Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Distal transradial access (TRA) and procedural anticoagulation (AC) are among the strategies to prevent radial artery occlusion (RAO) that have some gaps in evidence.
Aims: This study assessed the efficacy and safety of different radial access sites and procedural AC in patients undergoing coronary angiography (CAG).
Methods: The RAPID trial is a single-centre, open-label, 2x2 factorial study that randomised patients to procedural AC versus no procedural AC and also to distal versus conventional TRA with further stratification according to pre-existing oral AC. Patients with indicated percutaneous coronary intervention (PCI) were excluded from the analysis. The primary endpoints were the incidence of RAO, assessed by vascular ultrasound, and bleeding events.
Results: The trial was stopped early for efficacy by the data and safety monitoring board after the second preplanned interim analysis and inclusion of 600 participants. Excluding patients with indicated PCI, the final study population consisted of 439 patients. Distal TRA was associated with more access site crossovers (14.9% vs 8.3%; p=0.032) and a longer total procedure time (25 min vs 20 min; p=0.001) than conventional TRA. The rates of RAO (20.3% vs 21.2%; p=0.810) and bleeding events (4.1% vs 6.9%; p=0.188) were similar after distal and conventional TRA. In contrast, procedural AC reduced the incidence of RAO (7.3% vs 33.9%; p<0.001) without increasing bleeding risk (7.3% vs 3.6%; p=0.087). These results were consistent in patients on pre-existing oral AC and those with distal TRA.
Conclusions: While distal TRA did not reduce the risk of RAO, procedural AC proved effective in all patients undergoing transradial CAG including those on pre-existing oral AC. (Strategies to Maintain Radial Artery Patency Following Diagnostic Coronary Angiography [RAPID] trial; ClinicalTrials.gov: NCT04301921 [RAPID-1] and NCT04362020 [RAPID-2])
Transradial access (TRA) is the preferred approach for coronary angiography (CAG), considering the safety benefits over femoral access, with not only a reduced risk of bleeding and vascular complications but also improved survival particularly in high-risk patients with acute coronary syndrome1234. Radial artery occlusion (RAO) is the most frequent postprocedural complication after TRA, with an asymptomatic course in the majority of patients5. However, it restricts the use of the radial artery as an access route for future procedures and as a conduit for coronary artery bypass grafting or arteriovenous shunt creation. Therefore, strategies to prevent RAO are increasingly in the focus of radial operators. The primary mechanism of RAO is arterial thrombosis resulting from endothelial and vessel injury, a local hypercoagulable state, and decreased blood flow during haemostatic compression67. Consequently, adequate procedural anticoagulation (AC) is considered an important factor in preventing RAO and is recommended by expert consensus statements8910. Unfractionated heparin (UFH) is the most widely used agent, with substantial practice differences regarding dose and route of administration1112. While injection through the arterial sheath or intravenously does not seem to impact clinical efficacy13, several randomised trials and meta-analyses suggest benefits of a high-dose over a low-dose UFH strategy to prevent RAO51415. However, not all radial operators anticoagulate their patients routinely for diagnostic CAG, arguing that maintaining radial artery patency during haemostatic compression (patent haemostasis) is another important aspect of RAO prevention, which could be hampered by intensified AC12161718. Prolonged and more intense radial compression due to AC may also be associated with RAO. Furthermore, data are lacking regarding the need for procedural AC in patients with pre-existing oral AC and in case of distal TRA (dTRA), which has been associated with lower rates of RAO compared to conventional TRA (cTRA) in some investigations192021. Other studies, in turn, could not confirm benefits when using dTRA22, resulting in currently heterogeneous data. The randomised Strategies to Maintain Radial Artery Patency Following Diagnostic Coronary Angiography (RAPID) trial aimed to close these gaps in evidence.
Methods
Study design and oversight
The RAPID trial is a single-centre, open-label, 2x2 factorial, randomised study conducted at the University Heart Center Lübeck, Germany, between February 2020 and May 2023 in order to compare procedural AC versus no systemic AC and also to compare dTRA versus cTRA in patients undergoing diagnostic CAG. Therefore, patients were enrolled in one of the following treatment groups: (a) dTRA with procedural AC; (b) dTRA without procedural AC; (c) cTRA with procedural AC; or (d) cTRA without procedural AC. Randomisation was further stratified according to pre-existing oral AC using permuted blocks of variable size. Patients with an indication for percutaneous coronary intervention (PCI) or requiring anticoagulation for other procedures were excluded from the analysis. Randomisation and data collection and management were performed with REDCap (developed by Vanderbilt University) electronic data capture tools hosted at the University Heart Center Lübeck2324. The RAPID trial was approved by the ethical committee at the University of Lübeck, conducted according to the principles of the Declaration of Helsinki and registered at ClinicalTrials.gov (NCT04301921 [RAPID-1] and NCT04362020 [RAPID-2]).
Patient population and coronary angiography
Patients with an indication for diagnostic CAG and aged >18 years were eligible for enrolment. Exclusion criteria were scheduled PCI, pre-existing RAO or missing pulse at the potential puncture sites, intravenously administered AC prior to CAG, allergy/intolerance to anticoagulants, active bleeding or comorbidity with a significantly elevated bleeding risk, pregnancy, inability or refusal to sign informed consent, and participation in another trial. For patients on pre-existing oral AC, vitamin K antagonists were not interrupted for CAG with an international normalised ratio within the therapeutic range. Direct oral anticoagulants were paused on the morning of the procedure as per hospital practice.
All procedures were performed by experienced interventional cardiologists. Operators were required to have performed a minimum of 25 dTRA cases before being allowed to participate in the study. The technical aspects of CAG followed accepted standards and techniques. The choice of right or left TRA was left to the discretion of the operator, as was the use of ultrasound guidance for puncture. Intravenous access was recommended in the contralateral arm. For cTRA, the hand was positioned with the palm supinated and extended, and puncture of the radial artery was recommended 2 cm proximal to the styloid process. In case of randomisation to dTRA, the hand was positioned with the anatomical snuffbox upward, and puncture of the artery was recommended in the anatomical snuffbox or the dorsum of the hand, according to the strongest pulse and operator preference. The subcutaneous tissue was infiltrated with local anaesthetic, unless contraindicated because of patient intolerance. The radial puncture was performed with a 21G needle with a 30° to 45° entry angle to the skin using the Seldinger technique. A straight 0.021 inch wire was introduced into the vessel, and a 6 Fr sheath with a hydrophilic coating (Prelude EASE [Merit Medical Systems]) was used in all patients. Heparinised saline (2000 IU heparin added to 500 mL saline) was used to flush the sheath and catheters in all patients. In addition, patients randomised to procedural AC received UFH (bolus of 50 IU/kg body weight intravenous or intra-arterial [through the sheath] administration) or bivalirudin (bolus of 0.75 mg/kg body weight intravenous administration, followed by a maintenance infusion of 1.75 mg/kg/h throughout the procedure). Both anticoagulants are approved for use during CAG and were available in the RAPID trial, with the choice of the agent left to the interventionalist’s discretion. Patients in the control group did not receive systemic AC during CAG. Activated clotting time (ACT) was measured in all patients immediately after sheath placement and before sheath removal. Furthermore, repeated ACT assessments and dose adjustments were performed in patients receiving systemic AC to achieve a targeted ACT of 200 to 250 seconds. The administration of supportive medication (e.g., vasodilators to prevent radial artery spasm, analgesia, or sedation in anxious patients) was left to the operator's discretion.
The course of CAG was not regulated by the study protocol and followed standard practice. At the end of the procedure, the sheath was removed, and a dedicated air-filled compression band was applied with the least necessary pressure at the radial puncture site to achieve patent haemostasis. The applied pressure was adjusted 15 minutes after the initial placement to account for changes in blood pressure and ensure patent haemostasis. In case of cTRA, radial artery patency was assessed by checking the pulse distal to the puncture site while compressing the ulnar artery. Compression was sustained for 3 hours, followed by gradual pressure reduction.
Endpoints and definitions
The primary endpoints of the RAPID trial were forearm RAO and bleeding events. Duplex ultrasound was performed before hospital discharge by a blinded investigator not involved in the procedure or study analysis. The radial artery was considered occluded if no flow signal could be detected. In case of dTRA, both the forearm and the distal arteries were assessed. Furthermore, when RAO was present, occlusion length and compensation via ulnar circulation − defined as sufficient collateral blood flow and absence of clinical symptoms (e.g., pain or sensory/motor deficits) − were evaluated. Bleeding severity was classified according to the Bleeding Academic Research Consortium (BARC) definition25.
For the comparison of different access sites, additional secondary endpoint analyses included the number of puncture attempts, access site crossover, frequency of radial artery spasm, procedural and fluoroscopy times, dose area product, contrast volume, and comfort and pain during the exam assessed on a numerical rating scale from 1 (comfortable position, no pain) to 10 (uncomfortable position, severe pain). If vascular access failed at the randomised puncture site, any further attempt to obtain access at a different site – in the same or another limb − was considered crossover. Procedural time was defined as the interval between the first puncture attempt and final removal of the sheath.
Statistical analysis
The primary hypotheses of the study were that both systemic AC and dTRA reduce the risk of RAO compared to CAG without AC and cTRA. The sample size calculation estimated a between-group difference of 10% (5% vs 15%) resulting in a targeted population of 400 patients per group based on an α of 0.05, a 2-sided hypothesis test, and a statistical power of 80%. To account for a presumed dropout of one-third of patients due to indicated PCI, the total sample size was set to 1,200 patients. Sample size calculation was performed with nQuery Advisor, version 6.1 (Statistical Solutions).
According to the study protocol, preplanned interim analyses were scheduled after randomisation of 300, 600 and 900 participants. The data and safety monitoring board recommended early termination of the trial after the second interim analysis and inclusion of 600 participants due to a large difference in the primary endpoint favouring routine procedural AC.
All data were analysed according to the intention-to-treat principle. Continuous variables were assessed for normality with the Shapiro-Wilk test and are reported as mean±standard deviation or median (interquartile range [IQR]). Comparisons were made with the Student’s t-test or the non-parametric Mann-Whitney U test, as appropriate. Categorical variables are presented as frequencies and percentages. Comparisons were made with the chi-square test. The primary endpoints were evaluated in the overall study cohort and in the subgroups stratified by pre-existing oral AC, procedural AC, and/or access site, as appropriate. In terms of access site, an additional per-protocol analysis was performed after excluding patients with failed puncture at the intended site. Statistical analyses were performed with SPSS version 27.0 (IBM) and MedCalc version 19.6.4 (MedCalc Software). A 2-tailed p-value of <0.05 was considered statistically significant.
Results
During the study period, 600 participants were randomly assigned to undergo CAG via dTRA or cTRA and with or without procedural AC. All patients presented with chronic coronary syndrome. Excluding patients with indicated PCI (n=161), the final study population consisted of 439 patients. The median age of the cohort was 73 years old (IQR 60 to 82 years) with a slight predominance of male patients (n=249; 56.7%). A total of 162 patients (36.9%) were on oral AC with vitamin K antagonists (n=25; 5.7%) or direct oral anticoagulants (n=137; 31.2%), mostly apixaban (n=94; 21.4%) or rivaroxaban (n=29; 6.6%). The right radial artery was the targeted access site in the majority of patients (n=412; 93.8%), and local anaesthetic was given in 98.6% of cases (n=433) before skin puncture. In addition, 36 patients (8.2%) received sedative medication, and upstream nitroglycerine was administered in 147 cases (33.4%) to prevent radial artery spasm.
Distal versus conventional transradial access
Randomisation according to access site resulted in 222 patients assigned to dTRA and 217 patients assigned to cTRA (Figure 1). Baseline clinical and procedural characteristics showed only minor differences between the groups, with higher rates of diabetes mellitus (27.5% vs 18.0%; p=0.018) and prior stroke (10.4% vs 4.1%; p=0.012) in patients randomised to dTRA (Supplementary Table 1, Supplementary Table 2). Current medical therapy revealed a lower proportion of patients on calcium channel blockers in the dTRA group (19.4% vs 28.6%; p=0.024). Patent haemostasis was achieved in 201 patients (92.6%) in the cTRA group.
The rates of RAO (20.3% vs 21.2%; p=0.810) and puncture site-related bleeding events (4.1% vs 6.9%; p=0.188) were similar in the dTRA and the cTRA groups, respectively (Table 1, Central illustration). These results were consistent in the predefined subgroup analyses according to procedural or pre-existing AC, except for a lower bleeding risk after dTRA, compared to cTRA, in case of procedural AC (3.5% vs 11.7%; p=0.021) (Table 2). Among patients with RAO, compensation via the ulnar artery was greater in the cTRA group (p=0.046), driven by a significant difference in patients without procedural AC (p=0.015) (Table 1, Table 2). Secondary endpoint analysis showed significantly more puncture attempts (p<0.001) and failed punctures (p=0.005) in patients assigned to dTRA, which resulted in a higher rate of access site crossover compared to cTRA (14.9% vs 8.3%; p=0.032) (Table 1). Consequently, the total procedure time was longer when using dTRA (p=0.001), while fluoroscopy time (p=0.187) and dose area product (p=0.219) did not differ between the study groups. The subjective pain level during the exam was significantly higher in patients randomised to dTRA (p=0.012) (Table 2). An additional per-protocol analysis, excluding patients with failed puncture at the intended access site, confirmed the findings regarding all primary and secondary endpoints (Supplementary Table 3).
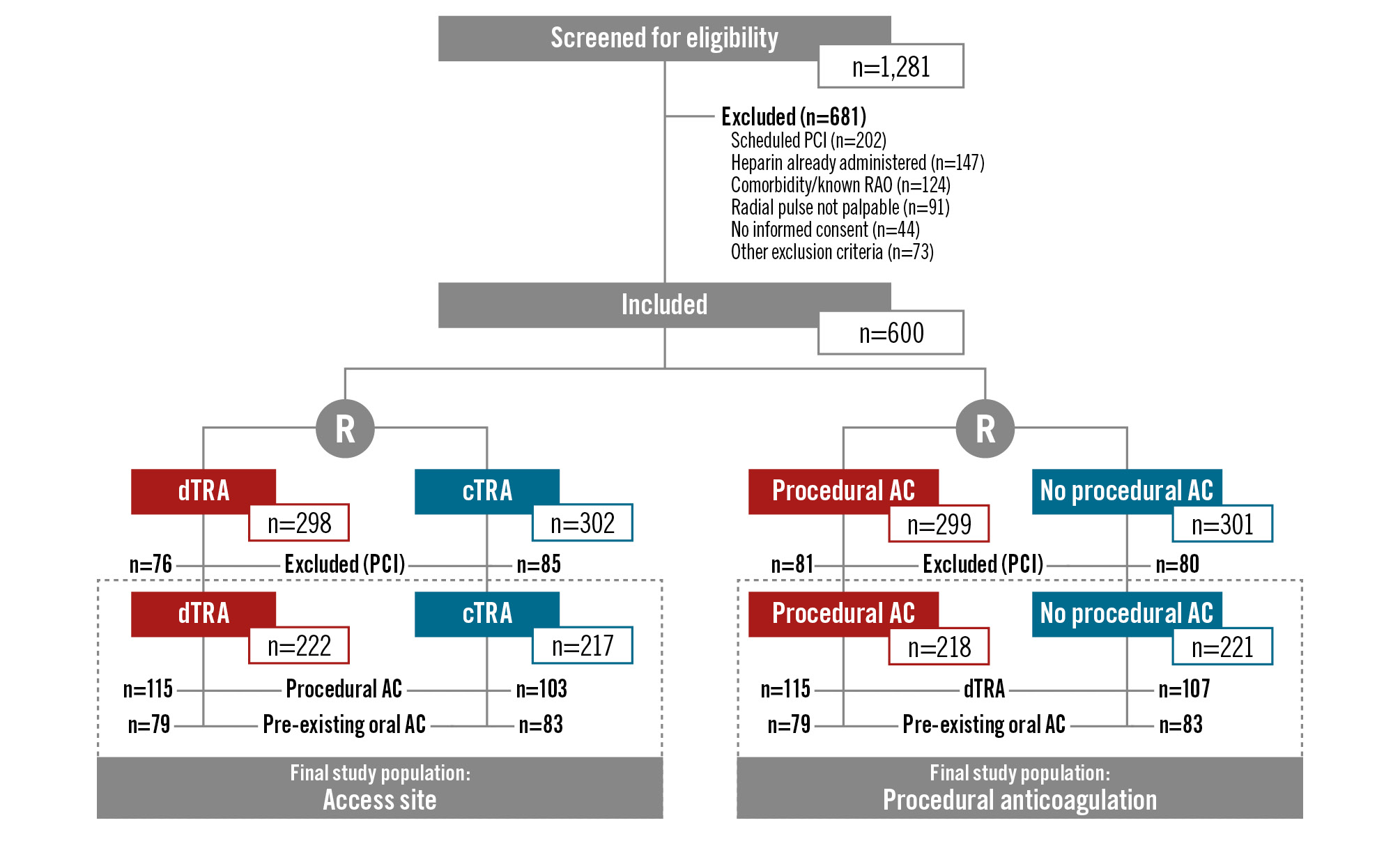
Figure 1. Study flowchart. AC: anticoagulation; cTRA: conventional transradial access; dTRA: distal transradial access; PCI: percutaneous coronary intervention; R: randomisation; RAO: radial artery occlusion
Table 1. Primary and secondary endpoints: dTRA versus cTRA.
| dTRA (n=222) | cTRA (n=217) | p-value | |
|---|---|---|---|
| Primary endpoints | |||
| Radial artery occlusion | 45 (20.3) | 46 (21.2) | 0.810 |
| Length of occlusion, mm | 22 (19, 103) | 30 (15, 120) | 0.514 |
| Occlusion compensated | 20/45 (44.4) | 30/46 (65.2) | 0.046* |
| Bleeding | 9 (4.1) | 15 (6.9) | 0.188 |
| BARC 1 | 3 (1.4) | 7 (3.2) | 0.188 |
| BARC 2 | 6 (2.7) | 8 (3.7) | 0.557 |
| BARC 3-5 | - | - | |
| Secondary endpoints | |||
| Number of skin punctures | 2.4±2.1 | 1.5±1.1 | <0.001* |
| Number of artery punctures | 1.3±0.8 | 1.2±0.6 | 0.012* |
| Access site crossover | 33 (14.9) | 18 (8.3) | 0.032* |
| Failed puncture | 25 (11.3) | 9 (4.1) | 0.005* |
| Anatomical limitations after successful sheath placement | 8 (3.6) | 9 (4.1) | 0.768 |
| Alternate access site | |||
| cTRA right | 17 (7.7) | - | |
| dTRA left | 1 (0.5) | 1 (0.5) | |
| cTRA left | 4 (1.8) | - | |
| Femoral | 11 (5.0) | 17 (7.8) | |
| Radial artery spasm | 21 (9.5) | 15 (6.9) | 0.331 |
| Total procedure time, min | 25 (18, 36) | 20 (15, 31) | 0.001* |
| Fluoroscopy time, min | 5 (3, 9) | 4 (3, 8) | 0.187 |
| Dose area product, cGy/cm² | 1,694 (901, 2,790) | 1,438 (830, 2,478) | 0.219 |
| Contrast volume, ml | 69 (50, 90) | 70 (50, 89) | 0.681 |
| Comfort during exam | 2 (1, 3) | 2 (1, 3) | 0.099 |
| Pain during exam | 3 (1, 5) | 2 (1, 4) | 0.012* |
| Data are presented as number (percentage), median (interquartile range) or mean±standard deviation. *Indicates a significant difference. BARC: Bleeding Academic Research Consortium; cTRA: conventional transradial access; dTRA: distal transradial access | |||
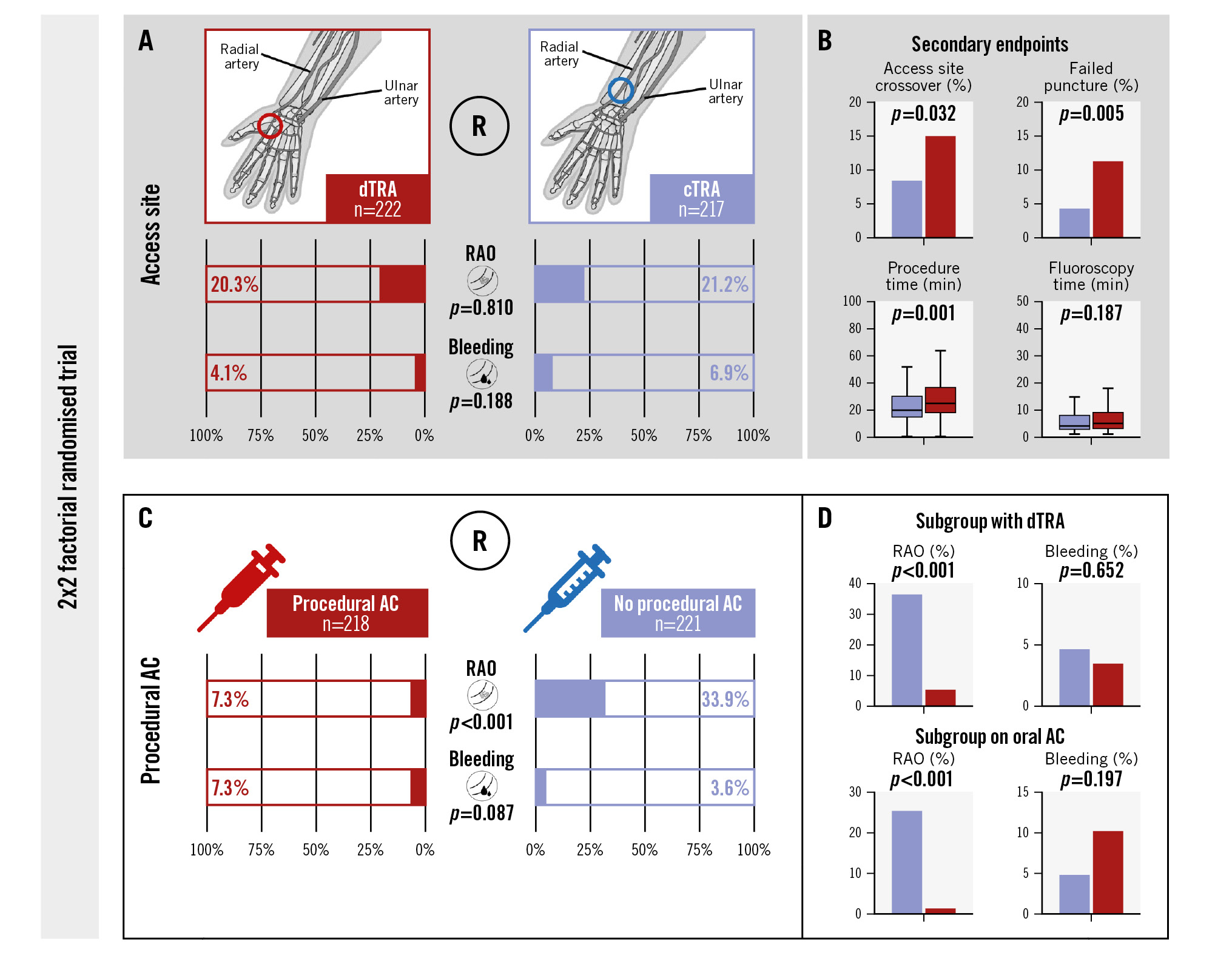
Central illustration. The RAPID trial: 2x2 factorial randomised trial. A) Comparison of dTRA with cTRA showed similar rates of RAO and bleeding events in both study groups. B) The frequency of failed puncture attempts and access site crossover were significantly increased in the dTRA group, resulting in an overall prolonged duration of the procedure without increasing fluoroscopy time. C) Procedural AC was associated with a substantially reduced risk of RAO, while rates of bleeding events were not significantly increased. These findings were consistent in all subgroups including patients on oral AC and with dTRA (D). AC: anticoagulation; cTRA: conventional transradial access; dTRA: distal transradial access; R: randomisation; RAO: radial artery occlusion
Table 2. Subgroup analyses: dTRA versus cTRA.
| dTRA | cTRA | p-value | |
|---|---|---|---|
| Procedural AC | n=115 | n=103 | |
| Radial artery occlusion | 6 (5.2) | 10 (9.7) | 0.204 |
| Occlusion compensated | 5/6 (83.3) | 6/10 (60.0) | 0.330 |
| Any bleeding | 4 (3.5) | 12 (11.7) | 0.021* |
| BARC 1 | 2 (1.7) | 6 (5.8) | 0.109 |
| BARC 2 | 2 (1.7) | 6 (5.8) | 0.109 |
| No procedural AC | n=107 | n=114 | |
| Radial artery occlusion | 39 (36.4) | 36 (31.6) | 0.445 |
| Occlusion compensated | 15/39 (38.5) | 24/36 (66.7) | 0.015* |
| Any bleeding | 5 (4.7) | 3 (2.6) | 0.417 |
| BARC 1 | 1 (0.9) | 1 (0.9) | 0.964 |
| BARC 2 | 4 (3.7) | 2 (1.8) | 0.364 |
| Pre-existing oral AC | n=79 | n=83 | |
| Radial artery occlusion | 11 (13.9) | 11 (13.3) | 0.901 |
| Occlusion compensated | 3/11 (27.3) | 6/11 (54.5) | 0.193 |
| Any bleeding | 6 (7.6) | 6 (7.2) | 0.929 |
| BARC 1 | 2 (2.5) | 2 (2.4) | 0.960 |
| BARC 2 | 4 (5.1) | 4 (4.8) | 0.943 |
| No pre-existing oral AC | n=143 | n=134 | |
| Radial artery occlusion | 34 (23.8) | 35 (26.1) | 0.652 |
| Occlusion compensated | 17/34 (50.0) | 24/35 (68.6) | 0.116 |
| Any bleeding | 3 (2.1) | 9 (6.7) | 0.059 |
| BARC 1 | 1 (0.7) | 5 (3.7) | 0.083 |
| BARC 2 | 2 (1.4) | 4 (3.0) | 0.365 |
| Data are presented as number (percentage). *Indicates a significant difference. AC: anticoagulation; BARC: Bleeding Academic Research Consortium; cTRA: conventional transradial access; dTRA: distal transradial access | |||
Procedural anticoagulation
The second part of the RAPID trial compared 218 patients randomised to procedural AC and 221 patients assigned to CAG without systemic AC (Figure 1). Clinical and procedural characteristics showed only minor variations in terms of prior myocardial infarction (3.7% vs 10.4%; p=0.006), treatment with aldosterone antagonists (12.4% vs 19.9%; p=0.032) and the use of nitroglycerine (38.5% vs 28.5%; p=0.026) when comparing the groups with and without procedural AC (Supplementary Table 4, Supplementary Table 5). Baseline ACT did not differ between the cohorts. All patients in the treatment group received UFH as an anticoagulative agent, resulting in a median ACT of 214 seconds (IQR 182 to 257 seconds) at the end of the procedure.
The incidence of RAO was significantly reduced in patients receiving procedural AC (7.3% vs 33.9%; p<0.001) (Table 3, Central illustration). This finding was consistent across all subgroups, including patients with dTRA (5.2% vs 36.4%; p<0.001) and those with pre-existing oral AC (1.3% vs 25.3%; p<0.001) (Table 4). The risk of bleeding complications was not significantly elevated in patients receiving procedural AC compared to controls (7.3% vs 3.6%; p=0.087) (Table 3, Central illustration). This also applied to patients on pre-existing oral AC (p=0.197) (Table 4). Only patients undergoing CAG via cTRA had an increased rate of bleeding events with procedural AC (11.7% vs 2.6%; p=0.009) (Table 4); however, all bleeding events in the trial were puncture site related and of minor severity (BARC 1 or 2).
Table 3. Primary endpoints: procedural AC versus no procedural AC.
| Procedural AC (n=218) | No procedural AC (n=221) | p-value | |
|---|---|---|---|
| Radial artery occlusion | 16 (7.3) | 75 (33.9) | <0.001* |
| Length of occlusion, mm | 23 (13, 75) | 30 (19, 118) | 0.517 |
| Occlusion compensated | 11/16 (68.8) | 39/75 (52.0) | 0.222 |
| Any bleeding | 16 (7.3) | 8 (3.6) | 0.087 |
| BARC 1 | 8 (3.7) | 2 (0.9) | 0.052 |
| BARC 2 | 8 (3.7) | 6 (2.7) | 0.569 |
| BARC 3-5 | - | - | |
| Data are presented as number (percentage) or median (interquartile range). *Indicates a significant difference. AC: anticoagulation; BARC: Bleeding Academic Research Consortium | |||
Table 4. Subgroup analyses: procedural AC versus no procedural AC.
| Procedural AC | No procedural AC | p-value | |
|---|---|---|---|
| dTRA | n=115 | n=107 | |
| Radial artery occlusion | 6 (5.2) | 39 (36.4) | <0.001* |
| Occlusion compensated | 5/6 (83.3) | 15/39 (38.5) | 0.039* |
| Any bleeding | 4 (3.5) | 5 (4.7) | 0.652 |
| BARC 1 | 2 (1.7) | 1 (0.9) | 0.604 |
| BARC 2 | 2 (1.7) | 4 (3.7) | 0.359 |
| cTRA | n=103 | n=114 | |
| Radial artery occlusion | 10 (9.7) | 36 (31.6) | <0.001* |
| Occlusion compensated | 6/10 (60.0) | 24/36 (66.7) | 0.695 |
| Any bleeding | 12 (11.7) | 3 (2.6) | 0.009* |
| BARC 1 | 6 (5.8) | 1 (0.9) | 0.039* |
| BARC 2 | 6 (5.8) | 2 (1.8) | 0.112 |
| Pre-existing oral AC | n=79 | n=83 | |
| Radial artery occlusion | 1 (1.3) | 21 (25.3) | <0.001* |
| Occlusion compensated | 1/1 (100) | 8/21 (38.1) | 0.219 |
| Any bleeding | 8 (10.1) | 4 (4.8) | 0.197 |
| BARC 1 | 3 (3.8) | 1 (1.2) | 0.288 |
| BARC 2 | 5 (6.3) | 3 (3.6) | 0.425 |
| No pre-existing oral AC | n=139 | n=138 | |
| Radial artery occlusion | 15 (10.8) | 54 (39.1) | <0.001* |
| Occlusion compensated | 10/15 (66.7) | 31/54 (57.4) | 0.518 |
| Any bleeding | 8 (5.8) | 4 (2.9) | 0.243 |
| BARC 1 | 5 (3.6) | 1 (0.7) | 0.101 |
| BARC 2 | 3 (2.2) | 3 (2.2) | 0.993 |
| Data are presented as number (percentage). *Indicates a significant difference. AC: anticoagulation; BARC: Bleeding Academic Research Consortium; cTRA: conventional transradial access; dTRA: distal transradial access | |||
Discussion
The steady rise in TRA for CAG in recent decades, along with the radial-first approach recommended in current guidelines, has led to an increasing interest in strategies to prevent RAO, its most frequent postprocedural complication46. In this regard, the randomised RAPID trial provides comprehensive evidence in terms of access site selection and periprocedural management, with several novel aspects. The main findings are as follows: (a) dTRA does not reduce the risk of RAO compared to cTRA; (b) procedural AC results in a significantly lower rate of RAO, including patients with pre-existing oral AC and in case of dTRA; and (c) cTRA with procedural AC is associated with the highest bleeding risk, even if the overall risk of bleeding complications after TRA is low and restricted to puncture site-related bleedings of minor severity.
Transradial access site selection
In recent years, dTRA via the anatomical snuffbox or the dorsum of the hand has emerged as a promising alternative to cTRA through the forearm radial artery. Apart from improved patient and operator comfort, potential advantages of dTRA compared to cTRA are easier and shorter haemostasis due to the more superficial course of the distal radial artery, fewer bleeding complications, and a reduced risk of RAO. The latter might be due to persistent anterograde flow in the forearm radial artery during haemostatic compression and, in case of distal RAO, a reduction in the risk of retrograde thrombus formation810. The first randomised studies supported this theory by showing a substantial reduction in RAO after dTRA compared to cTRA202627; this was also confirmed in meta-analyses1928. In contrast, the recent DISCO RADIAL trial reported a similar incidence of RAO and bleeding events in 1,307 patients randomised to dTRA or cTRA22. The RAO rates in this international, multicentre study were extremely low in both cohorts (<1%) due to strict operator selection, use of the thin-walled Glidesheath Slender sheath (Terumo), procedural AC, and detailed haemostasis protocols with plethysmographic monitoring of patent haemostasis1622. Without consistent application of these preventive factors − particularly procedural AC in only 50% of patients and a standardised but less extensive haemostasis protocol that is closer to resource-restricted real-world practice − event rates were considerably higher in the RAPID trial. However, the comparison of dTRA with cTRA in the RAPID and DISCO RADIAL trials arrived at the same conclusion: no benefit of dTRA over cTRA in terms of forearm RAO rates. Potential explanations include insufficient flow through the collateral circulation in some patients or microtrauma of the more proximal radial artery during wiring or sheath insertion.
Furthermore, the number of puncture attempts and the access site crossover rate were substantially increased in the dTRA group, resulting in an overall prolonged duration of the total procedure. This aspect is also reflected in the results of previous trials even after adjustment for operator experience2026. The smaller diameter and the more curved and angulated course of the distal part of the radial artery might hamper achieving success rates comparable to cTRA. In contrast to previous findings20, we did not observe an increased dose area product or fluoroscopy time after dTRA, hence the observed time delays were attributed to acquiring vascular access. From the patients’ perspective, dTRA was associated with a slightly higher pain level and did not affect comfort during the exam. Overall, evidence from the RAPID trial and previous studies does not suggest benefits of dTRA compared to cTRA.
Procedural anticoagulation
The mechanistic rationale for procedural AC to prevent RAO is sound, although prolonged and intensified haemostasis in patients receiving systemic AC could in turn favour the occurrence of RAO29. Supporting this hypothesis, the randomised PHARAOH study18 showed that a provisional approach with administration of heparin only if patent haemostasis could not be achieved was associated with similar RAO rates compared to routine procedural AC. The PROPHET studies further emphasise the decisive role of patent haemostasis to prevent RAO1617. However, the RAPID trial showed a clear and convincing benefit of procedural AC despite patent haemostasis in >90% of patients, which eventually led to early termination of the trial. Furthermore, the presented study provides unique insights into the optimal periprocedural management of patients with dTRA and pre-existing oral AC. Periprocedural AC significantly decreased the rates of RAO in all investigated subgroups, with a pronounced numerical difference observed with dTRA, suggesting that periprocedural AC is particularly important to prevent RAO when using this access site. Interestingly, additional systemic AC did not increase bleeding risk in patients on pre-existing oral anticoagulants. Only patients randomised to cTRA with procedural AC showed a higher incidence of minor, access site-related bleeding events (BARC type 1), potentially related to a least-pressure approach to ensure patent haemostasis, close proximity to superficial veins in some patients, and more extensive soft tissue that facilitates subcutaneous spreading of haematoma. Still, the sum of evidence, including data from the RAPID trial, now clearly supports routine procedural AC in patients undergoing transradial CAG, irrespective of access route and long-term anticoagulant therapy. The ideal intensity of procedural AC remains, however, unclear. Unfractionated heparin at a standard dose of 5,000 IU or body weight-adjusted 50 IU/kg is a common strategy, although clinical trial evidence suggests that higher doses up to 100 IU/kg may further improve radial artery patency rates15. In contrast, excessive AC with high ACT values >250 seconds resulted in a paradoxical increase in the incidence of RAO related to longer and more frequent occlusive haemostasis29. Therefore, a moderate approach, as used in the RAPID trial and recommended in consensus statements, seems appropriate for all patient subgroups, including those on oral AC9.
Limitations
The RAPID trial is limited by its single-centre design and the early termination after randomisation of half of the intended population. Numerical differences in RAO rates, for example, between dTRA and cTRA in the subgroup of patients with periprocedural AC, might have missed significance due to insufficient statistical power. Furthermore, study enrolment was slower than expected because of repetitive restrictions in terms of elective procedures during the COVID-19 pandemic. The study protocol did not demand routine ultrasound-guided puncture of the radial artery, and postprocedural care followed our standardised strategy with thorough attention to patent haemostasis but without objective plethysmographic monitoring. Nevertheless, this approach mirrors usual daily practice in most centres. Finally, the thin-walled Glidesheath Slender is associated with lower event rates compared to the standard radial sheaths used in the RAPID trial30. Unfortunately, the Glidesheath Slender was not available at the start of the trial because of delivery problems, and we decided to continue the study with one dedicated sheath to ensure the comparability and integrity of the data. Considering these aspects and procedural AC in only half of patients, the frequency of RAO was significantly higher than expected after TRA. However, the incidence in the subgroup of patients receiving procedural AC was in line with previously reported event rates61520.
Conclusions
According to this randomised trial, dTRA does not reduce the incidence of forearm RAO compared to cTRA but is associated with a longer procedure duration and increased access site crossover rates. In contrast, procedural AC reduces the risk of RAO and should be the standard of care in all patients undergoing transradial interventions, including those with pre-existing oral AC and dTRA.
Impact on daily practice
Procedural anticoagulation (AC) reduces the risk of radial artery occlusion (RAO) irrespective of distal or conventional access site and the presence or absence of pre-existing oral AC and should be standard of care in patients undergoing transradial coronary angiography. In contrast, distal transradial access (TRA) compared to conventional TRA does not seem to reduce the risk of RAO.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

