Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The ACURATE neo2 is a contemporary transcatheter aortic valve implantation (TAVI) system approved for the treatment of severe aortic stenosis in Europe. The ACURATE neo2 has not been evaluated in bicuspid aortic valve (BAV) stenosis.
Aims: We sought to evaluate the safety and efficacy of ACURATE neo2 in patients with BAV stenosis.
Methods: We retrospectively analysed consecutive severe BAV stenosis patients undergoing TAVI with ACURATE neo2 at 10 European centres. Imaging data from preprocedural multislice computed tomography, pre- and postprocedural echocardiography, and procedural cinefluoroscopy were evaluated by a core laboratory. Valve Academic Research Consortium 3 (VARC-3)-defined 30-day procedure safety and efficacy were the primary endpoints. Adverse events were site-reported according to VARC-3 criteria.
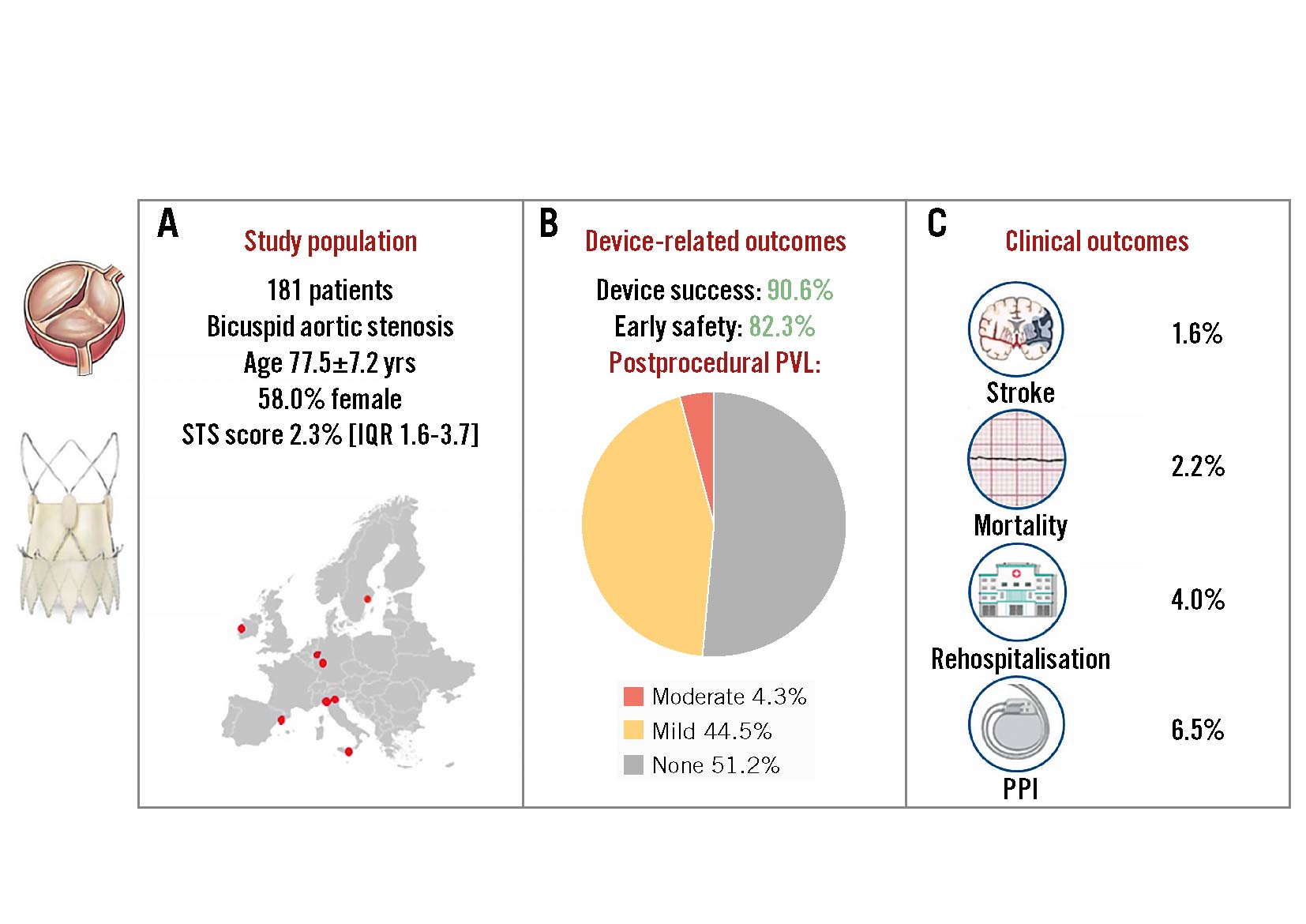
Results: Among 181 patients with BAV stenosis treated with the ACURATE neo2, the mean age was 77.5±7.2 years, 58.0% were female, and the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score was 2.3% (1.6-3.7%). Most procedures were transfemoral, and predilatation was performed in all cases. A second valve was required in 4 cases (2.2%). VARC-3-defined technical success was 95.6%. The primary endpoints of device success and early safety occurred in 90.6% and 82.3%, respectively. At 30 days, cardiovascular death occurred in 2.2% (N=4) and stroke in 1.6% (N=3). Core laboratory-adjudicated echocardiography reported an effective orifice area of 2.0 (1.7-2.5) cm2 and a mean transvalvular gradient of 6.5 (4.6-9.0) mmHg. Half of all cases (51.2%) had no paravalvular leak, while moderate leak occurred in 4.3%. A new permanent pacemaker was required in 11 patients (6.5%).
Conclusions: The ACURATE neo2 demonstrated favourable clinical outcomes and bioprosthetic valve performance at 30 days in selected patients with severe BAV stenosis.
Transcatheter aortic valve implantation (TAVI) is the standard-of-care treatment for elderly patients with symptomatic severe tricuspid aortic valve stenosis1. In contrast, the safety and efficacy of TAVI for patients with bicuspid aortic valve (BAV) stenosis remains unclear23. BAV stenosis is associated with heterogeneous anatomical features, including various morphological phenotypes, high leaflet calcium burdens, fibrotic or calcified raphe, abnormal coronary artery origins, and aortopathy4. These anatomical peculiarities represent a treatment challenge for TAVI and were not considered in the design of foundation TAVI devices5. BAV patients were thus systematically excluded from the seminal randomised TAVI trials6. Small prospective registries of selected BAV patients treated with balloon-expandable (SAPIEN 3 [Edwards Lifesciences]) and self-expanding (CoreValve Evolut [Medtronic]) TAVI devices have, however, suggested acceptable clinical outcomes78. Yet, there remain ongoing concerns around the incidence of paravalvular leak (PVL), the requirement for new permanent pacemaker implantation (PPI), and particularly, stroke when TAVI is used in BAV stenosis39. Given the heterogeneity of BAV anatomy, a class effect for TAVI systems cannot be assumed, and each device should be assessed based on its individual merits.
The ACURATE neo2 (Boston Scientific) is a contemporary TAVI system that has been approved for the treatment of severe aortic stenosis (AS) in Europe since April 2020. Accumulating evidence reports low rates of PVL and PPI and acceptable clinical outcomes in patients with tricuspid AS treated with this device1011. To date, the ACURATE neo2 has not been evaluated in patients with BAV stenosis.
Hence, we sought to evaluate the safety, effectiveness, and clinical performance of the ACURATE neo2 valve in patients with BAV stenosis.
Methods
Study design
The Neo2 BAV Registry (Neo2BAV) is an investigator-initiated, retrospective, international, multicentre study that evaluates the safety and effectiveness of the ACURATE neo2 system in patients with symptomatic severe BAV stenosis. We included consecutive BAV patients undergoing TAVI with the ACURATE neo2 at 10 high-volume European centres between October 2020 and March 2024. The decision to undergo TAVI and the final treatment strategy was determined by each institution’s Heart Team. Anonymised clinical, echocardiographic, and multislice computed tomographic (MSCT) data were collected centrally via a dedicated electronic case report form. Patients provided informed consent for the procedure and the use of their anonymised data. The study protocol complied with the Declaration of Helsinki and was approved by the ethics committee of Galway University Hospital and by the participating institutions.
Study device and procedures
The ACURATE neo2 is a self-expanding transcatheter heart valve (THV) constructed with a nitinol frame and a trileaflet porcine pericardial valve in the supra-annular position, with internal and external pericardial skirts. The device is available in three sizes (23, 25, and 27 mm) and is designed to treat annuli between 20.5 mm and 27 mm in diameter. The ACURATE neo2 has a 2-step, top-down deployment from a flexible delivery catheter introduced via a 14 Fr iSLEEVE (Boston Scientific). Device size and procedural strategy were determined by local operators. The systematic use of balloon predilatation, sized according to each individual patient’s anatomy (mean annular diameter - 1 mm) was strongly encouraged.
Patient assessment
All patients underwent routine preprocedural screening according to local protocols. Information on symptoms, medical history and comorbid illnesses, and surgical risk classification using the Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score were collected. Electrocardiographic data were evaluated by participating centres before and after TAVI. Imaging data (MSCT, pre- and postprocedural transthoracic echocardiography, and procedural cinefluoroscopy) were evaluated according to locally approved and validated standard operating procedures for core laboratory analysis, following current guidelines121314. Post-TAVI echocardiography was performed within 30 days of TAVI and was assessed for effectiveness and safety per Valve Academic Research Consortium (VARC)-3 criteria15. A 4-class grading scheme of none-trace, mild, moderate and severe was used to assess PVL. Prosthesis-patient mismatch was classified into mild, moderate, and severe according to indexed estimated orifice area and body mass index15. The 3Mensio Structural Heart software, version 10.3 (Pie Medical Imaging) was used for MSCT analysis. BAV type was identified according to Siever's classification (type 0, 1 and 2)16, and annular sizing and the Level of Implantation at the RAphe (LIRA) and Bicuspid Aortic Valve Anatomy and Relationship With Devices (BAVARD) sizing measurements were recorded1718. The total aortic valve calcification was reported using Agatston units (AU) and was obtained from non-contrast MSCT scans using a fixed threshold of 130 Hounsfield units (HU). In addition, calcium volume was estimated according to established protocols using the scan-specific method in which the calcium threshold was set as +4 standard deviations of a predefined aortic region of interest of HU, as described before19. In our population, the mean threshold for calcium was 653±164 HU. Furthermore, semiquantitative calcification severity grading was performed using previously published methodology, in which calcium severity was adjudicated as none, mild, moderate and severe20. Annular eccentricity was calculated as 1 minus the ratio of the minimum to maximum annular diameter.
Endpoints
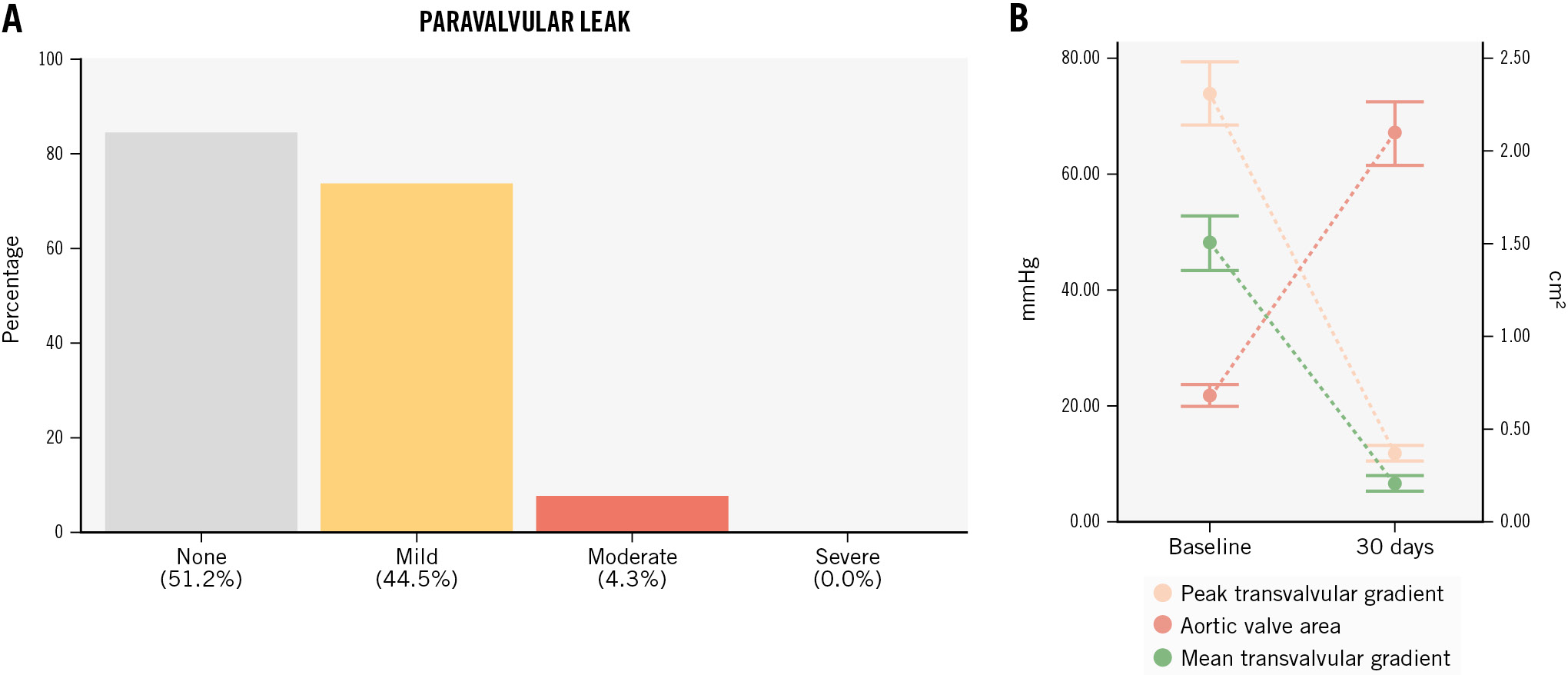
The primary endpoint of interest was 30-day device success, defined according to VARC-3 criteria as follows: the presence of technical success; freedom from all-cause mortality, surgery or intervention related to the device, or a major vascular, access-related or cardiac structural complication; and the intended performance of the THV (mean gradient <20 mmHg, peak velocity <3 m/s, Doppler velocity index ≥0.25, and Categorical variables are reported as counts and percentages. Continuous variables are reported as median and interquartile range (IQR) or mean±standard deviation (SD), according to the distribution assessed by the Shapiro-Wilk test. Primary and secondary endpoints were evaluated through descriptive statistics and survival analysis using R 4.1.2 software (R Foundation for Statistical Computing). A total of 181 consecutive patients with BAV stenosis treated with the ACURATE neo2 were included (Table 1). Patients were predominantly female (58.0%), with a mean age of 77.5±7.2 years, and were low surgical risk (STS-PROM 2.3% [1.6-3.7%]). Patients presented severe AS with a median aortic valve area of 0.7 (0.5-0.8) cm2 and a median aortic valve gradient of 47 (43-52) mmHg. Table 1. Baseline clinical characteristics. Among 160 analysable preprocedural MSCT scans, BAV phenotypes were characterised as Siever's type 0 (8.1%), type 1 (91.2%), and type 2 (0.6%), and the mean annulus diameter was 24.3±1.8 mm. The annular eccentricity index was 0.2±0.1, and the average ascending aortic diameter was 37.6±4.9 mm, with 40 cases (22.1%) presenting a mean aortic diameter >40 mm (Table 2). The mean aortic valve calcium score was 2,963±1,288 AU, and the mean calcium volume was 940.1±500.5 mm3. Semiquantitative aortic valve calcium assessment identified mild, moderate, and severe calcification in 6 (3.8%), 32 (20.1%), and 121 (76.1%) patients, respectively. Among 126 patients with MSCT suitable for raphe analysis, 96 (76.2%) had a calcified raphe, while 30 (23.8%) had a fibrotic raphe. The average raphe length was 7.7±2.7 mm. Raphe length/perimeter-derived annulus diameter ratio was 31±11%, and the median raphe calcium volume was 26 (2.5-70.0) mm3. Table 2. Preprocedural imaging analysis. Transfemoral TAVI was performed in most patients (98.9%) using the small (22.1%), medium (28.7%) and large (48.6%) ACURATE neo2 sizes, with annular-based sizing in all cases. Balloon predilatation was performed in all cases (non-compliant balloons were used in 50.8%) with an average balloon diameter of 22.8±1.9 mm, which represents a ratio of 0.9:1.0 with respect to the mean annulus diameter. Cerebral embolic protection was placed in 17 cases (9.4%), and commissural alignment of the ACURATE neo2 was performed in 82.1% (n=138/168) of patients with Siever's type 1 or 2 anatomy. Post-dilatation was performed in 104 patients (57.8%) using an average balloon diameter of 23.5±1.7 mm, which represents a ratio of 1.0:1.0 with respect to the mean annulus diameter (Table 3). Technical success was achieved in 173 cases (95.6%). Four patients (2.2%) required a second THV due to either high implantation or migration of the ACURATE neo2 with associated severe AR. Valve embolisation occurred in 3 cases (1.6%): 2 into the left ventricle (requiring surgery after failed snaring) and 1 into the aorta. Two patients (1.1%) experienced procedural death due to annular rupture after post-dilatation. Overall, conversion to surgery occurred in 3 cases (1.6%). A step-by-step case example is proposed in Figure 1. Table 3. Procedural characteristics. Figure 1. Bicuspid aortic stenosis treated with the ACURATE neo2 platform. Case example. A) Predilatation with residual balloon waist. B) THV positioned at the ideal depth (dashed arrow indicates annular plane). C) Upper crowns and stabilisation arches released with mild device parallax (dashed triangle). D) Post-release. E) Post-dilatation. F) Final result. THV: transcatheter heart valve The primary endpoint of interest, 30-day device success, was achieved in 164 (90.6%) cases (Central illustration, Table 4).Early safety was reported in 149 (82.3%) patients. The 30-day rates of cardiovascular death and stroke were 2.2% (n=4) and 1.6% (n=3), respectively. New PPI was required in 11 patients (6.5%), and major vascular complications occurred in 5 (2.9%). Seven patients (4.0%) underwent rehospitalisation for cardiovascular reasons. Central illustration. The Neo2 BAV Registry. A) Characteristics of the study population; (B) device-related outcomes including postprocedural PVL; (C) clinical outcomes. BAV: bicuspid aortic valve; IQR: interquartile range; PPI: permanent pacemaker implantation; PVL: paravalvular leak; STS: Society of Thoracic Surgeons Table 4. Thirty-day clinical and echocardiographic outcomes. Postprocedural echocardiographic data were available for core laboratory assessment in 164 (90.6%) patients. The peak and mean aortic gradients were 12.2 (9.0-16.7) mmHg and 6.5 (4.6-9.0) mmHg, respectively (Figure 2). The median effective orifice area was 2.0 (1.7-2.5) cm2. Aortic regurgitation, predominantly paravalvular, was trivial or absent in 51.2% (n=84), mild in 44.5% (n=73), and moderate in 4.3% (n=7). No patient had severe AR (Table 4). Figure 2. Valve performance: echocardiographic data. A) Postprocedural paravalvular leak; (B) median distribution of mean and peak transvalvular gradients and aortic valve area at baseline (left) and 30 days (right). Neo2BAV evaluated the safety and efficacy of the ACURATE neo2 THV among 181 patients with symptomatic severe BAV stenosis. The salient findings of the study are that VARC-3-defined 30-day device success was achieved in 90.6% and early safety in 82.3%. The short-term rates of death and stroke were 2.2% and 1.6%, respectively. Excellent haemodynamic results were achieved with a postprocedural echocardiographic transvalvular gradient of 6.5 (4.6-9.0) mmHg and moderate PVL in 4.3%. The rate of new PPI was low at 6.5%. The outcomes of TAVI in the setting of BAV stenosis have been considered inferior to those reported for tricuspid AS for more than a decade2. Most recently, the NOTION II study reported numerically higher rates of death and stroke at 30 days among patients with BAV stenosis (2.0% and 6.1%) undergoing TAVI compared to those with tricuspid AS (0.0% and 2.1%) or those treated with surgical aortic valve replacement (0.0% and 2.3%)3. The morphological features associated with BAV include more severe leaflet calcification, with calcific deposits higher in the leaflet architecture than with tricuspid AS, and the presence of a raphe of variable length and calcification often renders THV sizing, positioning, and expansion more challenging5. There is, however, a spectrum of bicuspid disease, and when selected, favourable BAV anatomies are treated with TAVI, acceptable outcomes are reported21. When compared to the results from the Bicuspid Aortic Stenosis With Evolut Platform International Experience (BIVOLUTX) registry, which evaluated TAVI with the self-expanding supra-annular Evolut PRO and Evolut R 34 mm devices (both Medtronic), the 30-day outcomes of the current study are comparable7. VARC-3-defined device success was 91.3% in BIVOLUTX and 90.6% in Neo2BAV, and early safety was 64.4% and 82.3%, respectively. Mortality at 30 days was 4% in BIVOLUTX7 and 2.2% in Neo2BAV. A small study with the balloon-expandable SAPIEN 3 valve in BAV stenosis also reported mortality of 3.9% at 30 days22. Stroke remains a much-feared complication of TAVI and appears to occur more frequently in BAV versus tricuspid patients undergoing the procedure. In an analysis of 2,691 propensity-matched pairs from the STS/American College of Cardiology Transcatheter Valve Therapies Registry, the 30-day stroke rate with TAVI was significantly higher for bicuspid versus tricuspid AS (2.5% vs 1.6%; hazard ratio 1.57, 95% confidence interval: 1.06-2.33)9. The rate of all stroke in the current study at 30 days was gratifyingly low at 1.6% and compared favourably with rates reported in both BIVOLUTX (4.6%) and the Medtronic Transcatheter Aortic Valve Replacement Low Risk Bicuspid Study (4.0%)723. The low stroke rate in the current study could be due to the inclusion of patients with less challenging anatomies; however, the simple top-down deployment of the ACURATE neo2 without need for THV repositioning, recapture, or undue manipulation within the calcified leaflets could also result in fewer stroke events. Surprisingly, embolic protection was used sparingly in Neo2BAV (9.4%), suggesting that the implanting teams are yet to be convinced by the true value of this therapy24. The ongoing randomised BHF PROTECT-TAVI trial (ISRCTN16665769) will hopefully provide clarity on the efficacy of the SENTINEL embolic protection system (Boston Scientific) in TAVI patients25. In the current study, relevant procedural complications were evident in 4.4% of patients and included the requirement for a second THV in 2.2%, procedural mortality in 1.1%, and conversion to surgery in 1.6%. These complications are rarely experienced in contemporary TAVI practice but seem to be more common among BAV patients. These complications underscore the complex nature of BAV morphology and the importance of Heart Team decision-making in treatment allocation for BAV stenosis. Moreover, we believe that an optimal procedural strategy is essential to successfully treat BAV with the ACURATE neo2, with mandatory optimal predilatation and near-universal post-dilatation to avoid frame malexpansion. Whether all BAV morphologies can be treated with ACURATE neo2, including those with very severe valvular calcification or excessively long leaflets, remains to be proven. In Neo2BAV, we report encouraging core laboratory-adjudicated haemodynamic results with a 30-day median gradient of 6.5 (4.6-9.0) mmHg and moderate PVL in 4.3% of cases. Core laboratory-adjudicated aortic regurgitation, predominantly paravalvular, was absent in 51.2% (n=84), mild in 44.5% (n=73), and moderate in 4.3% (n=7). In BIVOLUTX, PVL rates were trivial in 7.5%, mild in 81.1%, mild to moderate in 8.5%, and moderate in 2.8%7. In Neo2BAV, moderate and severe prosthesis-patient mismatch occurred in 16 (11.4%) and 1 (0.7%) cases, respectively. The 30-day rate of new PPI of 6.5% in the current study is the lowest reported to date in a TAVI-treated BAV population and is in line with prior reports of low new pacemaker rates with the ACURATE neo2 THV. The essential morphological differences between bicuspid and tricuspid aortic valve stenosis that influence outcomes after TAVI are the presence of a calcified raphe and the distribution and severity of leaflet calcification26. It is thus appropriate to consider BAV stenosis as a spectrum of disease with a higher risk of adverse events among patients with more severe calcification. The results of the current study need to be considered within this framework: did patients in Neo2BAV have more or less valve calcification than comparative studies? In Neo2BAV, we report an average calcium score of 2,963±1,288 AU, which is suggestive of severe valve calcification in our population27. We measured a mean calcium volume of 940.1±500.5 mm3 in our population based on a patient-specific threshold. Unfortunately, comparing this volume of calcification to other BAV series is not possible because of divergent calcium assessment methodologies. Most series report calcium volume based on contrast-enhanced MSCT with a unique HU threshold: in BIVOLUTX the mean calcium volume was 1,423.8±628.8 mm3 with a 650 HU threshold, while Yoon et al reported a median calcium volume of 382 (182-695) mm3 using an 850 HU threshold726. Using these fixed HU thresholds may introduce error in the evaluation of valvular calcification and lead to erroneous conclusions28, hence, we use the validated patient-specific threshold and acknowledge that between-study calcification comparisons are therefore impossible. It is hoped that the core laboratory-adjudicated calcium assessment in Neo2BAV should provide a reference for other comparative studies on this topic. The role of TAVI in the management of younger and lower surgical risk patients with severe BAV stenosis remains unresolved. TAVI technology, device-sizing algorithms, and implant techniques have been directed towards treating the less complex and more homogeneous tricuspid aortic valve. While the current study provides reassuring information on the use of theACURATE neo2 in BAV stenosis and reports similar outcomes with this device as for patients with tricuspid AS10, it is possible that less complex BAV patients were treated, and an appropriately powered randomised comparison between TAVI and surgical aortic valve replacement in BAV disease is essential before any technology is routinely applied to younger BAV populations. Our study has limitations, including its retrospective design, the absence of patient screening and clinical event committees, a short follow-up period, and limited sample size. Ongoing clinical follow-up is required to understand the longer-term clinical evolution of this patient population; however, the short-term outcomes of TAVI in patients with BAV stenosis are the most important to assess device safety. Indeed, outcomes between bicuspid and tricuspid morphologies are similar beyond 30 days3. The decision to proceed with TAVI using the ACURATE neo2 was at the local Heart Team’s discretion, and the selection of less calcified anatomies must be considered as well as patients with an aortic diameter >50 mm, who were not included. Screening logs to assess the number of patients with BAV treated with alternate devices during the study period are, unfortunately, not available. Adverse events were site-reported, with underreporting possible; however, the core laboratory assessment of all imaging data, especially procedural cineangiography and postprocedural echocardiography, represent distinct strengths of this study. Clearly, our findings should not be extended to other TAVI platforms. The Neo2 BAV Registry reports encouraging safety and clinical efficacy of the ACURATE neo2 THV in a select cohort of 181 patients with severe BAV stenosis. Technical success was achieved in 95.6% and early safety in 82.3% of participants, with low rates of stroke (1.6%), PPI (6.5%), and moderate PVL (4.3%). Large prospective studies and randomised comparative efficacy studies versus surgical aortic valve replacement are required to confirm these data. Selected patients with severe bicuspid aortic stenosis can be effectively and safely treated with transcatheter aortic valve implantation using the self-expanding ACURATE neo2 platform. Currently no other data are available on the subject. This paper was guest edited by Franz-Josef Neumann, MD, PhD; Department of Cardiology and Angiology II, University Heart Center Freiburg - Bad Krozingen, Bad Krozingen, Germany. The current study is funded by a grant from Boston Scientific. A. Rück reports grants and personal fees from Boston Scientific; and personal fees from Edwards Lifesciences, Abbott, and Anteris Technologies. W.-K. Kim reports proctor/speaker fees/advisory board participation for Abbott, Boston Scientific, Edwards Lifesciences, and Meril Life Sciences. M. Wagener received educational support from Medtronic. A. McInerney has received speaker fees from Shockwave Medical, Boston Scientific, and Novartis. M. Montorfano received consultant fees from Abbott, Boston Scientific, Kardia, and Medtronic. B. Bellini received consultant fees from Medtronic. H. Möllmann receives grants, consulting and lecture fees from Abbott, Boston Scientific, Edwards Lifesciences, Meril Life Sciences, and Medtronic; and support for attending meetings/travel from Boston Scientific, Abbott, and Edwards Lifesciences. G. Tarantini has received honoraria for lectures and consulting from Medtronic, Edwards Lifesciences, Boston Scientific, GADA group, and Abbott. M. Barbanti is a consultant for Edwards Lifesciences, Boston Scientific, and Medtronic. O. Soliman received institutional research grants, committee membership and chairmanship from Abbott, Biosensors, Boston Scientific, Cardiawave, and Meril Life Sciences. D. Mylotte reports institutional research grants from Boston Scientific and Medtronic; and is a consultant for Boston Scientific, Medtronic, and MicroPort. The other authors have no conflicts of interest to declare in relation to this manuscript. The Guest Editor reports consultancy fees from Novartis and Meril Life Sciences; speaker honoraria from Boston Scientific, Amgen, Daiichi Sankyo, and Meril Life Sciences; reports speaker honoraria paid to his institution from BMS/Pfizer, Daiichi Sankyo, Boston Scientific, Siemens, and Amgen; and research grants paid to his institution from Boston Scientific and Abbott.Statistical analysis
Results
Patients
Baseline data N=181 Age, years 77.5±7.2 Female sex 105 (58.0) BMI, kg/m² 25.7±4.7 STS-PROM, % 2.3 (1.6-3.7) Diabetes mellitus 34 (18.8) Hypertension 129 (71.3) Creatinine clearance, ml/min 59.5±23.0 Prior PCI 32 (17.7) Prior CABG 6 (3.3) Prior pacemaker 12 (6.6) Prior stroke 22 (12.2) Atrial fibrillation 47 (26.0) Peripheral vascular disease 22 (12.2) Chronic lung disease 33 (18.2) NYHA Functional Class III/IV 89 (49.2) Data are given as mean±standard deviation, n (%) or median (IQR). BMI: body mass index; CABG: coronary artery bypass graft; IQR: interquartile range; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality Baseline MSCT and sizing strategies
Echocardiographic data n=164 LVEF, % 57±10.1 Peak transaortic gradient, mmHg 74 (70-81) Mean transaortic gradient, mmHg 47 (43-52) Aortic valve area, cm² 0.7 (0.5-0.8) MSCT n=160 Mean annulus diameter, mm 24.3±1.8 Annulus area, mm² 461.2±66.9 Annulus perimeter, mm 77.5±5.7 Annular eccentricity index 0.2±0.1 Mean STJ diameter, mm 31.0±3.7 Mean sinus of Valsalva diameter, mm 32.7±3.7 Left coronary height, mm 14.6±3.4 Right coronary height, mm 18.3±3.3 Mean ascending aorta diameter, mm 37.6±4.9 Ascending aorta diameter ≥40 mm 40 (25.0) Mean LIRA, mm 14.8±3.2 LIRA area, mm² 151.7±57.2 LIRA perimeter, mm 66.9±8.8 LIRA raphe height, mm 8.7±2.3 BAVARD ICD at 4 mm, mm 26.1±2.4 Bicuspid morphology Type 0 13 (8.1) Type 1 146 (91.2) L-R 126 (78.8) R-N 18 (11.2) L-N 2 (1.2) Type 2 1 (0.6) Aortic valve calcium volume, mm3 940.1±500.5 Agatston score# 2,963.5±1,288.2 Raphe length, mm 7.7±2.7 Raphe length/perimeter-derived diameter ratio, % 31±11 Calcified raphe* 96 (76.2) Fibrotic raphe* 30 (23.8) Data are given as mean±standard deviation, median (IQR) or n (%). #Using a fixed threshold of 130 HU. *Percentage calculated over analysable raphe (N=126). BAVARD: Bicuspid Aortic Valve Anatomy and Relationship With Devices; HU: Hounsfield units; ICD: intercommissural distance; L-N: left-non-coronary; L-R: left-right; LIRA: Level of Implantation at the RAphe; LVEF: left ventricular ejection fraction; MSCT: multislice computed tomography; R-N: right-non-coronary; STJ: sinotubular junction Procedural details
Procedural data n=181 Access Transfemoral 179 (98.9) Transaxillary 2 (1.1) Valve size, mm Small (23 mm) 40 (22.1) Medium (25 mm) 52 (28.7) Large (27 mm) 88 (48.6) Mean annular oversizing, % 5.8±4.9 Cover index, % 5.3±4.5 Implantation view Cusp-overlap view used during deployment 98 (54.1) Commissural alignment performed* 138 (82.1) Predilatation 181 (100) Non-compliant balloon 92 (50.8) Balloon-to-annulus ratio 0.9:1.0 Mean balloon diameter, mm 22.8±1.9 Post-dilatation 104 (57.8) Non-compliant balloon 45 (43.3) Balloon-to-annulus ratio 1.0:1.0 Mean balloon diameter, mm 23.5±1.7 LV wire pacing 100 (55.5) CEPD 17 (9.4) Technical success 173 (95.6) Need for a second valve 4 (2.2) Procedural death 2 (1.1) Annular rupture 2 (1.1) Coronary obstruction 0 (0.0) Valve embolisation 3 (1.6) Conversion to surgery 3 (1.6) Data are given as n (%) or mean±standard deviation. *Percentage calculated across patients with Siever's type 1 or 2 anatomy. CEPD: cerebral embolic protection device; LV: left ventricle 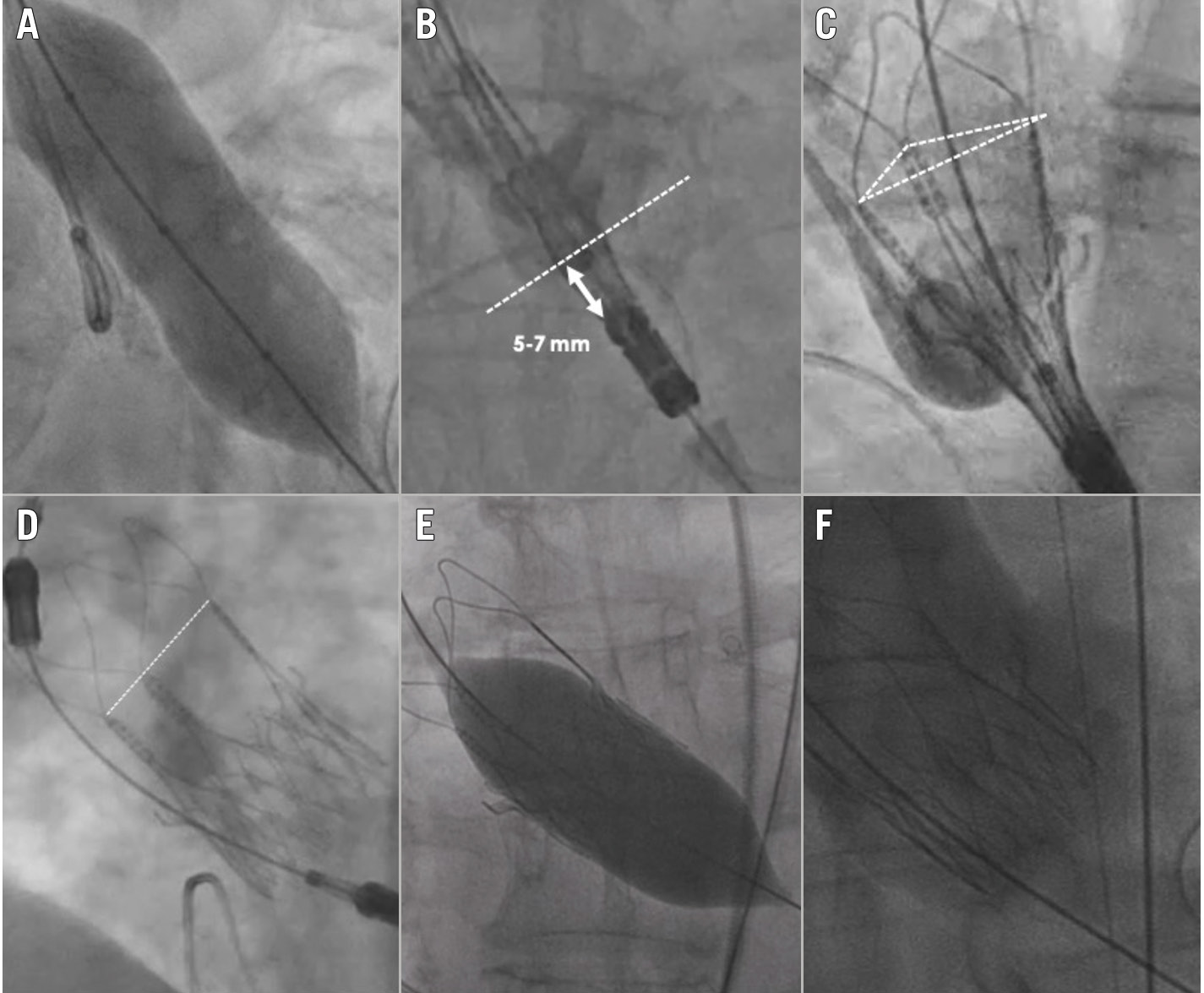
Thirty-day outcomes
Clinical outcomes (n=181) Device success 164 (90.6)* Early safety 149 (82.3)* Death 4 (2.2) All stroke 3 (1.6) Major bleeding 8 (4.4) Major vascular complication 5 (2.9) New pacemaker (total population) 11 (6.1)* New pacemaker (pacemaker naïve) 11 (6.5)** AV block II° 1 (0.6) AV block III° 9 (5.3) Sinus bradycardia 1 (0.6) Myocardial infarction 0 (0) Valve reintervention 0 (0) Acute kidney injury (stage 2-3) 0 (0) Rehospitalisation 9 (5.2) Cardiovascular rehospitalisation 7 (4.0) Echocardiography (n=164) LVEF, % 56.6±11.4 AVA (VTI), cm² 2.0 (1.7-2.5) Peak gradient, mmHg 12.2 (9.0-16.7) Mean gradient, mmHg 6.5 (4.6-9.0) Aortic regurgitation None 84 (51.2) Mild 73 (44.5) Moderate 7 (4.3) Severe 0 (0) Prosthesis-patient mismatch None 147 (89.6) Moderate 16 (11.4) Severe 1 (0.6) Data are given as n (%), mean±standard deviation or median (IQR). *Among entire population (n=181). **Among pacemaker-naïve patients (n=162). AV: atrioventricular; AVA: aortic valve area; IQR: interquartile range; LVEF: left ventricular ejection fraction; VTI: velocity time integral Echocardiographic outcomes
Discussion
Limitations
Conclusions
Impact on daily practice
Guest Editor
Funding
Conflict of interest statement

