE B-R)8.
The recent double-blinded Colchicine and Spironolactone in Patients with MI / SYNERGY Stent Registry (CLEAR SYNERGY) trial randomised over 7,000 patients with an acute MI (ST-segment elevation MI [STEMI] or large non-STEMI [NSTEMI]) within 72 hours of percutaneous coronary intervention (2x2 factorial design) to colchicine versus placebo (the other randomised strategy was spironolactone). The primary outcome was a composite of CV death, recurrent MI, stroke or ischaemia-driven revascularisation over a median of 3 years. Colchicine reduced CRP levels (mean difference of −1.28 mg per litre, 95% CI: −1.81 to −0.75) demonstrating its biological effect. However, there was no difference in the primary composite with colchicine versus placebo (9.1 vs 9.3 events; HR 0.99, 95% CI: 0.85-1.16; p=0.93) with near-identical overlap of the Kaplan-Meier curves. Moreover, there were no differences in the individual components of the composite events. The on-treatment analysis was consistent with the intention-to-treat analysis. Diarrhoea was significantly more common with colchicine (10.2% vs 6.6%; p<0.001)9.
So why the divergent results? Simply, CLEAR SYNERGY was a larger study powered for clinically meaningful endpoints with longer-term follow-up. CLEAR SYNERGY is the largest trial of colchicine in acute MI patients with double the events of its comparator, COLCOT. Reassuringly, there was no signal towards an increase in non-CV death, but the primary composite endpoint was completely neutral with no hint of benefit (despite showing a reduction in CRP levels with colchicine). This is a testament to the importance of conducting large clinical trials powered for important clinical endpoints to test the true treatment effect of an investigative agent. This situation is reminiscent of the aspiration thrombectomy story in STEMI in which two large clinical trials, TASTE (ClinicalTrials.gov: NCT05121883) and TOTAL (NCT01149044), stopped its routine use. Perhaps the dose is wrong or the bioavailability is questioned, and until further studies are completed, colchicine is “dead in the water” following an ACS. LoDoCo2 showed benefits for its use in SIHD; however, in CLEAR SYNERGY, there is no indication of clinical benefit during the chronic coronary syndrome phase after 1 year.
While the colchicine story in ACS appears to have reached a disappointing conclusion, the broader inflammatory hypothesis remains alive and well. The failure of colchicine, which targets a relatively upstream component of the inflammatory cascade, may simply indicate that a more downstream target, such as IL-6, is a more promising therapeutic avenue. High IL-6 levels are associated with worsening reperfusion injury and adverse left ventricular remodelling following STEMI10. In CANTOS, the benefits of canakinumab were directly related to the magnitude of IL-6 reduction achieved after the first dose11. Despite the setback with colchicine, the focus on inflammation in ASCVD remains a crucial area of research. The ongoing ARTEMIS trial (ClinicalTrials.gov: NCT06118281), targeting IL-6, represents a significant step in this direction and holds the potential to significantly advance our understanding and treatment of cardiovascular disease. This will be the largest clinical trial targeting inflammation in acute MI patients.
As it stands, colchicine has no established role in the routine treatment following ACS. Future ESC clinical guidelines will need to take the results of CLEAR SYNERGY into consideration and potentially downgrade its recommendation (Figure 1).
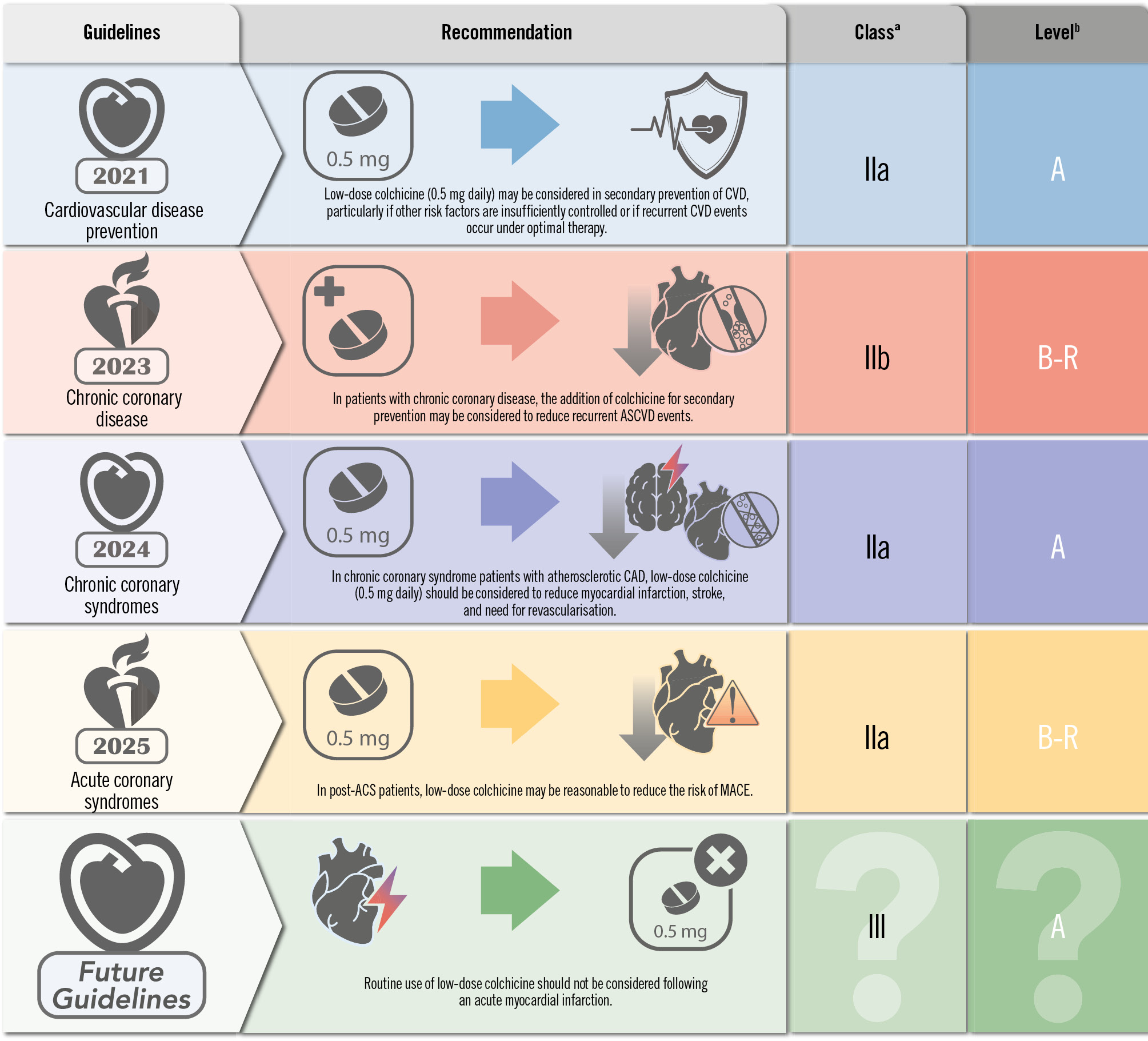
Figure 1. Current and potential future guidelines for low-dose colchicine. aClass of recommendation; bLevel of Evidence. ACS: acute coronary syndrome; ASCVD: atherosclerotic cardiovascular disease; CAD: coronary artery disease; CVD: cardiovascular disease; MACE: major adverse cardiac events
Acknowledgements
Lisa Soulard from the Canadian VIGOUR Centre for editorial assistance.
Conflict of interest statement
K.R. Bainey has no conflicts of interest to declare.
Cons
Xavier Rossello, MD, PhD
According to the 2023 ESC Guidelines for the management of acute coronary syndromes, low-dose colchicine (0.5 mg daily) may be considered, particularly if other risk factors are insufficiently controlled or if recurrent CV disease events occur under optimal therapy12. According to the 2024 ESC Guidelines for the management of chronic coronary syndromes, low-dose colchicine should be considered in CCS patients with atherosclerotic coronary artery disease to reduce myocardial infarction, stroke, and need for revascularisation. Of note, not all CCS patients are post-ACS7. Neither Class IIa nor IIb recommendations can be considered strong recommendations. They are usually worded as "should be considered" and "may be considered", respectively. Class II recommendations are not summarised in the ESC guideline table summarising “what to do” and “what not to do” and are therefore not as unambiguous Class I and III recommendations13.
Several recent RCTs have tested the role of the anti-inflammatory agent colchicine in ACS and CCS34. COLCOT tested whether low-dose colchicine was superior to placebo in 4,745 patients with recent (<30 days) acute MI regardless of C-reactive protein values3. After a median of 2.3 years, the primary composite of CV death, resuscitated cardiac arrest, MI, stroke, or unstable angina-driven revascularisation occurred in 131 (5.5%) and 170 (7.1%) participants in the colchicine and placebo groups, respectively (HR 0.77, 95% CI: 0.61-0.96; p=0.02). There was therefore a difference in 39 primary events. Among these primary outcomes, 25 (64%) were urgent revascularisations. Notably, a p-value of 0.02 falls into the category of “some evidence”, but not into the category of strong (0.001≤p<0.01) or overwhelming (p<0.001) evidence14. In addition to the interpretation of clinical benefit, there were some safety concerns. Both nausea and pneumonia, although infrequent, were more often reported with colchicine than placebo.
The LoDoCo2 Trial randomised 5,522 patients with CCS (84% of whom had prior ACS) to low-dose colchicine or placebo4. After a median of 2.4 years, the primary endpoint (composite of CV death, MI, stroke, or ischaemia-driven coronary revascularisation) occurred in 187 (6.8%) and 264 (9.6%) in the colchicine and placebo arms, respectively (HR 0.69, 95% CI: 0.57-0.83; p<0.001). There was therefore a difference in 77 primary events. Among these primary outcomes, 42 (55%) were urgent revascularisations. In this case, a simplified interpretation of the p-value would classify the evidence as “overwhelming”. There were no substantial differences in the rates of pneumonia or gastrointestinal disorders. Counterintuitively, the number of non-CV deaths (n=88) outweighed the number of CV deaths (n=45), and the incidence of non-CV death was 53 (2%) in the colchicine arm and 35 (1.3%) in the placebo arm (HR 1.51, 95% CI: 0.99-2.31). Although we cannot rule out a small competing risk phenomenon15, the non-cardiovascular mortality did not neutralise the reduction in CV mortality. Hence, the incidence of all-cause death was 73 (0.7%) in the colchicine arm and 60 (2.2%) in the placebo arm (HR 1.21, 95% CI: 0.86-1.71).
More recently, the CLEAR SYNERGY trial randomised 7,062 patients with ACS to low-dose colchicine or placebo9. After a median of 3 years, the primary endpoint (composite of CV death, MI, stroke, or unplanned ischaemia-driven coronary revascularisation) occurred in 322 (9.1%) and 327 (9.3%) in the colchicine and placebo arms, respectively (HR 0.99, 95% CI: 0.85-1.16; p=0.93). There was therefore a difference in 5 primary events. This evidence was not available at the time when both the 2023 and 2024 guidelines for ACS and CCS were published.
Inflammation plays a central role in the pathogenesis of atherosclerosis and acute coronary events. Although stable for long periods, patients with CCS are frequently progressive and may destabilise at any moment with the development of an ACS. Based on the totality of evidence, it might well be that the anti-inflammatory effect of colchicine plays a role in preventing ischaemic events (mostly unplanned revascularisations) in the chronic setting but not in the acute scenario, where other prognostic determinants might prevail over inflammation. As evidence accrues, future guidelines might provide different recommendations to the current Class IIb and IIa for patients with ACS and CCS, respectively.
Conflict of interest statement
X. Rossello has no conflicts of interest to declare.

