Cory:
Unlock Your AI Assistant Now!
Abstract
BACKGROUND: Limited data are available on transcatheter patent foramen ovale (PFO) closure outcomes in the elderly.
AIMS: Through this study, we aimed to determine the incidence and predictors of adverse events (recurrent cerebrovascular events [CVE] and atrial fibrillation [AF]) post-PFO closure in older patients with cryptogenic events.
METHODS: This multicentre international study included patients over 60 years undergoing PFO closure for cryptogenic thromboembolic events. A dedicated database compiled baseline, procedural, and follow-up data. Competing risk and adjusted outcome predictor analyses were conducted.
RESULTS: A total of 689 patients were included (median age 65 years, 41.2% female, mean Risk of Paradoxical Embolism [RoPE] score 4.5). The procedural success rate was 99.4%. After a median follow-up of 2 (interquartile range 1-5) years, 66 patients (9.6%) had died. CVE and stroke rates were 1.21 and 0.55 per 100 patient-years, respectively. Diabetes (hazard ratio [HR] 3.89, 95% confidence interval [CI]: 1.67-9.07; p=0.002) and atrial septal aneurysm (ASA; HR 5.25, 95% CI: 1.56-17.62; p=0.007) increased the CVE risk. New-onset AF occurred at a rate of 3.30 per 100 patient-years, with 51.3% within one month post-procedure. Older age (HR 1.05 per year, 95% CI: 1.00-1.09; p=0.023) and the absence of hypertension (HR 2.04, 95% CI: 1.19-3.57; p=0.010) were associated with an increased risk of AF.
CONCLUSIONS: Older patients undergoing PFO closure had a relatively low rate of CVE and new-onset AF after a median follow-up of 2 years. The presence of diabetes, ASA, and a more advanced age determined an increased risk of adverse clinical events. These factors may be considered in the clinical decision-making process regarding PFO closure in this challenging population.
Patent foramen ovale (PFO) closure is known to be effective in reducing the risk of recurrent ischaemic stroke among patients suffering from PFO-associated cryptogenic thromboembolic events. However, this benefit has been proven only among patients <60 years old, as most randomised PFO closure trials were restricted to this younger population1. Consequently, in current PFO management guidelines, PFO closure is either not considered or its degree of recommendation is weak and conditional in older (>60 years) patients2.
Interestingly, observational data have shown a higher prevalence of PFO among older patients diagnosed with cryptogenic stroke3, and its presence has been associated with an increased risk of recurrent cerebrovascular events (CVE)4. In addition, several studies have shown the safety and preliminary efficacy of transcatheter PFO closure in older cryptogenic stroke patients5678910. However, older patients with potential PFO-related events are a more complex group compared to their younger counterparts, with a much higher prevalence of atherothrombotic risk factors along with a higher risk for atrial fibrillation (AF), thus a higher risk of CVE11. In fact, a higher rate of recurrent thromboembolic events and new-onset AF episodes at follow-up have been observed following PFO closure in older patients compared to their younger counterparts5610. A proper evaluation of the factors associated with recurrent CVE and AF episodes following PFO closure in elderly patients is key to improving patient selection and identifying those patients who would benefit the most from PFO closure. Thus, the objectives of our study were to determine the incidence and factors associated with adverse clinical events (recurrent CVE, new-onset AF) in older patients with cryptogenic thromboembolic events undergoing transcatheter PFO closure.
Methods
This multicentre, international cohort study included consecutive patients older than 60 years diagnosed with a cryptogenic thromboembolic event (ischaemic stroke, transient ischaemic attack [TIA], or peripheral embolism) who underwent transcatheter PFO closure between 1 August 2000 and 24 February 2023, at 14 university centres in Canada, France, Spain, and Denmark. The study protocol was performed according to the ethics board at each participating centre, and all patients provided informed consent for the procedures. The data supporting this study’s findings are available from the corresponding author upon reasonable request.
The diagnosis of PFO was established based on the presence of a right-to-left shunt during the transoesophageal echocardiography (TOE) examination (using an agitated saline test with and without Valsalva manoeuvres). An atrial septal aneurysm (ASA) was defined as an excursion of the septal tissue greater than 10 mm from the plane of the atrial septum into the right or left atrium or a combined total excursion right and left of 15 mm12. The relationship between the index thromboembolic event and the PFO was established after excluding other potential causes for the thromboembolic event via comprehensive screening, including brain computed tomography (CT) and/or magnetic resonance imaging (MRI), 24-hr (or more) Holter monitoring, transthoracic echocardiography, TOE, and carotid Doppler. Transcatheter PFO closure was performed under fluoroscopic and echocardiographic guidance, and the choice of the closure device and antithrombotic treatment at hospital discharge was left to the physician responsible for the procedure.
Patients were followed up by clinical visits or over-the-phone medical appointments at 1 to 3 months, at 12 months, and yearly thereafter. Data gathered included baseline and procedural characteristics as well as PFO closure-related clinical events. In cases where a clinical event was identified, the complete medical record was reviewed as needed. For each clinical event, the primary care physician, cardiologist, or neurologist was consulted if additional information was required.
During follow-up, the stroke diagnosis was confirmed with a CT or MRI scan of the brain. Recurrent strokes were classified as lacunar or non-lacunar (cardioembolic, cryptogenic, large artery related, or other) following recent stroke guidelines13. Transient ischaemic attack was diagnosed following the tissue-based definition and was based on the presence of a transient episode of neurological dysfunction caused by focal brain, spinal cord, or retinal ischaemia without acute infarction14. All cerebrovascular events were confirmed by a neurologist at each centre. Procedural AF was defined as any new-onset AF episode occurring during the PFO closure periprocedural period, while follow-up AF was considered when new AF episodes occurred after hospital discharge. All bleeding events were classified according to the Bleeding Academic Research Consortium (BARC) criteria15. The STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) cohort reporting guidelines were used for writing our report16.
STATISTICAL ANALYSIS
Categorical variables are reported as counts and percentages, and continuous variables as mean±standard deviation for normally distributed data, and median (interquartile range [IQR]) otherwise. The incidence of follow-up clinical events is reported as the number of events per 100 patient-years. Curves for time-to-event variables were performed using Kaplan-Meier estimates, and the log-rank test was used when performing group comparisons. Competing-risks survival regression analyses using the Fine and Gray subdistribution hazard model (competing with the mortality outcome) were performed to evaluate the predictors of CVE (stroke or TIA), stroke, new-onset AF, and bleeding. For multivariable analyses, threshold values of 0.05 and 0.2 were set for covariates' entry and exit, respectively, with the final decision based on their clinical relevance. Cumulative incidence function curves were used to illustrate the cumulative incidence of factors associated with cerebrovascular events and stroke. Results were considered significant at p<0.05. All statistical analyses were performed using Stata 14 software (StataCorp).
Results
BASELINE CHARACTERISTICS
In total, 689 consecutive patients were included. Patients had a median age of 65 (IQR 62-69) years, with 41.2% female, and a mean Risk of Paradoxical Embolism (RoPE) score of 4.5±1.1. The PFO closure indication was stroke in 552 (80.1%), TIA in 121 (17.6%), and peripheral embolism in 16 (2.3%) patients. A total of 63 (9.1%) patients had diabetes mellitus, and ASA was present in 398 (60.9%) patients (Table 1). The prevalence of thrombophilia was 7.4%, and its subtypes are described in Supplementary Table 1.
Table 1. Baseline characteristics of the study population.
| Characteristic | n=689 |
|---|---|
| Age, years | 65 [62-69] |
| Female | 284 (41.2) |
| BSA, m2 | 1.87±0.19 |
| Smoker | 126 (18.3) |
| Current | 83 (12.0) |
| Former | 43 (6.2) |
| Diabetes | 63 (9.1) |
| Hypertension | 305 (44.3) |
| Dyslipidaemia | 301 (43.7) |
| Chronic kidney disease | 29 (4.2) |
| Coronary artery disease | 40 (5.8) |
| Myocardial infarction | 27 (3.9) |
| COPD | 29 (4.2) |
| Migraine | 78 (11.3) |
| DVT/PE | 89 (12.9) |
| Thrombophilia | 51 (7.4) |
| LVEF, % | 60 [60-65] |
| ASAa | 398 (60.9) |
| PFO closure indication | |
| Stroke | 552 (80.1) |
| TIA | 121 (17.6) |
| Peripheral embolism | 16 (2.3) |
| RoPE score | 4.48±1.14 |
| Continuous variables are expressed as mean±SD or median [interquartile range]. Categorical variables are expressed as n (%). aData available for 654 patients. ASA: atrial septal aneurysm; BSA: body surface area; COPD: chronic obstructive pulmonary disease; DVT: deep venous thrombosis; LVEF: left ventricular ejection fraction; PE: pulmonary embolism; PFO: patent foramen ovale; RoPE: Risk of Paradoxical Embolism; SD: standard deviation; TIA: transient ischaemic attack | |
PROCEDURAL CHARACTERISTICS
The procedural success rate was 99.4%, and the most frequently used device (58.9%) was the Amplatzer PFO Occluder (Abbott), with a median disc size of 25 (IQR 25-35) mm. The overall complication rate was 2.6%, with an incidence of periprocedural AF of 1.2%. The most frequent antithrombotic regimen at discharge was dual antiplatelet therapy (aspirin+clopidogrel), in 38.2% of patients, followed by aspirin alone in 21.6%. The residual shunt rate at short-term (1-3 months) follow-up was 14.9% (Table 2).
Table 2. Procedural characteristics and in-hospital outcomes.
| Characteristic/outcome | n=689 |
|---|---|
| Success rate | 685 (99.42) |
| PFO closure device | |
| Amplatzer PFO Occluder1 | 406 (58.93) |
| Premere2 | 15 (2.18) |
| Amplatzer ASD1 | 43 (6.24) |
| Amplatzer Cribriform1 | 22 (3.19) |
| GORE CARDIOFORM3 | 39 (5.66) |
| Cardia Intrasept4 | 43 (6.24) |
| STARFlex5 | 49 (7.11) |
| Occlutech6 | 54 (7.84) |
| Cocoon7 | 18 (2.61) |
| Disc diameter, mm | 25 [25-35] |
| ≤25 mm | 346 (50.22) |
| >25 mm | 343 (49.78) |
| Fluoroscopy time, min | 7 [5-10] |
| Complications | |
| Major vascular complication | 1 (0.15) |
| Atrial fibrillation | 8 (1.16) |
| Stroke | 1 (0.15) |
| Myocardial infarction | 2 (0.29) |
| Tamponade | 1 (0.15) |
| Device embolisation | 4 (0.58) |
| In-hospital death | 1 (0.14) |
| Antithrombotic treatment at discharge | |
| Aspirin | 149 (21.63) |
| P2Y12 inhibitor | 73 (10.60) |
| OAC | 105 (15.24) |
| DAPT | 263 (38.17) |
| TAT | 57 (8.27) |
| Aspirin+OAC | 19 (2.76) |
| P2Y12 inhibitor+OAC | 10 (1.45) |
| Residual shunta | 85 (14.89) |
| Continuous variables are expressed as mean±SD or median [interquartile range]. Categorical variables are expressed as n (%). 1Abbott; 2St. Jude Medical, now Abbott; 3W.L. Gore & Associates; 4Cardia; 5Nitinol Medical Technologies; 6Occlutech; 7Sahajanand Medical Technologies. aShort-term follow-up echocardiogram (available in 571 patients). DAPT: dual antiplatelet therapy; OAC: oral anticoagulation; PFO: patent foramen ovale; SD: standard deviation; TAT: triple antithrombotic therapy | |
FOLLOW-UP AND CLINICAL EVENTS
The median follow-up was 2 (IQR 1-5) years and was completed in 98.3% (n=677) of patients. The incidence of CVE (stroke or TIA) was 1.21 per 100 person-years (n=32), with a stroke rate of 0.55 per 100 person-years (n=15) (Table 3). One stroke was haemorrhagic, while the other 14 were ischaemic. Among the 14 recurrent ischaemic stroke events, 8 (57%) were non-lacunar, 2 (14%) were lacunar, and 4 (29%) remained unclassified. Among the non-lacunar strokes, 6 (75%) were cryptogenic, 1 (12.5%) was due to large artery atherosclerosis, and 1 (12.5%) was cardioembolic because of late PFO closure device thrombosis with secondary thrombus embolisation. None of the patients with recurrent cryptogenic strokes had interatrial residual shunt or new-onset AF events at follow-up.
The incidence rate of new-onset AF during follow-up was 3.30 per 100 person-years (n=83), and 51.4% of AF episodes (n=38) occurred during the month following the PFO closure procedure (Table 3). The incidence rate of bleeding events was 0.77 per 100 person-years (n=21). The severity of bleeding events is summarised in Supplementary Table 2 and Supplementary Table 3. The incidence rate of death was 2.38 per 100 person-years (n=66), with the leading cause being cancer-related death (Table 3). The Kaplan-Meier time-to-event curves of CVE, AF, bleeding, and death events are shown in Figure 1.
Table 3. Incidence rate of clinical events following PFO closure (n=677).
| Event | n | Incidence rate (per 100 person-years) |
|---|---|---|
| Stroke/TIA | 32 | 1.21 |
| Stroke | 15 | 0.55 |
| TIA | 17 | 0.63 |
| DVT | 15 | 0.55 |
| AF | 83 | 3.30 |
| MI | 7 | 0.25 |
| Bleeding | 21 | 0.77 |
| Death | 66 | 2.38 |
| AF: atrial fibrillation; DVT: deep venous thrombosis; MI: myocardial infarction; PFO: patent foramen ovale; TIA: transient ischaemic attack | ||
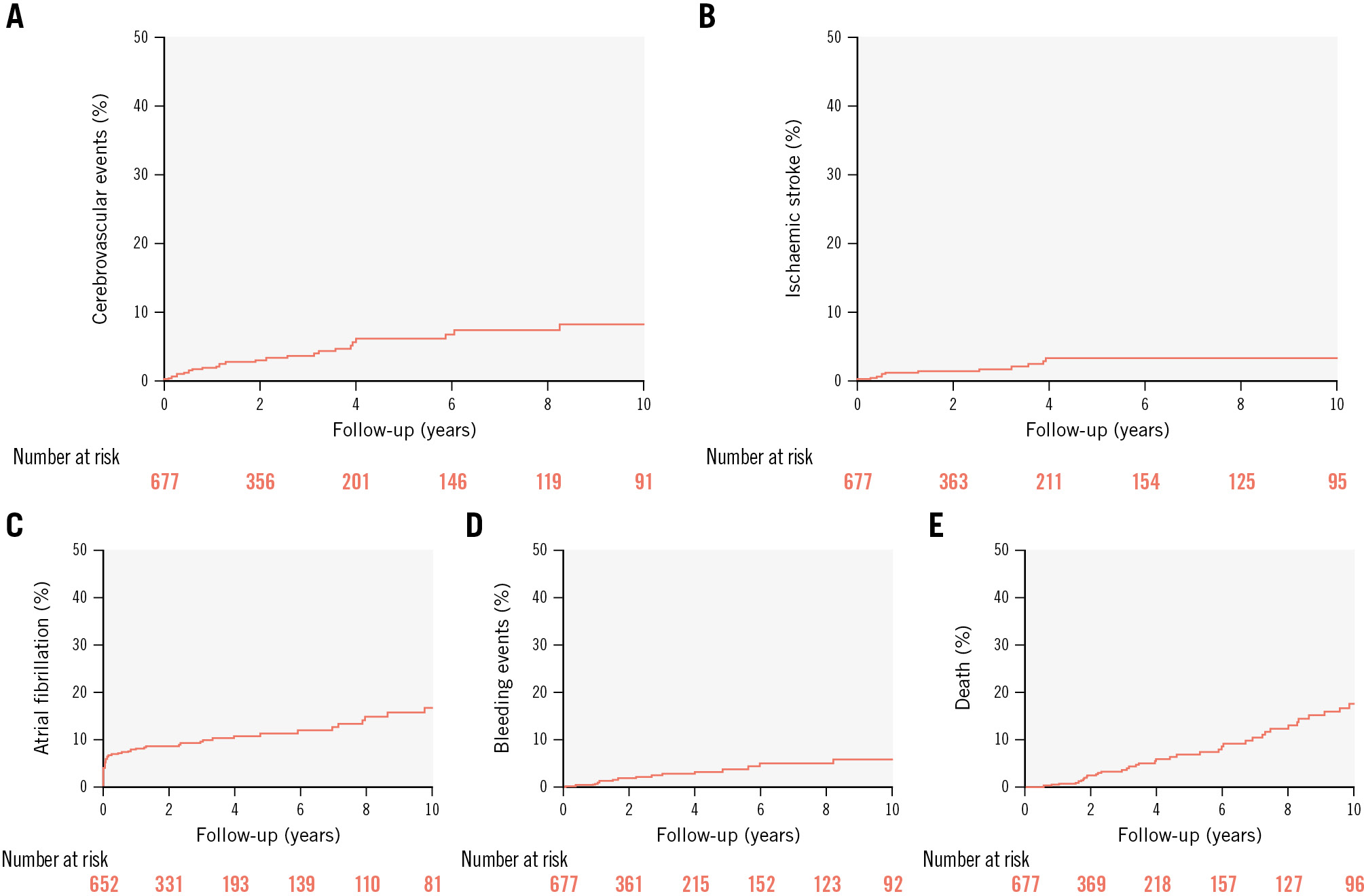
Figure 1. Clinical outcomes among older patients undergoing transcatheter patent foramen ovale closure. Time-to-event curves of clinical outcomes among older patients undergoing transcatheter patent foramen ovale closure: (A) recurrent combined (stroke or transient ischaemic attack) cerebrovascular events; (B) recurrent ischaemic stroke; (C) new-onset atrial fibrillation; (D) bleeding events; (E) death.
RECURRENT CEREBROVASCULAR EVENTS
In the univariable analysis, the factors associated with CVE at follow-up were diabetes (hazard ratio [HR] 3.73, 95% confidence interval [CI]: 1.66-8.36; p<0.001) and the presence of ASA (HR 5.48, 95% CI: 1.65-18.13; p=0.005), and both remained associated with an increased risk in the multivariable analysis (HR for diabetes: 3.89, 95% CI: 1.67-9.07; p=0.002; HR for ASA: 5.25, 95% CI: 1.56-17.62; p=0.007) (Table 4).
The factors associated with stroke events at follow-up were diabetes (HR 7.64, 95% CI: 2.69-21.69; p<0.001) and ASA (HR 3.65, 95% CI: 0.81-16.33; p=0.090). However, only diabetes (HR 6.77, 95% CI: 2.22-20.62; p=0.001) remained associated with recurrent stroke in the multivariable analysis (Table 4). The Kaplan-Meier 10-year time-to-CVE and time-to-stroke comparative curves for patients with diabetes and ASA are shown in Figure 2. The cumulative incidence function curves of CVE and stroke and the estimated contribution of each are shown in Figure 3.
Table 4. Factors associated with recurrent cerebrovascular events (stroke or transient ischaemic attack), stroke, and new-onset symptomatic atrial fibrillation following PFO closure.
| Cerebrovascular events (Stroke/transient ischaemic attack) | ||||
|---|---|---|---|---|
| Univariable | Multivariable | |||
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Diabetes | 3.73 (1.66-8.36) | 0.001 | 3.89 (1.67-9.07) | 0.002 |
| ASA | 5.48 (1.65-18.13) | 0.005 | 5.25 (1.56-17.62) | 0.007 |
| Stroke | ||||
| Diabetes | 7.64 (2.69-21.69) | <0.001 | 6.77 (2.22-20.62) | 0.001 |
| ASA | 3.65 (0.81-16.33) | 0.090 | 4.07 (0.87-19.09) | 0.075 |
| New-onset symptomatic atrial fibrillation | ||||
| Age | 1.02 (0.99-1.06) | 0.113 | 1.05 (1.00-1.09) | 0.023 |
| Male | 2.07 (1.27-3.36) | 0.003 | 1.42 (0.77-2.61) | 0.252 |
| BSA | 3.44 (1.15-10.26) | 0.027 | 3.11 (0.70-13.82) | 0.135 |
| HTN | 0.57 (0.36-0.91) | 0.020 | 0.49 (0.28-0.84) | 0.010 |
| MI | 1.94 (0.86-4.39) | 0.110 | 1.94 (0.79-4.76) | 0.145 |
| ASA | 1.72 (1.02-2.91) | 0.041 | 1.70 (0.98-2.94) | 0.057 |
| Univariable and multivariable competing risks regression analyses for the combined outcome of recurrent cerebrovascular events (stroke or transient ischaemic attack) and stroke only. The outcome of new-onset symptomatic atrial fibrillation (AF) included procedural and long-term follow-up AF (n=83), and only variables with a p-value<0.20 were considered for the multivariate analysis. ASA: atrial septal aneurysm; BSA: body surface area; CI: confidence interval; HR: hazard ratio; HTN: hypertension; MI: myocardial infarction; PFO: patent foramen ovale | ||||
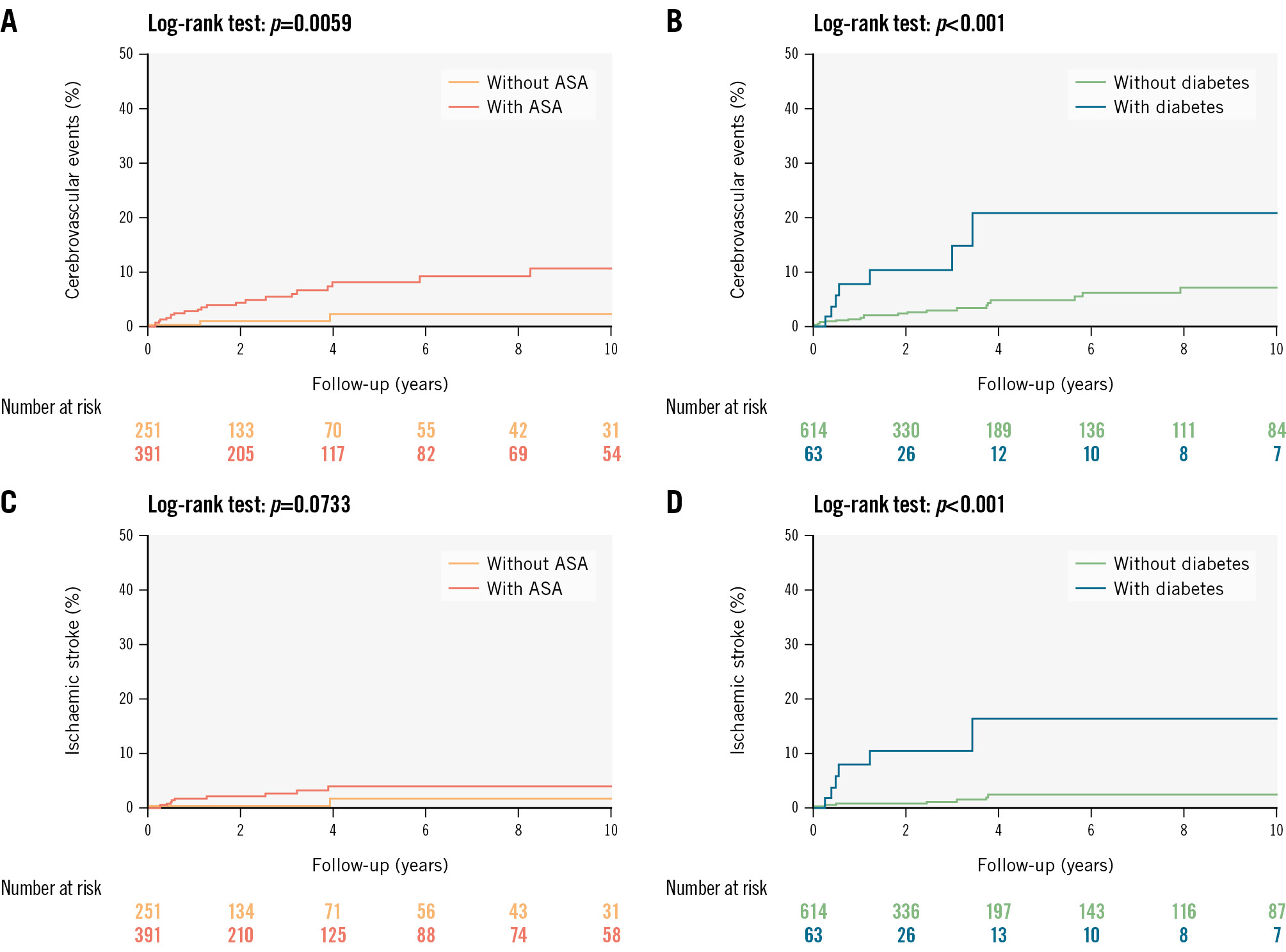
Figure 2. Cerebrovascular events during follow-up in older patients undergoing transcatheter patent foramen ovale closure. Time-to-event curves of cerebrovascular events following transcatheter patent foramen ovale closure among older patients: (A) cerebrovascular events according to the presence of atrial septal aneurysm; (B) cerebrovascular events according to the presence of diabetes; (C) stroke according to the presence of atrial septal aneurysm; (D) stroke according to the presence of diabetes. ASA: atrial septal aneurysm
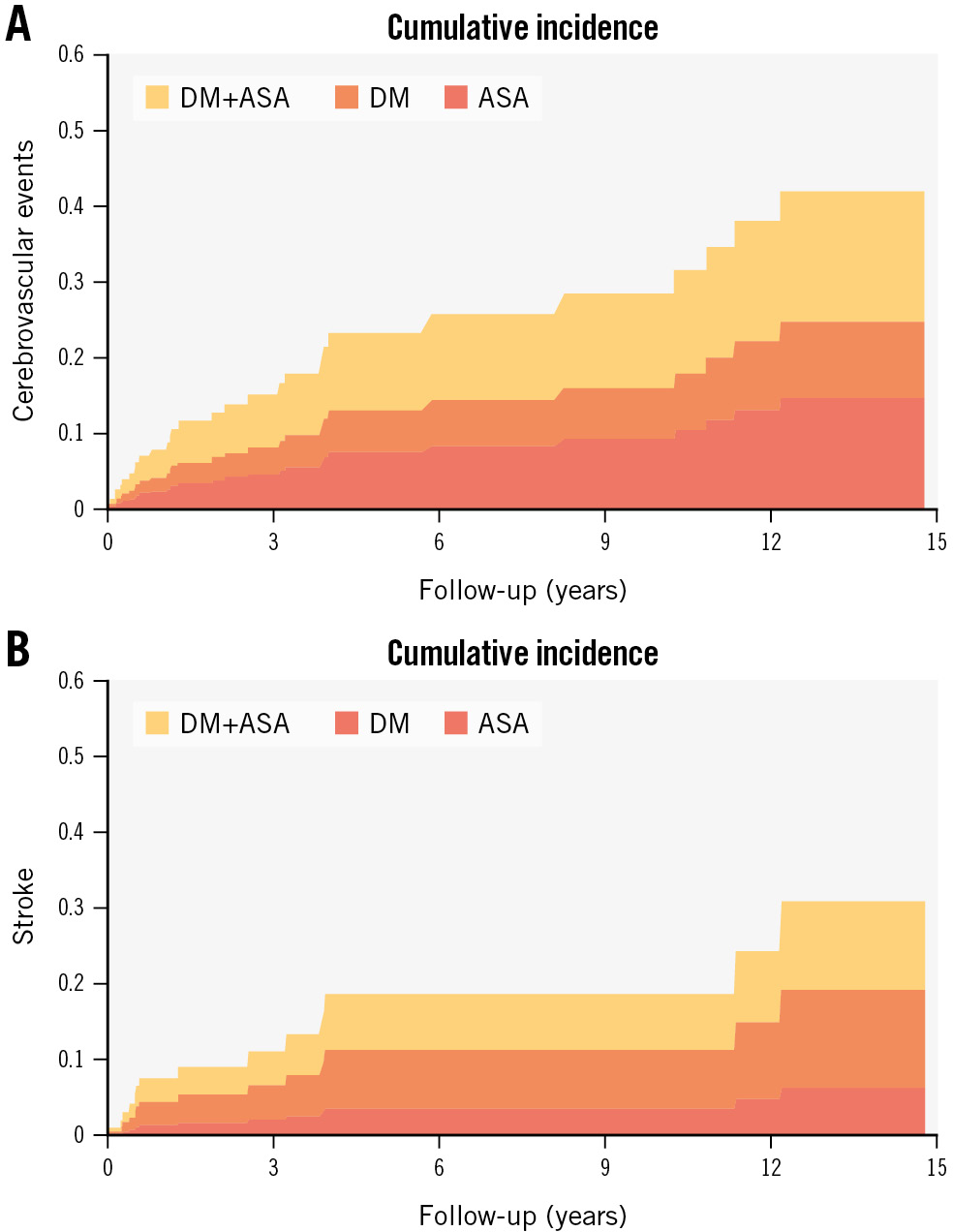
Figure 3. Cumulative incidence function curves of cerebrovascular events among elderly patients. Cumulative incidence curves of cerebrovascular events and stroke among elderly patients undergoing transcatheter patent foramen ovale closure: (A) cerebrovascular events (stroke or transient ischaemic attack) in patients with atrial septal aneurysm (red), diabetes (orange), and both (yellow); (B) stroke in patients with atrial septal aneurysm (red), diabetes (orange), and both (yellow). Both probabilities consider the possibility that a death event could occur instead. ASA: atrial septal aneurysm; DM: diabetes mellitus
NEW-ONSET ATRIAL FIBRILLATION
In the univariable analysis, the factors associated with new-onset AF (including procedural AF) were older age (HR 1.02 per 1-year increase, 95% CI: 0.99-1.06; p=0.113), male sex (HR 2.07, 95% CI: 1.27-3.36; p=0.003), larger body surface area (HR 3.44, 95% CI: 1.15-10.26; p=0.027), the absence of hypertension (HR 1.73, 95% CI: 1.09-2.75; p=0.020), and ASA (HR 1.72, 95% CI: 1.02-2.91; p=0.041). After the multivariable analysis, age (HR 1.05, 95% CI: 1.00-1.09; p=0.023) and the absence of hypertension (HR 2.02, 95% CI: 1.18-3.45; p=0.010) remained as independent associated factors (Table 4).
BLEEDING EVENTS
The factor associated with bleeding events in the univariable and multivariable analysis was AF (HR 6.45, 95% CI: 2.10-19.73; p=0.001) (Supplementary Table 4).
Discussion
The findings of this study, which represents the largest international cohort of older patients undergoing PFO closure to date, can be summarised as follows: (1) older patients undergoing PFO closure for cryptogenic thromboembolic events exhibit a high prevalence of traditional age-related cardiovascular risk factors and ASA; (2) the PFO closure procedure showed a very high success rate and a very low rate of periprocedural complications; (3) after a median follow-up of 2 years, the rate of CVE and stroke were 1.21 and 0.55 per 100 patient-years, respectively, and the presence of diabetes mellitus or ASA determined an increased risk (4- and 5-fold, respectively) of CVE, with diabetes remaining as the only factor associated (~7-fold increased risk) with recurrent stroke events; (4) the incidence of new-onset AF following PFO closure was 3.30 per 100 patient-years, with half of the episodes occurring within the month following the procedure, and a more advanced age and the absence of a history of hypertension determined an increased risk (Central illustration).
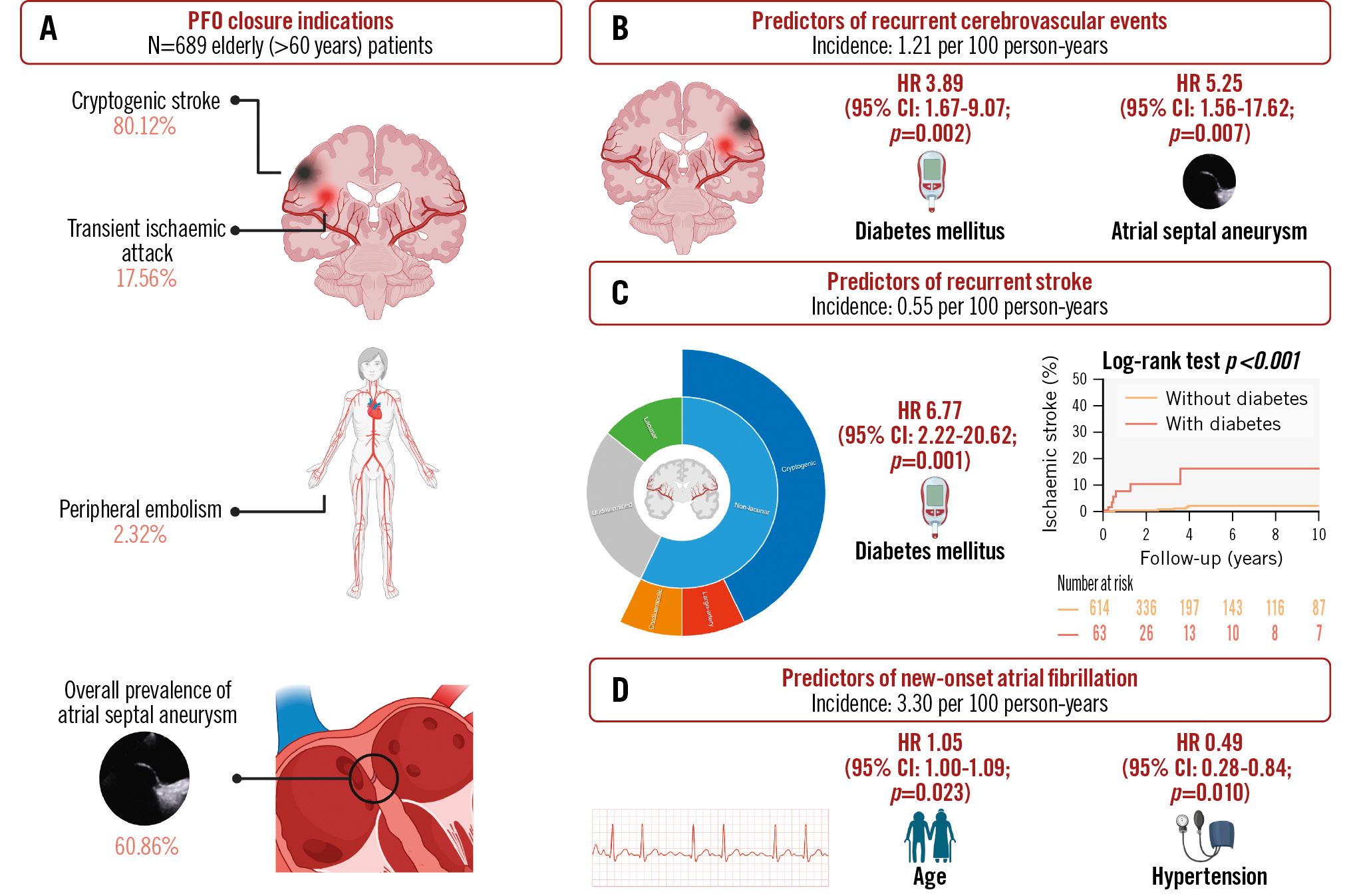
Central illustration. Patent foramen ovale closure among older patients: incidence and predictors of adverse clinical events. A) Older (>60 years) patients undergoing patent foramen ovale (PFO) closure due to cryptogenic thromboembolic events (cryptogenic stroke, transient ischaemic attack, or peripheral embolism) had an overall high prevalence of atrial septal aneurysm. After a median follow-up of 2.21 years following PFO closure, the incidence of cerebrovascular events (stroke or transient ischaemic attack) (B), stroke (C), and new-onset symptomatic atrial fibrillation (D) were 1.21, 0.55, and 3.30 per 100 person-years, respectively. After multivariable analysis, the predictors of recurrent cerebrovascular events were diabetes mellitus and atrial septal aneurysm; however, the only remaining predictor of recurrent stroke was diabetes mellitus. The only predictor of new-onset atrial fibrillation was increasing age, whereas hypertension under medical treatment was associated with a decreased incidence of events. Created with BioRender.com. CI: confidence interval; HR: hazard ratio
BASELINE AND PROCEDURAL CHARACTERISTICS
The mean age of our study population was 65 years, 20 years older than the mean age of patients undergoing PFO closure in randomised clinical trials (~45 years)1. In accordance with previous observational studies on PFO closure among older patients67910, our study population had a high prevalence of traditional age-related cardiovascular risk factors and a high prevalence of high-risk PFO anatomical features (particularly ASA), higher than that observed in younger (<60 years) patients undergoing PFO closure6710. Also, older patients have additional age-related risk factors that increase the risk for PFO-related stroke; these include a higher rate of thrombotic disorders, gradually increasing pulmonary pressure, and an increasing incidence of pacemaker leads – all of which predispose to paradoxical embolisation2. The mean RoPE score (4.5) could be explained by the overall older population and the high prevalence of traditional cardiovascular risk factors, which are key elements in the RoPE score calculation.
The overall procedural success rate was very high, similar to that reported by older and contemporary observational studies on PFO closure678910. Interestingly, most studies have shown that transcatheter PFO closure is a very safe procedure, even among older patients678910.
CEREBROVASCULAR EVENTS FOLLOWING PATENT FORAMEN OVALE CLOSURE
The incidence of recurrent CVE in our study population was comparable to that reported in previous studies810. In a subgroup analysis of the DEFENSE-PFO trial17, in older patients ≥60 years, there were 6 recurrent CVE at 3-year follow-up, and all of them occurred in the medical treatment group (none in the PFO closure group). Also, the rates of recurrent stroke in previous studies ranged from 0.6 to 2.56910 per 100 person-years, similar to the low stroke rate observed in our study. Furthermore, a recent population-based study and systematic review reported a mean incidence of recurrent stroke of 3.27 per 100 patient-years in patients older than 60 years with cryptogenic stroke and PFO left under medical treatment18, a much higher incidence than that observed after PFO closure. This may indicate a protective effect of PFO closure in these patients, similar to what has already been well demonstrated in randomised trials, including younger patients1234. The ongoing CLOSE-2 (ClinicalTrials.gov: NCT0538795419) and STOP (ClinicalTrials.gov: NCT0590769420) trials, comparing medical treatment versus PFO closure in patients older than 60 years diagnosed with cryptogenic stroke, should provide definitive data about the efficacy of PFO closure in older patients.
Diabetes is a well-established risk factor for stroke. It determines a 2-fold higher risk compared to people without diabetes of the same age, in addition to poorer post-stroke outcomes and a greater risk for stroke recurrence21. Diabetes-related strokes are not restricted to lacunar strokes22; in our study population, the largest proportion of strokes following PFO closure were non-lacunar cryptogenic. This has several important implications – first, the efficacy of PFO closure itself. All recurrent strokes occurred in patients without residual shunt, and only 1 was subclassified as cardioembolic, highlighting the PFO closure efficacy.
Interestingly, a high-risk anatomical feature, such as the presence of an ASA, showed a strong association with CVE following PFO closure and a tendency towards an increased risk for stroke recurrence. Previous studies have shown an association between ASA and PFO-related stroke in younger patients before (but not after) PFO closure23. The reason the ASA increases the risk of thromboembolic events following PFO closure remains unknown. ASA has been associated with larger right-to-left shunts, but residual shunting after PFO closure failed to determine an increased risk in our study. The presence of ASA has been associated with an increased atrial vulnerability, which may determine an increased rate of AF24. In fact, the presence of ASA was associated with a tendency towards a higher risk of AF episodes following PFO closure in our study. Also, some imaging and pathology studies have shown the presence of thrombus in situ at the level of the ASA, and this could be associated with an increased risk of embolic events25. The higher prevalence of ASA in elderly (vs younger) patients undergoing PFO closure5610 may be related to a selection bias (i.e., only high-risk patients are referred to PFO closure among the elderly population). However, the possibility that ASA represents a specific anatomical feature, in addition to right-to-left shunting, portending a higher risk of thromboembolic events in this population cannot be excluded and should be further evaluated in future studies.
NEW-ONSET ATRIAL FIBRILLATION FOLLOWING PATENT FORAMEN OVALE CLOSURE
The incidence of AF observed in our study (3.3 per 100 patient-years) appears to be higher than that reported in a general population of elderly people (reported at 1.42 per 100 person-years in a population aged between 65 and 69 years26). In a recent meta-analysis in a younger population undergoing PFO closure, the incidence of AF after PFO closure was 3.7 per 100 patient-years, much higher than the incidence observed in patients treated medically27. In accordance with our study results, the increased risk of AF episodes was observed mainly in the first 45 days post-procedure, with a higher risk among older patients.
A more advanced age and the absence of (diagnosed) systemic hypertension were the 2 factors associated with an increased risk of AF after PFO closure in our study. The increasing incidence of AF with age is well known in the overall population28. It is also known that PFO closure is associated with a substantially increased risk of procedure-related AF29. The results of our study go in this same direction, further highlighting the importance of prolonged (likely no less than 2 weeks) ambulatory electrocardiogram (ECG) monitoring before referring older patients to PFO closure.
Although counterintuitive, our results showed that patients with a history of systemic hypertension were at a decreased risk of new AF following PFO closure. This could be partially related to patient selection bias since our study included only older patients, thus perhaps not reflecting overall population studies' results. Additionally, several meta-analyses have shown that non-pharmacological and pharmacological interventions can delay the progression from prehypertension to hypertension and may prevent the occurrence of new-onset AF30. Also, patients diagnosed with systemic hypertension under medical treatment exhibit a significant risk reduction of new-onset atrial fibrillation31. The association found in our study could be partially explained by a closely followed population under adequate medical treatment of this cardiovascular risk factor.
Limitations
Our study was a retrospective, non-prespecified analysis of prospectively collected data. The proportion of patients undergoing specific arterial imaging protocols in their CT and/or MRI workup was not collected. Although moderate or severe carotid artery disease was excluded, information on the proportion of patients with mild atherosclerotic disease was not collected. Procedural AF was not classified as transient or persistent, and no postprocedural long-duration loop recorder was explicitly used to identify the short-term rate of AF or the AF burden; additionally, data on changes in antithrombotic treatment following a diagnosis of AF were not collected. Although our observational data suggest an association, we cannot establish how much of the new-onset AF burden is attributable to PFO closure itself versus otherwise age-related AF. Transthoracic echocardiography at follow-up was missing in 17% of patients, and this may have biased both the incidence of residual shunting and its potential impact on clinical outcomes. While most patients completed the follow-up, we recognise that some minor events could have been missed; however, this would have likely had a similar impact on the obtained results and the incidence and risk factors of the reported PFO-related outcomes. Although all CVE were confirmed by a neurologist and classified according to the most recent stroke guidelines13, there was no event adjudication committee for this study. Finally, selection bias should be considered when interpreting our results since only high-risk patients were referred to PFO closure among this elderly population.
Conclusions
Older patients undergoing PFO closure due to cryptogenic thromboembolic events presented a high prevalence of traditional cardiovascular risk factors and high-risk anatomical features like ASA. PFO closure in such patients was safe and associated with high rates of successful complete abolition of the interatrial shunting. The incidence of recurrent CVE after a median follow-up of 2 years was relatively low and appeared to be lower than expected (in medically treated patients) according to age, risk factors, and the presence of PFO. However, the rate of new AF episodes was >3 per 100 patient-years, which seems higher than the annual rate observed in a general elderly population. Diabetes mellitus and the presence of ASA determined an increased risk of CVE events at follow-up, and a more advanced age determined a higher risk of AF. While awaiting definitive data from randomised trials, this observational single-arm retrospective study without an independent adjudication committee suggests that PFO closure may be a reasonable alternative in selected patients older than 60 years diagnosed with cryptogenic CVE. However, careful screening with prolonged ambulatory ECG monitoring and consideration of specific risk factors, particularly diabetes and ASA, in the clinical decision-making process and postprocedural management of these patients would be recommendable.
Impact on daily practice
In older patients (>60 years) with cryptogenic thromboembolic events undergoing patent foramen ovale (PFO) closure, the presence of diabetes, atrial septal aneurysm, and a more advanced age demonstrated an increased risk of adverse clinical events. This study’s findings may provide important insights for future clinical trial designs in older patients presenting with PFO-associated stroke. Careful consideration of diabetes mellitus status and systematic predefined atrial fibrillation detection and monitoring should be encouraged.
Acknowledgements
Dr Rodés-Cabau holds the Research Chair “Fondation Famille Jacques Larivière” for the development of Structural Heart Disease Interventions (Laval University).
Conflict of interest statement
J. Abtan reports consulting fees from Abbott, Gore Medical, and Occlutech. B. Hibbert has received institutional research support from Abbott and Occlutech. L. Sondergaard has received consultant fees and institutional research grants from Abbott and W.L. Gore & Associates. E. Horlick has received research grants from Abbott and Occlutech. G. Montalescot reports research funds for the institution or fees from Abbott. J. Rodés-Cabau received an institutional research grant from Abbott. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

