The collaborative paper from the multinational ILIAS Registry on hyperaemic stenosis resistance (HSR) by Boerhout et al in this issue of EuroIntervention1 provides the occasion to discuss a key distinction between flow in the epicardial coronary arteries versus its subsequent distribution across the layers of the myocardium. In short, measures of regional average transmural perfusion do not capture clinically essential information for managing many patients.
Inflow versus distribution
An important mechanistic paper from over 50 years ago offers insights regarding transmural flow distribution across the left ventricular (LV) wall2. In an animal model, the investigators studied simultaneous epicardial blood flow and its subendocardial versus subepicardial distribution. Across a range of perturbations, coronary flow increased or decreased relative to baseline. For each condition, the relative ratio of subendocardial to subepicardial blood flow was computed in tissue samples from the inner third to outer third circumferentially across the LV wall. Figure 1 plots data from Table 1 of their manuscript, demonstrating two major points.
First, when coronary blood flow decreases below baseline levels, subendocardial flow always decreases faster than subepicardial blood flow. Indeed, this differential sensitivity of the innermost LV layers to reduced flow explains the vast majority of what might be best called “no stenosis” angina3. Second, when coronary blood flow increases above baseline, the balance can favour either the subendocardium (ratios >1) or the subepicardium (ratios <1). In the absence of vascular dysfunction, hyperaemic epicardial inflow may boost subendocardial perfusion with an endocardial-to-epicardial flow ratio >1 due to subendocardial layers generating predominant contractive force. However, for diffuse yet non-stenotic epicardial coronary disease, hyperaemic epicardial coronary blood flow increases subepicardial perfusion but also decreases coronary pressure that reduces subendocardial perfusion and can cause angina or ST-segment depression. Figure 1 provides examples of each scenario using cardiac positron emission tomography (PET) imaging.
Finally, this mechanistic animal study2 matches findings from patients showing a wide scatter and heterogeneity between fractional flow reserve (FFR) and transmural perfusion gradients by non-invasive cardiac magnetic resonance4 and PET5 imaging. These cumulative results extend to clinical application the experimental conclusions that “methods that measure only total left ventricular flow⦠give limited information”2.
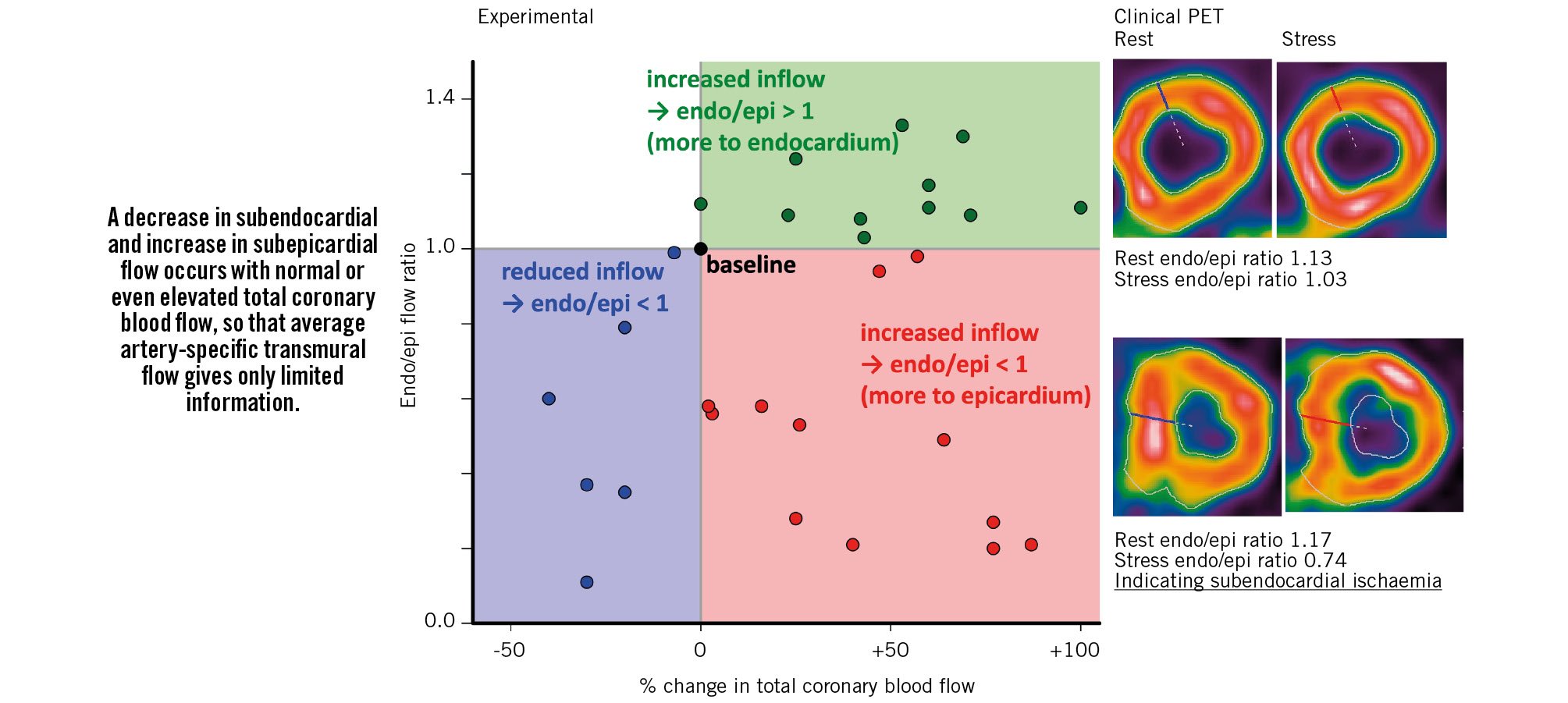
Figure 1. Relationship between changes in coronary blood flow and its distribution between subendocardial (endo) and subepicardial (epi) layers. Paraphrased summary and plotted data from a prior publication2, with our own clinical examples using cardiac positron emission tomography (PET) imaging, demonstrating that average regional transmural coronary blood flow gives limited information.
Hyperaemic stenosis resistance
These experimental and clinical data establish the physiological basis for critiquing all epicardial coronary measurements of pressure and flow, including HSR, as fundamentally incomplete or inadequate. Part of the ILIAS analysis compares the ability of HSR, FFR, and invasive coronary flow reserve (CFR) to predict abnormal non-invasive stress test results. Notably, the modest difference in the area under the receiver operating characteristic curve (from 0.63 for CFR, to 0.66 for FFR, to 0.71 for HSR – a delta of 0.08 among invasive tests) was dwarfed by the much larger difference with non-invasive testing itself (value of 1.00 – a 0.29 delta for HSR). Consequently, a basic clinical question remains: if the goal is to predict the results of a stress test, why not simply do the non-invasive imaging first? Healthcare systems need to provide and support stronger gatekeepers to invasive cardiac catheterisation.
For deferred patients in the ILIAS Registry, composite event rates were remarkably uncommon. Over 5 years of follow-up, just 13 spontaneous myocardial infarctions occurred in the 1,050 target vessels studied – an incidence of 13/1,050/5=0.2%/year, dwarfed by the 5-fold higher rate of approximately 1%/year after stenting6. As explained previously6, stenting stable lesions does not reduce all-cause death; also, separating immediate revascularisation at the time of physiological assessment from delayed revascularisation during follow-up obfuscates that upfront stenting of all lesions leads to more total stents compared to initial medical therapy. Even for these endpoints, the ILIAS Registry of deferred lesions found a very low incidence after initial medical treatment (cardiac death 0.5%/year and 1%/year revascularisation during follow-up)1.
Inflow or distribution?
In conclusion, fundamental and clinical pathophysiology over 50 years, from controlled animal studies2 to multimodality human imaging345, reminds us that focusing only on epicardial inflow misses complementary information regarding its transmural myocardial distribution. Subendocardial perfusion establishes the diagnosis and management of diffuse, non-obstructive coronary disease, microvascular disease, and non-ischaemic versus ischaemic cardiomyopathy not provided by the average transmural perfusion. Rather than parsing the fine differences among invasive coronary metrics1, we recommend the more complete view of clinical coronary pathophysiology to guide interventions afforded by quantitative perfusion imaging and transmural perfusion gradients.
Conflict of interest statement
N.P. Johnson and K.L. Gould report no direct relationships, but outside of the present work receive internal funding from the Weatherhead PET Imaging Center; and have patents pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology, and on methods to correct pressure tracings from fluid-filled catheters. N.P. Johnson receives significant institutional research support from Neovasc/Shockwave Medical (PET core lab for COSIRA-II, ClinicalTrials.gov: NCT05102019). K.L. Gould is the 510(k) applicant for several cardiac PET software packages approved by the U.S. FDA (K113754, K143664, K171303, K202679, K231731) but does not receive any licensing fees paid to UTHealth by Bracco Diagnostics or GE HealthCare.

