Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The Myval series is the first commercially available balloon-expandable transcatheter aortic valve implantation (TAVI) system designed as an alternative to the SAPIEN series. The LANDMARK trial recently demonstrated its non-inferiority compared to contemporary systems. However, the long-term durability of the Myval series remains unknown.
Aims: We aimed to evaluate the 4-year durability of the Myval series using Valve Academic Research Consortium (VARC)-3-defined endpoints.
Methods: We carried out a multicentre ambispective study of patients with severe aortic stenosis who underwent TAVI with the Myval series between December 2017 and April 2020. Baseline characteristics were prospectively recorded in a dedicated database. Clinical and echocardiographic follow-up was performed at 4 years. Outcomes included haemodynamic valve deterioration (HVD), bioprosthetic valve failure (BVF), and patient-prosthesis mismatch (PPM) as defined by the VARC-3 criteria, assessed at a central echocardiography laboratory.
Results: A total of 366 patients from 9 institutions were included, all of whom completed 4-year follow-up or were followed up until death. The 4-year survival rate was 81.8%, with residual ≥moderate aortic regurgitation observed in 9.2% of patients. BVF criterion 1 (symptomatic valve failure) occurred in 3.3%, while no cases of BVF criteria 2 or 3 were reported. Stage 2 HVD was observed in 9.7% of patients and stage 3 HVD in 0.7% at 4 years. Moderate and severe PPM were identified in 3.6% and 2.1% of patients at 1 year, respectively.
Conclusions: In a real-world cohort, 4-year outcomes with the balloon-expandable Myval series demonstrated acceptable valve durability, low haemodynamic deterioration, and comparable performance to contemporary TAVI systems.
With the advent of new-generation transcatheter aortic valve implantation (TAVI) devices, transcatheter therapy has become the primary alternative for treating aortic stenosis in a broad range of patients. Advances in technology have led to the development of several transcatheter heart valves (THVs) that incorporate features such as lower profiles, improved positioning and repositioning capabilities, and recapturability. These innovations aim to reduce procedural complications including paravalvular leak (PVL), vascular injuries, and conduction disturbances, ultimately improving clinical outcomes123.
The Myval series (Meril Life Sciences) is the only commercially available balloon-expandable THV designed as an alternative to the SAPIEN series (Edwards Lifesciences). The LANDMARK trial demonstrated the non-inferiority of the Myval series compared to contemporary THVs including the SAPIEN and Evolut series (Medtronic)4.
Mid- and long-term durability are critical factors for the success of TAVI, particularly as the procedure is increasingly adopted for younger and lower-risk patients who require durable solutions. Although early clinical outcomes with the Myval device have been promising, data on its mid- and long-term performance remain limited. This study aims to evaluate the 4-year durability of the Myval series based on Valve Academic Research Consortium (VARC)-3-defined parameters.
Methods
Objective
The primary endpoints of this study were haemodynamic valve deterioration (HVD; stages 2-3), bioprosthetic valve failure (BVF; criteria 1-3), and patient-prosthesis mismatch (PPM), as defined by the VARC-3 criteria5. Stage 2 HVD was defined as a mean transvalvular gradient increase of ≥10 mmHg, resulting in a mean gradient ≥20 mmHg, or an increase of ≥1 grade of intraprosthetic aortic regurgitation (AR) leading to ≥moderate AR. Stage 3 HVD was characterised by a mean transvalvular gradient increase of ≥20 mmHg, resulting in a mean gradient ≥30 mmHg, or an increase of ≥2 grades of intraprosthetic AR resulting in severe AR.
BVF was categorised as follows: criterion 1, any bioprosthetic valve dysfunction (BVD) with new-onset or worsening symptoms or irreversible stage 3 HVD; criterion 2, BVD requiring aortic valve reintervention; criterion 3, valve-related death6. PPM was defined using body mass index-adjusted thresholds according to VARC-3 guidelines.
Device description
The Myval device is a next-generation, balloon-expandable THV constructed from a nickel-cobalt frame. Its hybrid honeycomb structure, consisting of single-element hexagons, supports three bovine pericardium leaflets treated with the proprietary anticalcification process “AntiCa”. To minimise paravalvular leak, the lower cells are lined with polyethylene terephthalate, internally and externally. Myval valves are available in a range of sizes, including conventional (20 mm, 23 mm, 26 mm, 29 mm), intermediate (21.5 mm, 24.5 mm, 27.5 mm), and extra-large sizes (30.5 mm, 32 mm), all compatible with a 14 Fr sheath. The MyVal-1 study previously demonstrated the safety and efficacy of this valve in intermediate- to high-risk patients78.
Study design and population
This ambispective multicentre study included all consecutive patients who underwent TAVI with the Myval series across 9 centres between December 2017 and April 2020. Baseline clinical and procedural characteristics were prospectively recorded. Informed consent was waived by the central ethics committee, although specific consent was obtained from those patients who required an additional echocardiographic study to complete their 4-year follow-up, which was necessary in 56.8% of the sample. Follow-up was scheduled for 4 years or until death, with clinical and echocardiographic assessments performed at each visit to ensure accurate monitoring of haemodynamic function. A total of 442 patients underwent TAVI with a Myval series device in the aortic position. Of these, 366 patients completed 4 years of follow-up and were evaluated.
Imaging analysis
Echocardiographic examinations were performed according to the guidelines of the American Society of Echocardiography. The following measurements were obtained: left ventricular outflow tract diameter; left ventricular ejection fraction (Simpson method); mean/peak transvalvular gradients; area by continuity equation; and presence, degree, and type (transvalvular, paravalvular, global) of AR. AR severity was evaluated using a multiparametric approach and classified following the VARC-3 recommendations. Images were centrally analysed (www.icicorelab.es) by two independent experienced cardiologists blinded to the type of prostheses; the absence of significant interobserver differences was assessed. The Xcelera Cardiology Information System (Philips Medical Systems) was used for all offline analysis. Although data were gathered retrospectively, patients without adequate 4-year imaging follow-up were scheduled for echocardiographic testing after obtaining informed consent.
Statistical analysis
Categorical variables are presented as absolute numbers and percentages, while continuous variables are expressed as median (interquartile range). Normality was assessed using the Kolmogorov-Smirnov test and Q-Q plots. Categorical variables were compared using the chi-square or Fisher’s exact test, as appropriate, and continuous variables were compared using the Mann-Whitney U test. Kaplan-Meier survival analysis was performed to evaluate all-cause mortality and stroke. Statistical significance was defined as a p-value<0.05. All analyses were performed using R software, version 3.6.1 (R Foundation for Statistical Computing).
Results
The study cohort consisted of 366 patients who had completed 4-year follow-up (or until death) after TAVI. The mean age was 75.9±9.1 years, 32.5% were female, and the mean European System for Cardiac Operative Risk Evaluation (EuroSCORE) II was 4.2±3.5. Of the procedures, 98.9% were transfemoral, and post-dilatation was used in 7.9%. Baseline demographic, clinical, electrocardiographic and imaging characteristics are shown in Table 1.
Table 1. Main clinical, electrocardiographic, and imaging characteristics at baseline of study population.
| Clinical characteristics | |
|---|---|
| Age, years | 75.9±9.1 |
| Female sex | 32.5% |
| BSA, m2 | 1.8±0.2 |
| BMI, kg/m2 | 26.5±4.3 |
| Diabetes mellitus | 136 (37.2) |
| Prior pacemaker | 32 (8.7) |
| Chronic kidney disease | 137 (37.4) |
| Haemodialysis | 4 (1.1) |
| Chronic pulmonary disease | 44 (12.0) |
| Peripheral artery disease | 42 (11.5) |
| Previous stroke/TIA | 28 (7.7) |
| Porcelain aorta | 8 (2.2) |
| Coronary artery disease | 202 (55.2) |
| Prior heart surgery | 58 (15.8) |
| Prior CABG | 48 (13.1) |
| Prior valvular surgery | 23 (6.3) |
| Prior atrial fibrillation | 80 (21.9) |
| NYHA III-IV | 50.4% |
| STS-PROM score, % | 4.8±4.3 |
| EuroSCORE II, % | 4.2±3.5 |
| Electrocardiography | |
| Sinus rhythm | 252 (68.9) |
| Atrial fibrillation | 54 (14.8) |
| LBBB | 27 (7.4) |
| RBBB | 23 (6.3) |
| AVB (1st degree) | 18 (4.9) |
| Computed tomography | |
| Maximum AA diameter, mm | 28.6±4.7 |
| Minimum AA diameter, mm | 22.3±4.8 |
| Eccentricity index | 0.30±0.19 |
| Mean AA diameter, mm | 25.1±3.9 |
| AA area, mm2 | 458.4±114.6 |
| Agatston score | 2,553.6±1,419.4 |
| Echocardiography | |
| LVEF, % | 54.4±12.2 |
| Peak aortic gradient, mmHg | 77.8±23.5 |
| Mean aortic gradient, mmHg | 48.1±16.4 |
| ≥Moderate aortic regurgitation | 72 (19.7) |
| ≥Moderate mitral regurgitation | 52 (14.2) |
| ≥Moderate tricuspid regurgitation | 43 (11.7) |
| Data are %, n (%), or mean±standard deviation. AA: aortic annulus; AVB: atrioventricular block; BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass graft; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LBBB: left bundle branch block; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association; RBBB: right bundle branch block; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TIA: transient ischaemic attack | |
Periprocedural and 30-day clinical outcomes
The mean aortic valve gradient was 10.3±4.7 mmHg at 30-day follow-up, and the mean aortic valve area was 1.7±0.4 cm2. The peak aortic valve velocity and gradient were 2.12±0.47 m/s and 18.90±7.96 mmHg, respectively. At 30 days, 5.5% of patients had moderate aortic regurgitation, and 0.5% had severe AR (Table 2).
Table 2. Thirty-day, 1- and 4-year (VARC-3) outcomes of patients with Myval implantation.
| Outcome | 30 days | 1 year | 4 years |
|---|---|---|---|
| Freedom from death | 98.6% | 93.6% | 81.8% |
| Freedom from stroke | 99.5% | 99.2% | 98.5% |
| Valve-related hospitalisation | 0 (0) | 5 (1.4) | 6 (1.6) |
| Severe patient-prosthesis mismatch | 2.0% | 2.1% | 2.1% |
| Mean aortic gradient, mmHg | 10.3±4.7 | 11.2±5.6 | 12.0±6.2 |
| Peak aortic gradient, mmHg | 18.9±7.9 | 20.7±9.1 | 21.6±10.3 |
| AVA, cm2 | 1.7±0.4 | 1.7±0.5 | 1.7±0.4 |
| LVEF, % | 56.4±10.3 | 56.4±9.7 | 56.8±9.4 |
| ≥Moderate aortic regurgitation | 5.9% | 7.9% | 9.2% |
| Early safety | 83.0% | - | - |
| Device success | 95.5% | - | - |
| Leaflet thrombosis | 0.5% | - | - |
| Endocarditis | 0 (0) | - | - |
| Data are %, n (%) or mean±standard deviation. AVA: aortic valve area; LVEF: left ventricular ejection fraction; VARC: Valve Academic Research Consortium | |||
Clinical outcomes
Figure 1 shows the survival rate of 81.8% and freedom from stroke rate of 98.5% at 4-year follow-up. The main outcomes are summarised in Table 3.
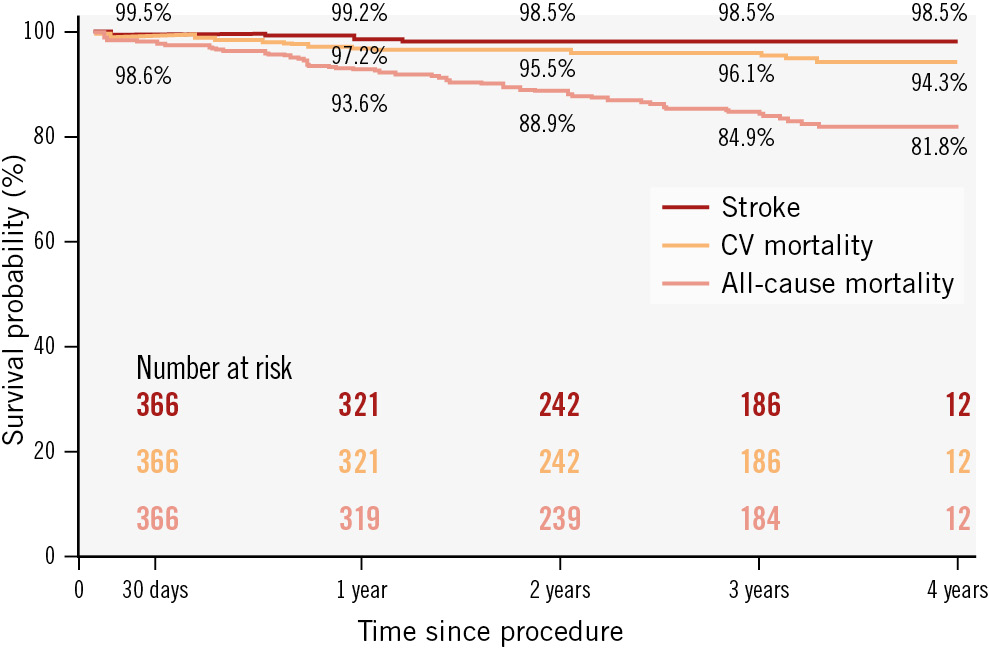
Figure 1. Kaplan-Meir survival curves for all-cause mortality, cardiovascular mortality and stroke at 4 years. CV: cardiovascular
Table 3. Main procedural and in-hospital (VARC-3) outcomes of the study population.
| Procedural outcomes | |
|---|---|
| Transfemoral approach | 362 (98.9) |
| More than 1 prosthesis implanted | 4 (1.1) |
| Balloon predilatation | 199 (54.4) |
| Balloon post-dilatation | 29 (7.9) |
| Procedural complications | |
| Valve embolisation | 2 (0.5) |
| Annulus rupture | 0 (0) |
| Coronary artery occlusion | 1 (0.3) |
| Tamponade | 1 (0.3) |
| Aortic dissection | 1 (0.3) |
| Conversion to open surgery | 0 (0) |
| Arrythmia | 21 (5.7) |
| Need for haemodynamic support | 5 (1.4) |
| Procedural death | 1 (0.3) |
| Technical success | 355 (97) |
| In-hospital outcomes | |
| Major bleeding | 8 (2.2) |
| Major vascular complication | 9 (2.5) |
| Acute kidney injury | 8 (2.2) |
| New permanent pacemaker implantation | 29 (7.9) |
| Stroke | 2 (0.5) |
| Myocardial infarction | 1 (0.3) |
| Data are n (%). VARC: Valve Academic Research Consortium | |
Haemodynamic valve performance and durability
The mean aortic valve gradients were 11.18±5.59 mmHg and 11.96±6.21 mmHg at 1- and 4-year follow-up, respectively (p<0.001 for progression), and the mean aortic valve areas were 1.75±0.47 cm2 and 1.66±0.38 cm2 at 1- and 4-year follow-up, respectively (p=0.010 for progression). At 1 and 4 years, the peak aortic valve gradients were 20.75±9.15 mmHg and 21.56±10.32 mmHg, respectively. A total of 6.8% and 7.8% of the patients had moderate aortic regurgitation, and 1% and 1.4% had severe AR, at 1 and 4 years, respectively (p=0.206 for progression) (Central illustration, Supplementary Table 1).
As presented in Supplementary Figure 1, during follow-up, BVF criterion 1 (symptomatic failure) occurred in 3.3%, and there were no instances of BVF criterion 2 (aortic valve reintervention) or BVF criterion 3 (valve-related death). Finally, the rates of stage 2 and stage 3 HVD were 9.7% and 0.7%, respectively, with severe PPM present in 2.0% and 2.1% of patients at 30 days and 1 year (Figure 2).
The rate of stage 2 HVD developing over time was almost equally attributable to the increases in the aortic gradient and AR. At 1 year, 6.2% of patients developed stage 2 HVD (48% due to the increase in the gradient and 52% due to the increase in AR); at 2 years, 7.1% (54% due to the increase in gradient, 46% due to the increase in AR); at 3 years, 8.9% (48% due to the increase in gradient, 52% due to the increase in AR); and at 4 years, 9.7% (45% due to the increase in gradient, 55% due to the increase in AR). All cases of stage 3 HVD were due to increased gradient. Structural BVF was attributed to 1.9% of all cases, and a non-structural cause of BVF was seen in 1.4% of cases.

Central illustration. Summary of the study population and main findings. A) Device description. B) Rates of aortic regurgitation at follow-up. C) Study population. D) Aortic mean gradient and aortic valve area at follow-up. E) Clinical and haemodynamic outcomes at 4 years. BVF: bioprosthetic valve failure; HVD: haemodynamic valve deterioration

Figure 2. Haemodynamic valve deterioration and patient-prosthesis mismatch at follow-up. A) Prevalence of stage 2 and stage 3 haemodynamic valve deterioration (HVD) over 4 years. B) Rates of moderate and severe patient-prosthesis mismatch at 30 days and 1 year.
Discussion
A summary of the durability reported for the main current balloon-expandable and self-expanding devices is presented in Table 49101112131415. To our knowledge, this study represents one of the largest midterm follow-up analyses of the Myval prosthesis, with a median follow-up of nearly 4 years and a robust dataset of echocardiographic assessments. In our cohort of 366 patients, we observed a 4-year survival rate of 81.8%, comparable to similar high-risk populations16. This survival rate reflects the advanced age and significant comorbid burden of our study group, including high rates of coronary artery disease and chronic kidney disease.
The durability of the Myval prosthesis appears promising, with favourable haemodynamic performance sustained over the follow-up period. At 4 years, the rates of stage 2 and 3 HVD were 9.7% and 0.7%, respectively. This performance at 4-year follow-up is in line with contemporary devices and is likely related to an adequate prosthesis design and the anticalcification process of the bovine pericardium leaflets. The study found the valve had low susceptibility to endocarditis, a complication that can significantly impair valve durability1718. Clinically overt leaflet thrombosis was rare in our cohort, occurring in only 0.5% of patients. However, no comment can be made on hypoattenuated leaflet thickening (HALT) as systematic computed tomography was not performed at follow-up. These findings highlight the importance of maintaining appropriate antithrombotic therapy post-TAVI to further enhance valve durability.
The rate of BVF criterion 1, which includes symptomatic valve dysfunction, was 3.3% at 4 years. No cases of BVF criterion 2 (requiring reintervention) or BVF criterion 3 (valve-related death) were reported, underscoring the valve’s structural integrity. Additionally, procedural success was high, with technical failure occurring in only 3% of patients, largely due to rare complications such as valve embolisation and aortic dissection which likely did not differ to the complications reported for alternative contemporary alternatives, as highlighted by LANDMARK4.
Balloon pre- or post-dilatation, a strategy to optimise valve seating and minimise PPM, was required in only 7.9% of cases. Severe PPM was noted in 2% of patients at 30 days and in 2.1% at 1 year, rates that compare favourably to other intra-annular TAVI devices19.
The rates of mortality, stroke, and valve-related rehospitalisation at 1 year and 4 years align with recent findings from the ACURATE IDE trial, where comparable rates for these endpoints were observed with other contemporary TAVI devices20.
The Myval valve’s design has been previously associated with optimised transvalvular gradients and effective orifice areas421, which might be a determinant factor in the sustained haemodynamic performance reported herein. Our study found mean transvalvular gradients and aortic valve areas at 4 years that were comparable to those with other balloon-expandable and self-expanding devices, emphasising its competitive haemodynamic profile. However, direct comparisons with other studies should be approached with caution due to differences in patient populations and the criteria used to define structural valve deterioration and BVF. Importantly, this is one of the first durability studies that used VARC-3 criteria. A similar reflection can be made regarding the rate of moderate or severe PVL; although in our research, it was proportionally greater than in other low-risk studies such as SMART or PARTNER 3, from our perspective one of the main reasons is the treatment of extra-large annuli (indeed for the 32 mm valve, the rate of PVL of moderate or severe degree was 25% at 4 years). This kind of anatomy is usually excluded in other trials and might be the baseline anatomical condition explaining the higher rate of PVL rather than device-related technical aspects, since Myval seems to perform comparably to other devices according to LANDMARK (where the extra-large sizes were also excluded). Regarding the new Myval iterations, the Octacor and Octapro devices (Meril Life Sciences), a technical modification in the skirt might help to mitigate the degree of PVL, but this has not been explored yet.
Long-term data from surgical bioprostheses show rates of 10-year freedom from valvular failure rates of between 60% and 95%222324. However, these studies often include younger, lower-risk patients who may not be directly comparable to TAVI cohorts. Interestingly, recent analyses show no significant difference in outcomes between TAVI and surgical aortic valve replacement after 5 years, supporting the expanding role of TAVI in diverse patient populations2526.
Our study, the largest focused solely on midterm Myval outcomes, highlights stable valve performance at 4 years, with acceptable rates of regurgitation, HVD, and PPM.
Table 4. TAVI durability data.
| Study | Number of patients | Definition of BVD/BVF | TAVI device | Stage 2-3 SVD-related BVD | All-cause BVF | Duration of follow-up |
|---|---|---|---|---|---|---|
| PARTNER II-S3i Pibarot et al92020 | 1,665 | VARC-3 | SAPIEN XTaSAPIEN 3aSAVR | 9.5%3.9%3.5% | 4.7%2.6%1.3% | 5 years |
| Ferreira-Neto et al102020 | 212 | VARC-3 | TAVI-SAPIENa/ SAPIEN XT | 30.2% | 8 years | |
| Rheude et al11 2020 | 691 | VARC-2 | SAPIEN 3 | 10.3% | 1.88% | 1 year |
| UK TAVI Ali et al122023 | 221 | EAPCI/EACTS | TAVI-SAPIEN/ SAPIEN XTTAVI-COREVALVEb | 22.4%9.8% | 7 years | |
| PARTNER 3 Mack et al132023 | 280(low-risk patients) | VARC-3 | SAPIEN 3aSAVR | 4.2%3.8% | 3.3%3.8% | 5 years |
| NOTION Thyregod et al142024 | 280(low-risk patients) | VARC-3 | TAVI-COREVALVESAVR | 12.5%13.9% | 9.7%13.8% | 10 years |
| Rück et al15 2024 | 433 | VARC-3 | ACURATE neoc | 2.2% | 1.4% | 39 months |
| aBy Edwards Lifesciences; bby Medtronic; cby Boston Scientific. BVD: bioprosthetic valve dysfunction; BVF: bioprosthetic valve failure; EACTS: European Association for Cardio-Thoracic Surgery; EAPCI: European Association of Percutaneous Cardiovascular Interventions; SAVR: surgical aortic valve replacement; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation; VARC: Valve Academic Research Consortium | ||||||
Limitations
This study has several limitations. As a non-randomised, observational analysis, its findings may be influenced by selection bias. Additionally, despite our central analysis of echocardiographic images at follow-up, the exams did not follow a standardised protocol, and operators varied widely. However, the inclusion of consecutive patients and the rigorous review of health records enhance data reliability. The symptomatic evaluation at follow-up is subjective, as it was reported by the patient and the clinician; this may have affected the rates of BVF.
Conclusions
Four-year follow-up in real-world patients undergoing TAVI with the Myval prosthesis demonstrates acceptable durability, with results comparable to contemporary balloon-expandable and self-expanding devices. Further studies with extended follow-up and multimodal imaging are warranted to confirm these findings and explore predictors of valve deterioration.
Impact on daily practice
So far, data regarding the midterm prevalence of haemodynamic structural valve deterioration after balloon-expandable transcatheter aortic valve implantation (TAVI) remain scarce. Sustained haemodynamic performance without significant aortic regurgitation contributes to the overall 4-year durability of the Myval transcatheter heart valve, as it minimises the risk of complications that could require reintervention. Thus, favourable midterm durability data could help to extend the indication of TAVI to a younger population.
Conflict of interest statement
I.J. Amat-Santos is a proctor for Meril Life Sciences; the institution received an unrestricted grant for the LANDMARK trial. M. Montorfano received consultant fees from Abbott, Boston Scientific, AliveCor, and Medtronic. G. Sengottuvelu is a proctor for Meril Life Sciences and Boston Scientific; and has received honoraria from Meril Life Sciences and Boston Scientific. A. Seth is a proctor for Meril Life Sciences, Boston Scientific, and Sentinel Devices; Principal Investigator of the MyVal-1 study; and has received honoraria for speaker’s bureau from Meril Life Sciences and Boston Scientific. A. Jain received consultant fees from Meril Life Sciences. The other authors have no conflict of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

