Cory:
Unlock Your AI Assistant Now!
Contemporary coronary intervention strategies primarily focus on anatomical obstructions and physiological flow limitations. In the context of chronic coronary syndromes, increasing clinical evidence has demonstrated that revascularising flow-limiting coronary obstructions does not substantially reduce the risk of myocardial infarction or cardiac death. Furthermore, rehospitalisation due to a new acute coronary syndrome frequently occurs within the first year from a percutaneous coronary intervention (PCI) targeting flow-limiting plaque disease1. This underscores the unmet need to identify the non-obstructive vulnerable plaques, which could be treated to improve clinical outcomes, as demonstrated in the recent PREVENT trial2.
Inflammation is a critical process in both the development and rupture of atherosclerotic plaques3. Among patients undergoing routine clinical coronary computed tomography angiography (CCTA), coronary inflammation can be non-invasively detected by mapping the radiological changes in the perivascular adipose tissue, and quantified using the fat attenuation index (FAI)-Score45. Indeed, the FAI-Score measured around any coronary artery demonstrated significant prognostic value among patients with or without obstructive coronary atherosclerosis in the ORFAN study6. Previous studies have demonstrated that pericoronary FAI7, or even the uncorrected pericoronary adipose tissue (PCAT) mean attenuation8, adds significant value in predicting acute coronary syndromes, in addition to the morphological features of high-risk plaque seen on CCTA. This has shifted the paradigm from treating coronary obstruction to identifying vulnerable patients with inflamed plaques (or inflamed coronaries in the absence of plaque) who are at risk of cardiovascular events, enabling timely interventions at any stage of the disease process, to improve clinical outcomes (Figure 1).
In this issue of EuroIntervention, Naniwa et al demonstrate that coronary inflammation (measured using pericoronary fat attenuation measurements on CCTA) and high-risk plaque features identified before PCI have additive value in predicting the primary composite outcome of cardiovascular death, non-fatal myocardial infarction (MI), revascularisation, and stroke, while high coronary inflammation might identify those individuals who would respond well to statin treatment, thereby reducing the clinical event rate post-PCI9.
Atherosclerotic plaques were characterised on CCTA, and PCAT was used to quantify coronary inflammation at the lesion, target vessel, and patient levels. The addition of high-risk plaque features (positive remodelling, spotty calcification and napkin-ring sign) improved the prediction of outcomes over cardiovascular risk factors alone. Adding PCAT led to further significant improvement of the ability to predict the primary composite endpoint, achieving an overall area under the curve of just over 0.80. This is in line with previous observations underlying the importance of coronary inflammation in identifying a high-risk patient and high-risk plaque45678.
Importantly, patients with high coronary inflammation at the patient level exhibited a significantly higher risk of target vessel failure and target lesion failure following PCI, accompanied by an increased incidence of target vessel/lesion revascularisation. Notably, these associations remained significant even after adjustment for cardiovascular risk factors and quantitative plaque burden, highlighting the detrimental effect of residual inflammatory risk that persists after PCI9. Importantly, patients with high coronary inflammation responded very well to statin treatment (hazard ratio [HR] 0.46, 95% confidence interval [CI]: 0.24-0.88) for the composite endpoint post-PCI, while those with low coronary inflammation did not respond to statin treatment (HR 0.94, 95% CI: 0.19-4.61). This important finding is in line with a recent study demonstrating that coronary inflammation-guided statin management could lead to an overall ~30% reduction of fatal and non-fatal major adverse cardiovascular events (MACE) over a lifetime horizon10.
In recent years, the benefit of low-dose anti-inflammatory agents like colchicine in lowering MACE has been demonstrated in the setting of stable coronary disease (LoDoCo2 trial; Australian New Zealand Clinical Trials Registry: ACTRN12614000093684) and after myocardial infarction (COLCOT; ClinicalTrials.gov: NCT02551094). A deeper understanding of the timing and choice of anti-inflammatory agents among patients with acute presentation is warranted in light of the results from the recent CLEAR SYNERGY trial11. Very early initiation of low-dose colchicine in patients presenting with acute MI had no impact on the risk of MACE. This probably reflects the relatively limited role of inflammation in the development of early clinical events after PCI, which are primarily attributed to atherothrombosis, and possibly the fact that high-dose statin treatment is very effective at reducing inflammatory risk during the early post-PCI period. In the current study, it is noteworthy that the rate of clinical events in the high coronary inflammation group began to accelerate after 12 months compared to the low coronary inflammation group, coinciding with the end of the dual antiplatelet treatment period after PCI, or reduction of the high-dose statin if not tolerated. This highlights the importance of measuring coronary inflammation when routine medication settles to a long-term regime (e.g., a year after PCI), and deciding at that point whether specific anti-inflammatory agents (like colchicine) could be considered as long-term risk modifiers.
A limitation of the study by Naniwa et al is the use of PCAT (the mean perivascular fat attenuation) with a fixed cutoff as a reading of coronary inflammation instead of the standardised FAI-Score45. The latter takes into account several technical, local anatomical and biological factors in generating a standardised metric of coronary inflammation. Even in a research context where scan parameters are kept stable and the results are analysed consistently, PCAT provides meaningful information only when measured around the right coronary artery, while the FAI-Score is applicable across all coronary arteries with equally prognostic results, as it takes into account the local anatomy for its calculations6.
In summary, the study by Naniwa et al demonstrates the importance of residual inflammatory risk in predicting future cardiac events among patients undergoing PCI. The increasing use of CCTA for diagnosing coronary obstruction provides an opportunity for comprehensive risk assessment, by combining quantitative assessment of coronary inflammation and atherosclerotic plaque burden with traditional risk factors using prognostic models. Such a prognostic model that uses artificial intelligence to integrate this information into a clinical-grade risk assessment tool has recently been introduced in clinical practice with striking performance10, allowing personalised management of patients in both primary and secondary care.
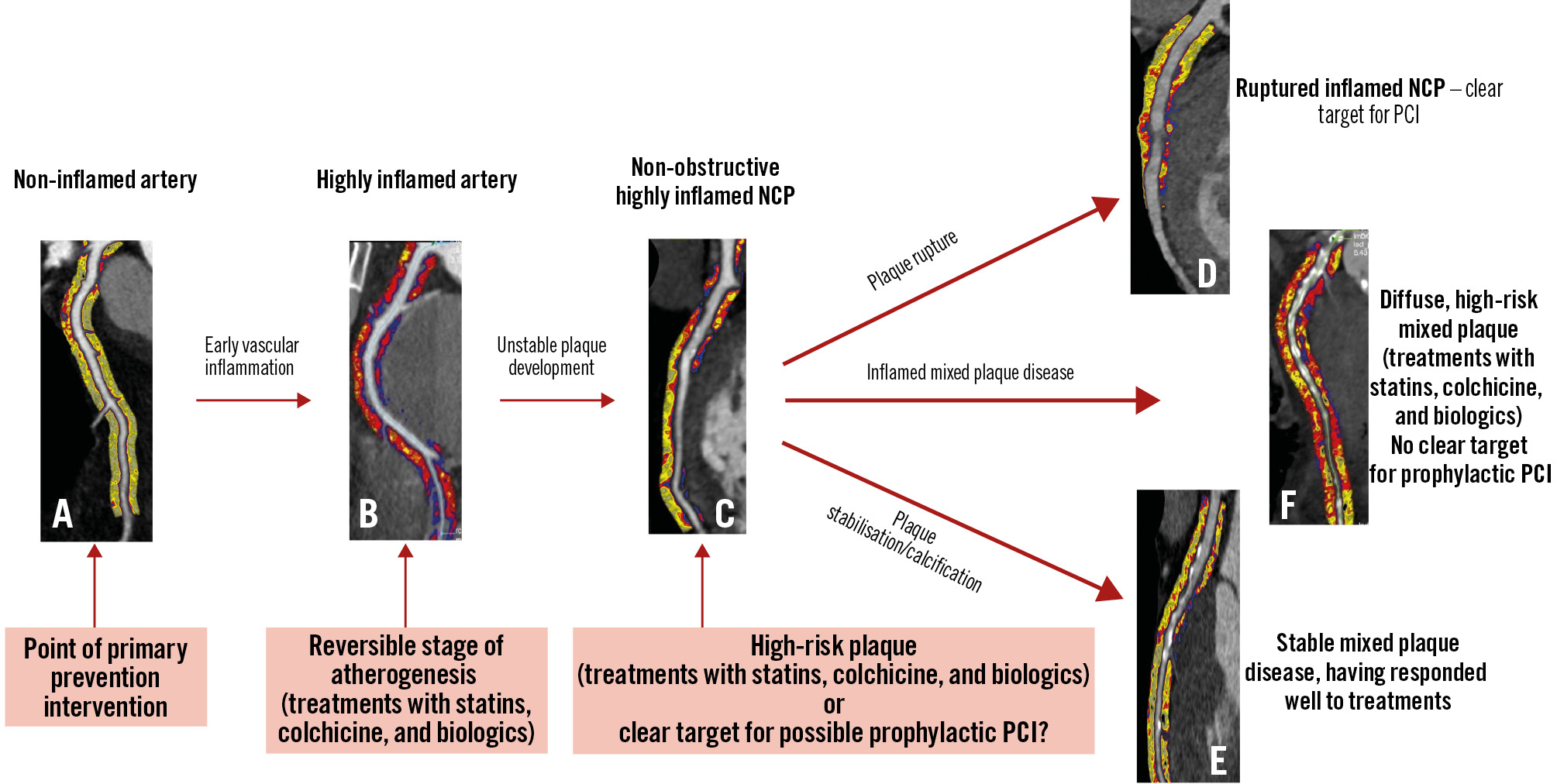
Figure 1. Natural history of coronary atherosclerosis and the role of coronary inflammation, demonstrated using perivascular fat attenuation index on routine coronary CT angiography. A) Non-inflamed coronary arteries (yellow colour on fat attenuation index [FAI] maps) form the target of primary prevention. B) Coronary inflammation, as identified by abnormal FAI (red colour on FAI maps), is the first step of atherogenesis, and still reversible with anti-inflammatory agents (e.g., statins, colchicine, biologics, etc.). C) Coronary inflammation leads to the development of high-risk inflamed non-calcified plaque (NCP), which could be a target of medication or possibly preventive PCI. This inflamed plaque disease may either lead to plaque rupture (D) or stabilisation of the plaques via calcification (E) or diffuse, mixed plaque disease which can still be inflamed/high risk (F). CT: computed tomography; PCI: percutaneous coronary intervention
Funding
C. Antoniades is supported by the British Heart Foundation (CH/F/21/90009, TG/19/2/34831, and RG/F/21/110040); Innovate UK (Grant 104472); the EU Research and Innovation Action MAESTRIA (grant agreement ID: 965286); the NIHR Oxford Biomedical Research Centre (Cardiac and Imaging themes); and the Oxford British Heart Foundation, Oxford Centre of Research Excellence. K. Chan is supported by the British Heart Foundation (FS/CRTF/24/24704).
Conflict of interest statement
C. Antoniades has received honoraria/consulting fees from Amarin, Covance, Silence Therapeutics, Amgen, Abcentra, Nodthera, Novartis, Eli Lilly, Caristo, and UCB; he has received research grants from Sanofi, Novo Nordisk, AstraZeneca, Caristo, and Lexicon; is a founder, shareholder, and non-executive director of Caristo Diagnostics; and has a leadership role at the British Atherosclerosis Society. K. Chan has no conflicts of interest to declare.

