Cory:
Unlock Your AI Assistant Now!
Subendocardial and transmural perfusion improve after aortic valve replacement in aortic stenosis. Accordingly, in this issue of EuroIntervention, the publication by Gallinoro et al1 on longitudinal coronary physiology before and after transcatheter aortic valve implantation (TAVI) raises a critical point regarding the quantification of absolute myocardial perfusion. As summarised in Figure 1, we argue that the algorithm for determining absolute myocardial mass from computed tomography (CT) imaging needs validation and currently appears to be inaccurate.
As clearly stated by the developers of invasive continuous thermodilution: “Absolute flow, expressed in mL/min is meaningless because the myocardial distribution to be perfused is unknown and varies widely between different arteries and different subjects”2. Two possible normalisations exist. First, flow during stress conditions can be expressed as a unitless multiple of resting conditions, i.e., coronary flow reserve (CFR). Second, absolute flow (mL/min) can be divided by the amount of distal myocardial mass (g) to calculate perfusion (mL/min/g).
Cardiac positron emission tomography (PET) or magnetic resonance imaging (CMR) intrinsically quantify myocardial perfusion due to their data acquisition and flow models. Other techniques, such as invasive continuous thermodilution, require an external and separate measurement of myocardial mass to transform flow into perfusion, as done in this paper1.
While epicardial blood flow using continuous thermodilution has been extensively validated in mL/min, downstream myocardial mass using the Voronoi algorithm has only been compared against relative mass (percentage of the left ventricle, not absolute grams of tissue)3. Consequently, what might be called “hybrid” perfusion quantification (invasive absolute flow in an epicardial vessel plus non-invasive myocardial mass of its supplied territory) has never been verified to produce valid results.
To our knowledge, only two publications of just over 90 patients report absolute myocardial perfusion in mL/min/g using the hybrid technique combining invasive continuous thermodilution for absolute flow with CT imaging for distal myocardial mass14. But, do the results appear believable?
Among 15 normal controls and 25 patients with mild atherosclerosis, hyperaemic perfusion reached approximately 5 mL/min/g, as detailed in Figure 14. This rate of perfusion substantially exceeds the average stress value of 3 mL/min/g noted in almost 550 normal subjects and 30 publications using cardiac PET5. Moreover, in the current paper, 51 subjects without prior myocardial infarction undergoing TAVI had a baseline perfusion rate of 0.3-0.4 mL/min/g1 that implausibly equals the levels in proven transmural infarcts as demonstrated by microspheres, PET, and CMR6.
Therefore, the <100 published patients who underwent the hybrid perfusion technique do not agree with long-standing results from the wide, multimodal literature. Inaccurate measurement of myocardial mass likely explains this disagreement, which is also noted by the authors who describe “tr[ying] to solve an apparent divergence (likely due to [left ventricular] concentric hypertrophy driven by [aortic stenosis]) that was found between CT- and echocardiography-derived myocardial masses” (further details can be found in their Supplementary data)1.
Even with this key caveat regarding hybrid perfusion, the current manuscript1 contributes to a growing literature regarding how aortic stenosis and its treatment impact coronary physiology7. They studied 51 patients immediately before and after TAVI, as well as 20 patients who returned after 6 months for repeat assessment. Resting flow did not change acutely (68 vs 69 mL/min; p=0.78), whereas hyperaemic flow increased, albeit not significantly (159 vs 172 mL/min; p=0.45), translating into non-significantly higher CFR (2.38 vs 2.47; p=0.88) and lower fractional flow reserve (FFR; 0.84 vs 0.83; p=0.34). During the next half year, the total myocardial mass decreased as expected after successful TAVI (208 vs 164 g; p<0.001), with the same trends in greater hyperaemic flow (significant when accounting for regression of left ventricular hypertrophy) and non-significantly higher CFR and lower FFR. The direction of these changes agrees with the prior literature, albeit with a smaller magnitude of effect7.
The current manuscript1 has two limitations that require subsequent attention. First, the findings of discordant perfusion results1456, divergent estimates of myocardial mass1, and lack of validation for the Voronoi algorithm to calculate absolute myocardial mass3 indicate that the “hybrid perfusion” technique is not yet ready for scientific or clinical application. Second, pooling all manuscripts regarding coronary physiology in aortic stenosis into a single analysis would help mitigate the risk of underpowered, smaller studies, as they are currently scattered in the literature7.
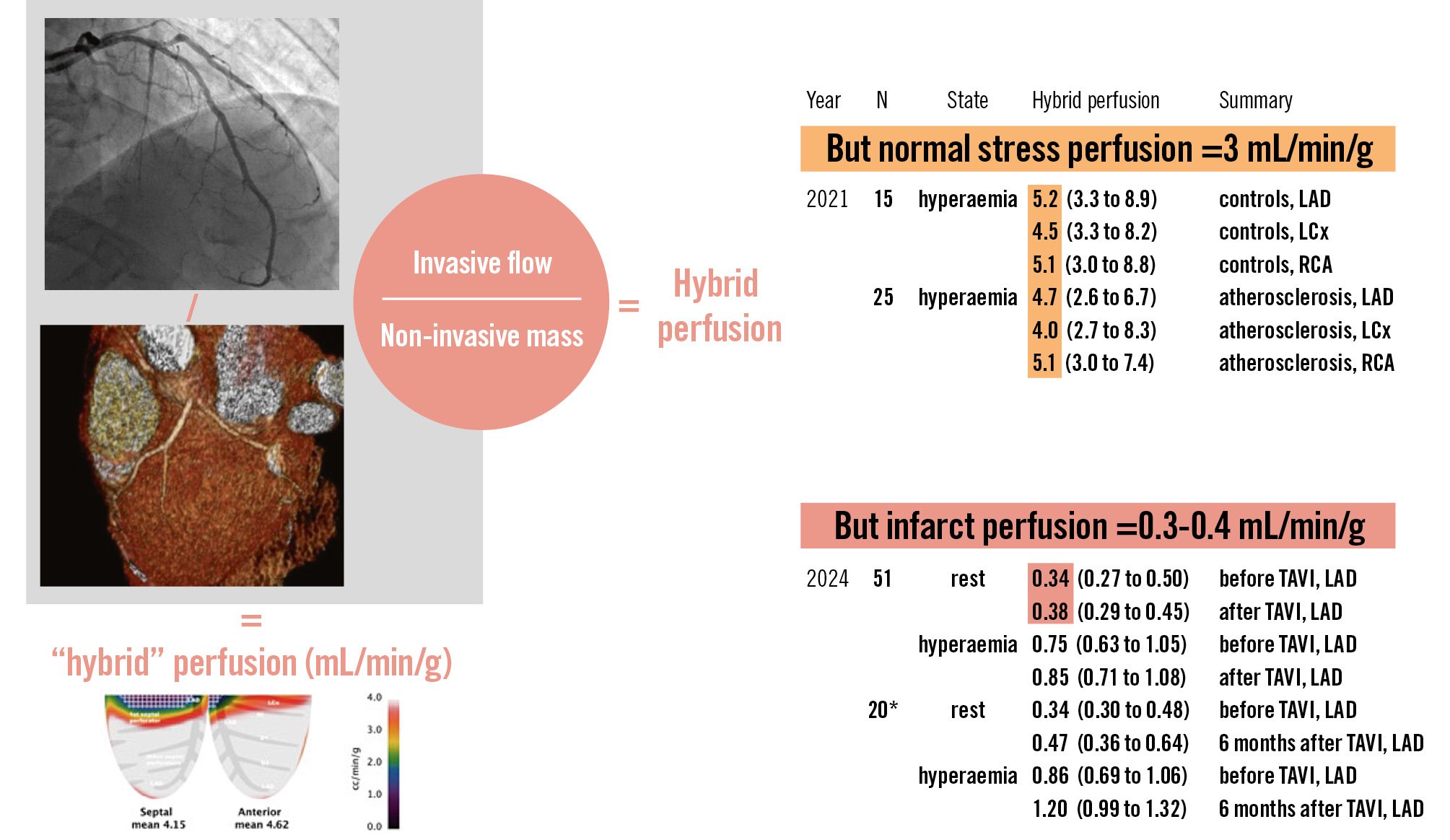
Figure 1. Hybrid perfusion quantification. Combining absolute epicardial flow (mL/min) from invasive continuous thermodilution with absolute downstream myocardial mass (g) from non-invasive imaging yields “hybrid” perfusion quantification (mL/min/g). However, published results14 disagree with a broad existing literature56. *20-subject subset of the larger 51 subjects studied acutely. CMR: cardiac magnetic resonance imaging; CT: computed tomography; LAD: left anterior descending artery; LCx: left circumflex artery; PET: positron emission tomography imaging; RCA: right coronary artery; TAVI: transcatheter aortic valve implantation
Conflict of interest statement
N.P. Johnson and K.L. Gould report no direct relationships, but outside of the present work, they receive internal funding from the Weatherhead PET Imaging Center; and have patents pending on diagnostic methods for quantifying aortic stenosis and TAVI physiology, and on methods to correct pressure tracings from fluid-filled catheters. N.P. Johnson receives significant institutional research support from Shockwave Medical (PET core lab for COSIRA-II, ClinicalTrials.gov: NCT05102019); and lectures frequently for academia and industry, but donates any honoraria to his institution. K.L. Gould is the 510(k) applicant for several cardiac PET software packages approved by the U.S. FDA (K113754, K143664, K171303, K202679, K231731) but does not receive any licensing fees paid to UTHealth by Bracco Diagnostics and GE HealthCare.

