Cory:
Unlock Your AI Assistant Now!
In the ACURATE neo AS study, the ACURATE neo2 valve (Boston Scientific) demonstrated good clinical outcomes in patients with severe aortic stenosis (AS), with significant improvement in valve haemodynamics up to 12 months, including a low overall rate of paravalvular leak (97% had mild or less at 30 days; 97.5% had mild or less at 1 year)1. Here, we evaluate the 5-year clinical outcomes from the ACURATE neo AS study. An evolution of the ACURATE neo valve, the ACURATE neo2 is a transcatheter heart valve (THV) system that retains key design features from its predecessor: the self-expanding nitinol frame with axial, self-aligning stabilisation arches, and supra-annular porcine pericardium leaflets. The integrated internal and external porcine pericardium sealing skirts have been updated to further reduce paravalvular leak (Figure 1A).
ACURATE neo AS is a prospective, multicentre single-arm clinical study. Patients were enrolled at 9 European centres between December 2016 and November 2017. The primary endpoint of the study, the incidence of all-cause mortality at 30 days, was 3.3%1. Clinical events as defined per Valve Academic Research Consortium (VARC)-2 guidelines2 were also evaluated annually up to 5 years. The present report is the longest-term published data for this self-expanding THV system. Baseline and outcome variables were summarised using descriptive statistics. The study was conducted in accordance with International Conference for Harmonisation Good Clinical Practice (ICH-GCP) regulations and guidelines and the ethical principles outlined in the Declaration of Helsinki, and all patients gave written informed consent. An independent data monitoring committee (DMC) was initially responsible for the review of aggregate safety data up to 12 months, and then, beginning in April 2018, an independent clinical events committee (CEC) assumed responsibility for the adjudication of all reported VARC-2 endpoint events up to 5 years. Study methods have been described previously in a manuscript reporting 30-day and 1-year outcomes1, and the study was registered at ClinicalTrials.gov: NCT02909556.
All enrolled patients were implanted with the ACURATE neo2 THV (n=120), and 94.2% (113/120) were evaluable for clinical endpoints at 5 years. Evaluable patients were those who either had an event within 5 years post-procedure or who were event-free with their last follow-up at least 4 years and 9 months after the procedure. This evaluable sample includes 50 patients who died prior to 5-year follow-up. The mean age was 82 years, and 68% were female. The mean Society of Thoracic Surgeons (STS) risk score was 4.8±3.8%; the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 4.7±3.8%. The procedural success rate was 97.5% (117/120). Procedural success was not achieved in 3 subjects; 2 cases were adjudicated as transcatheter aortic valve (TAV)-in-TAV deployments, and 1 case was converted to surgery. Time-to-event analyses for mortality and other VARC-2 event rates for years 1 to 5 are presented in Figure 1B and Figure 1C, respectively. The 5-year all-cause mortality rate was 44.0% (49/120); cardiovascular mortality was 31.5% (33/120). The stroke rate was 14.2% (13/120); disabling stroke was 9.9% (9/120). The rate of hospitalisation due to valve-related symptoms or worsening heart failure was 14.6% (14/120); in 13 patients, hospitalisation was heart failure related, and 1 patient had mitral insufficiency. Among the heart failure-related hospitalisations, 1 patient presented with mild-to-moderate paravalvular leak at 2 years and was successfully treated with balloon valvuloplasty. Notably, this is the only instance of repeat intervention, so the rate remained low, with a value of 1.1% at 5 years. During 5 years of follow-up, of the patients who did not have a previous pacemaker, 25 received a new pacemaker, resulting in a 5-year rate of 24.5%. A total of 84% (21/25) of new pacemaker implantations occurred within 1 year of the index procedure, including 16 patients who received new pacemakers during the periprocedural window (at discharge or 7 days post-index procedure, whichever was less). At 30-day follow-up, 2 additional patients had received new pacemakers after being discharged, and at 6-month follow-up, 1 additional patient had received a new pacemaker since their last follow-up at 30 days. Among the 4 patients who had new pacemakers implanted after 1 year, indications included third degree atrioventricular block (2/4), bifascicular bundle block (1/4), and dilated cardiomyopathy (1/4). There were no cases of clinical valve thrombosis nor endocarditis up to 5 years.
This self-expanding THV system demonstrated favourable clinical outcomes up to 5 years. Evolution of the population of patients treated with TAVI and improvements in valve design make it difficult to compare studies over time. However, 5-year event rates for the outcomes reported in the present study are comparable to or lower than those seen in contemporary studies for a similar surgical risk population. The rates of all-cause mortality and all stroke (44.0% and 14.2%, respectively) are lower in the present study compared to the rates reported with the balloon-expandable SAPIEN XT valve (Edwards Lifesciences) in the PARTNER II Trial (PII A)3 (all-cause mortality rate=46%, all stroke rate=15.3%). The present study rates are also lower than those observed for the SAPIEN XT and the original self-expanding CoreValve (Medtronic) in the CHOICE4 study (balloon-expandable valve: all-cause mortality rate=53.4%, all stroke rate=17.4%; self-expanding valve: all-cause mortality rate=47.6%, all stroke rate=16.5%). Rehospitalisation rates in the present study (14.6%) are also lower than those seen with the balloon-expandable valve in the aforementioned studies (33.3% in PII A; 28.9% in CHOICE), as well as with the self-expanding valve in the CHOICE (22.5%) and SURTAVI5 (23.9%) studies. Furthermore, the 5-year repeat intervention rate in the ACURATE neo AS study (1.1%) is lower compared to the PII A (3.2%) and SURTAVI studies (3.5%). The 5-year new pacemaker implantation rate (22.9%, including patients who had a previous pacemaker) is lower compared to the SURTAVI (35.8%) and CHOICE (25.4% for the balloon-expandable valve; 40.4% for the self-expanding valve) studies.
Although the 5-year ACURATE neo AS data provide important updates on clinical outcomes over multiple years, we acknowledge several limitations of the present study. Events are reported according to VARC-2 criteria; this was standard convention during the initial trial design and enrolment but has since been succeeded by VARC-3. Most critically, this study cannot adequately address the bioprosthetic valve dysfunction component of VARC-3, as echocardiography data were not collected after 1 year, and computed tomography (CT) was not systematically done after screening. Therefore, the rates of valve dysfunction may be underestimated; also, the relationship between valve dysfunction and clinical events cannot be determined from this data set. Additionally, this study does not include other endpoints of contemporary interest: haemodynamics up to 5 years, coronary reaccess, and commissural alignment. Quality-of-life measures were not collected in this study, and New York Heart Association (NYHA) classification data were only collected up to 1 year, thus limiting our ability to assess patient improvement over time. These limitations will be addressed in the ACURATE IDE randomised controlled trial (ClinicalTrials.gov: NCT03735667) which includes patients of all surgical risks. Enrolment for this trial has been completed, and 10-year follow-up will include echocardiography and CT as well as NYHA classification and quality-of-life questionnaires.
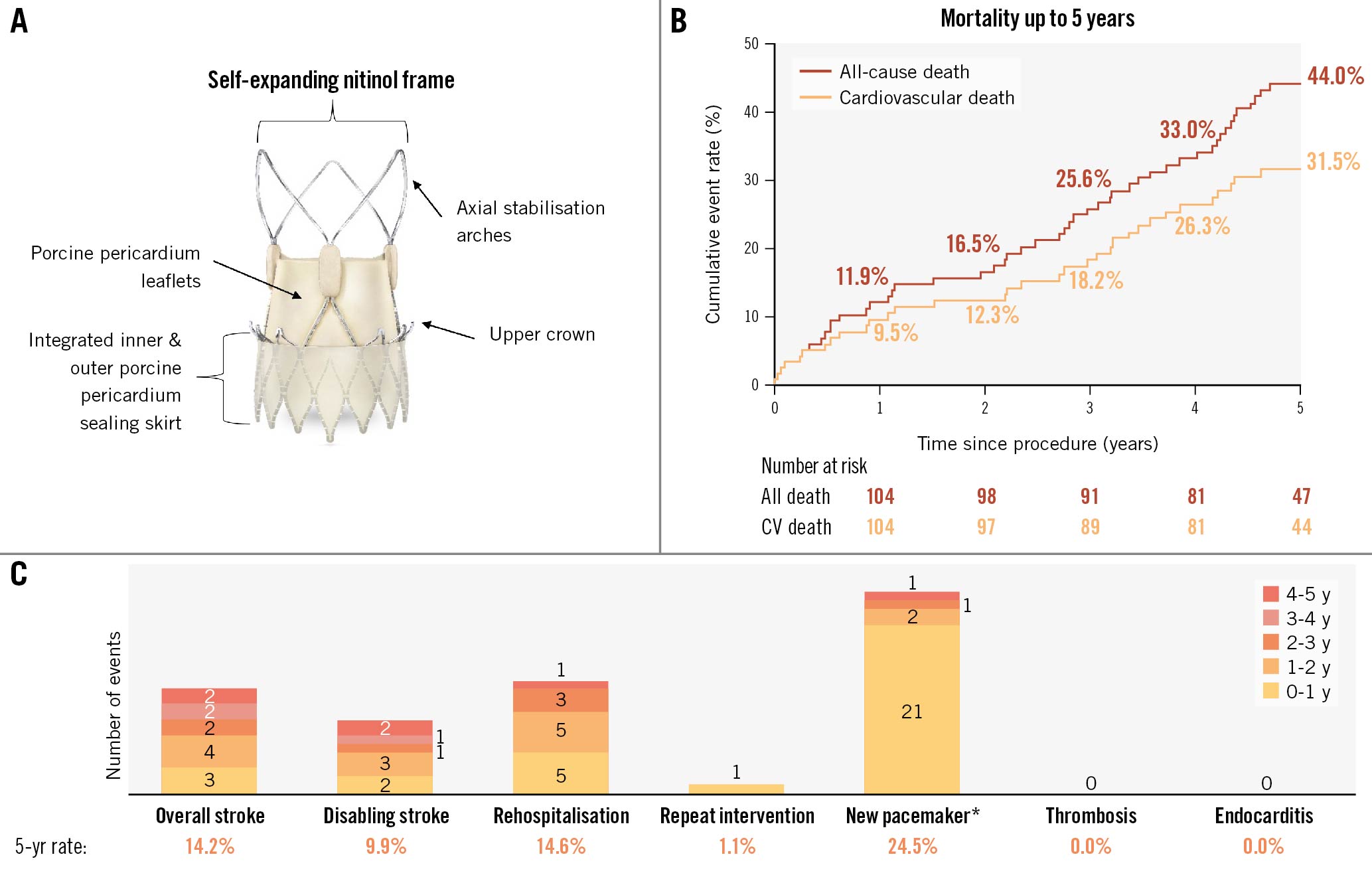
Figure 1. ACURATE neo2 valve and 5-year results from the ACURATE neo AS study. A) ACURATE neo2 is a transcatheter self-expanding bioprosthetic aortic valve with supra-annular leaflet positioning and a porcine sealing skirt, allowing for lower gradients and reduced paravalvular leak. B) Kaplan-Meier event rates for all-cause and cardiovascular death up to 5 years. C) Data monitoring committee/clinical events committee-adjudicated VARC-2 endpoint event rates up to 5 years. *in patients who did not previously have a pacemaker. CV: cardiovascular; VARC: Valve Academic Research Consortium
Acknowledgements
The authors thank Songtao Jiang, MS (Boston Scientific) for their statistical analysis; and Krystal Leger, PhD (Boston Scientific) and MaryEllen Carlile Klusacek, PhD (Boston Scientific) for their assistance in manuscript preparation.
Funding
The ACURATE neo AS study was sponsored and funded by Symetis S.A., a subsidiary of Boston Scientific.
Conflict of interest statement
H. Möllmann has received honoraria or consultation fees from Abbott, Biotronik, Boston Scientific, Edwards Lifesciences, Medtronic, and SMT. T. Noack has received speaker honoraria from Abbott, Edwards Lifesciences, and Medtronic. M. Hilker has served as a proctor for Boston Scientific. L. Conradi has been an advisory board member for Abbott, Medtronic, and JenaValve; and a consultant for Edwards Lifesciences, Boston Scientific, Pi-Cardia, MicroPort, and Venus Medtech. S. Toggweiler has served as a consultant and proctor for Medtronic, Boston Scientific, Edwards Lifesciences, and Biosensors; as a proctor for Edwards Lifesciences and Abbott; as a consultant for Medira, Shockwave, Teleflex, atHeart Medical, VeoSource, Cardiac Dimensions, and Polares Medical; has received institutional research grants from Boston Scientific, Fumedica, and Novartis; has received speaker honoraria from Sanofi, AstraZeneca, ReCor Medical, and Daiichi Sankyo; and has held equity in Hi-D Imaging. R. Modolo and D.J. Allocco have been employees of and shareholders in Boston Scientific. W. Kim has received honoraria or consultation fees from Abbott, Boston Scientific, Edwards Lifesciences, Meril Life Sciences; institutional fees from Boston Scientific; and has been an advisory board member for Hi-D Imaging. The other authors have no conflicts of interest to declare.

