Cory:
Unlock Your AI Assistant Now!
Abstract
Background: There is a lack of evidence to guide treatment of patients with a concomitant indication for transcatheter aortic valve implantation (TAVI) and complex, high-risk percutaneous coronary intervention (PCI).
Aims: We aimed to assess different strategies of PCI timing in this high-risk TAVI cohort.
Methods: The ASCoP registry retrospectively included patients with a clinical indication for both TAVI and PCI with at least 1 criterion of complex or high-risk PCI. The primary endpoint was a composite of all-cause death and unplanned rehospitalisation for cardiovascular causes. The secondary endpoint was a composite of all-cause death, stroke, acute myocardial infarction, major bleeding, major vascular complication and unplanned revascularisation. Multivariable analysis was used to adjust for possible confounders.
Results: A total of 519 patients were included: 363 (69.9%) underwent staged procedures and 156 (30.1%) concomitant TAVI and PCI. After 441 (interquartile range 182-824) days, the primary endpoint occurred in 151 (36.5%) cases, without any significant difference between the 2 groups (p=0.98), while the secondary endpoint occurred more frequently in the concomitant group (n=36 [25.8%] vs n=57 [17.4%]; p=0.014).
Conclusions: In patients undergoing TAVI and complex/high-risk PCI, a concomitant strategy is associated with a higher rate of adverse events and increased procedural risk. (ClinicalTrials.gov: NCT05750927)
Transcatheter aortic valve implantation (TAVI) is recommended by international guidelines to treat severe, symptomatic aortic stenosis (AS)12, regardless of procedural risk, and its use is predicted to increase as the global population ages. Concomitant coronary artery disease (CAD) is common3 and a predictor of worse outcome4, and recent evidence suggests that treatment of CAD can be staged after TAVI in selected cases5. Nonetheless, there is a lack of evidence regarding patients that require complex percutaneous coronary intervention (PCI), as they are often excluded from clinical trials and underrepresented in retrospective registries. High-risk PCI risk can affect the TAVI procedure, as ongoing coronary ischaemia might compromise an optimal TAVI outcome. On the other hand, ongoing severe aortic stenosis increases the procedural risk during high-risk PCI, and TAVI placement introduces further procedural challenges. The Aortic Stenosis with COmplex PCI (ASCoP) registry is an observational, multicentre, investigator-initiated study, designed to collect data of patients with severe AS scheduled for TAVI and PCI of coronary artery lesions with complex and/or high-risk clinically indicated PCI (ClinicalTrials.gov: NCT05750927).
Methods
Study design and objectives
All consecutive patients that underwent complex PCI before or after TAVI between January 2013 and June 2023 at 14 centres in Europe and the United States were evaluated for inclusion in the registry. Data concerning patients’ baseline clinical and instrumental characteristics, procedural information, and clinical follow-up status were retrospectively collected by each participating centre using a shared electronic database. The inclusion of patients in the study was approved at each institution by a local ethics committee or per local practice for the collection of retrospective data.
The aims of this study were (i) to describe the prevalence and clinical features of patients with severe AS undergoing TAVI and concomitant clinically indicated, complex and/or high-risk PCI; (ii) to depict the different possible strategies that are currently employed in this context and to retrospectively analyse outcomes; and (iii) to investigate predictors of better outcomes.
Of note, the study also tried to capture differences in the time management of TAVI and complex, high-risk PCI.
Patient selection and inclusion criteria
To be included in the ASCoP registry, patients were required to have severe symptomatic aortic stenosis with an indication for transfemoral TAVI according to international guidelines12 and concomitant CAD with a clinical indication for complex and/or high-risk PCI, defined as including one or more of the following features67:
a) left main (LM) CAD or proximal left anterior descending/left circumflex lesions requiring LM PCI;
b) three-vessel disease PCI;
c) PCI of a last remaining vessel;
d) bifurcation PCI (defined as “true bifurcation” including any proximal branch);
e) severely calcified lesions requiring calcium debulking;
f) lesion length ≥30 mm;
g) severely depressed left ventricular ejection function (≤30%);
h) any condition requiring mechanical circulatory support during PCI.
Indication for PCI was given by each operator based on angiographic, haemodynamic or anatomical characteristics (obtained by intravascular imaging techniques) of coronary lesions, following the recommendations of current international guidelines on acute and chronic coronary syndromes78; patients were excluded if emergent/urgent revascularisation was deemed necessary (e.g., in the setting of ST-elevation myocardial infarction [MI]), if the time between PCI and TAVI was greater than 6 months, or in cases of valve-in-valve TAVI.
Patients were stratified into two groups: if they were scheduled to undergo TAVI and PCI in the same procedure or in separate staged procedures. The decision on patient treatment and on valve model was solely clinical and at the physician’s discretion.
Study endpoints
The primary endpoint of the present study was the composite adverse event rate of all-cause death and first unplanned hospitalisation for cardiovascular causes. The secondary endpoint was a composite of major adverse cardiac and cerebrovascular events (MACCE), including all-cause death, stroke, acute MI, major bleeding, major vascular complication and unplanned revascularisation. The occurrence of both endpoints was evaluated at discharge and at 1 year after the index procedure, with the latter defined as the first intervention performed (PCI and/or TAVI). Moreover, a landmark analysis was performed, considering events occurring in the first 30 days and from 30 days to 1 year after the index procedure, to exclude a possible excess of early vascular complications in patients undergoing staged procedures with TAVI first (compared to those undergoing PCI first).
We also investigated the occurrence of each component of the two study endpoints at 1 year and the rate of in-hospital events (all-cause death, bleeding, vascular complications, stroke or transient ischaemic attack [TIA], and acute kidney injury). For patients who underwent staged procedures during two different hospitalisations, in-hospital events occurring during both procedures were considered. All events were categorised according to the Valve Academic Research Consortium-3, Bleeding Academic Research Consortium and Academic Research Consortium-2 consensuses91011.
Statistical analysis
Results are presented as mean±standard deviation or as median (25th-75th percentiles) for continuous variables and as absolute numbers (percentages) for categorical data; all variables were tested for normality with the Shapiro-Wilk normality test and reported accordingly. One-way analysis of variance and the Student’s unpaired t-test were used to compare normally distributed continuous variables; for non-normally distributed continuous variables, the Kruskal-Wallis test was used. The Chi-square and Fisher’s exact tests were used to compare categorical variables. Event rates of primary and secondary composite endpoints were estimated using the Kaplan-Meier method; comparisons between study groups were performed using the log-rank test. To account for possible confounders in baseline characteristics, clinical, procedural and anatomical characteristics were tested by means of univariate analysis with Cox proportional hazards regression. All variables with a p-value<0.10 and those considered clinically relevant (e.g., established risk factors) were inserted in a multivariable Cox regression model to assess the hazard ratio (HR) and 95% confidence interval (CI) for the relationship between predictors and the primary and secondary endpoints. The rate of missing values was below 20% for variables included in the final model; the convention of limiting the number of independent variables to 1 for every 10 events was followed. The final model was the result of a stepwise (both backward and forward) regression with the minimal Akaike information criterion. Goodness of fit of the Cox regression model was assessed using the Cox-Snell residual test. To address potential additional sources of bias, two sets of sensitivity analyses were conducted. First, considering the small number of patients undergoing PCI after TAVI, a sensitivity analysis excluding this group was performed to ascertain the role of a possible selection bias. Second, the E-value was calculated, which is the minimum strength of association that an unmeasured confounder would need to explain away the treatment-outcome association12. The statistical analysis, the Kaplan-Meier mortality curves, and the graphs were performed with the use of Stata, version 14 (StataCorp), and Prism software, version 6 (GraphPad Software).
Results
Patients
Between January 2013 and May 2023, 18,333 patients underwent TAVI at 14 international centres (Central illustration, Supplementary Table 1). Of them, 519 (2.83%) had concomitant severe aortic stenosis and an indication for complex or high-risk PCI; temporal trends are shown in Supplementary Figure 1. Of these 519 patients, 156 (30.1%) underwent both TAVI and PCI in the same procedure, while 363 (69.9%) underwent staged procedures. In both groups, PCI was more commonly performed before TAVI (n=135, 86.7% of those undergoing concomitant procedures; n=333, 91.7% of those undergoing staged procedures). In the staged group, the median time between TAVI and PCI was 10.5 (interquartile range [IQR] 3-55) days, and 150 (41.3%) subjects underwent both procedures in the same hospital stay. The baseline characteristics of our population are reported in Table 1.
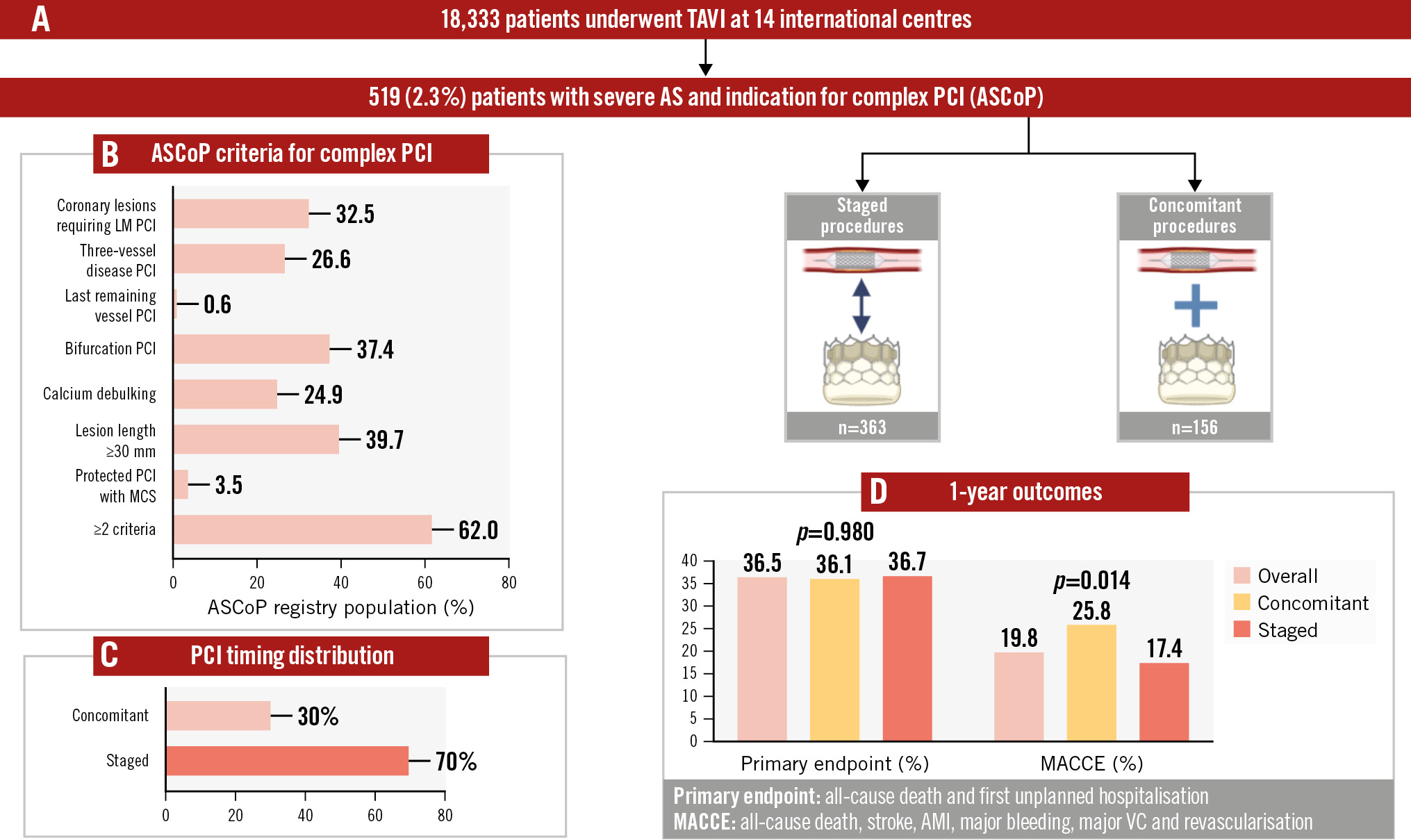
Central illustration. The Aortic Stenosis with COmplex PCI (ASCoP) registry. A) Flowchart illustrating the study; (B) the population in terms of ASCoP criteria for complex PCI; (C) PCI timing distribution; and (D) the main results at 1 year. AMI: acute myocardial infarction; LM: left main; MACCE: major adverse cardiac and cerebrovascular events; MCS: mechanical circulatory support; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation; VC: vascular complication
Table 1. Baseline characteristics.
| Entête ajoutée | ||||
|---|---|---|---|---|
| Overall (n=519) | Concomitant (n=156) | Staged (n=363) | p-value | |
| Age, years | 81.5 (77.5-85.2) | 82.4 (78.6-85.4) | 81.3 (77.4-85.1) | 0.260 |
| Male sex | 327 (63) | 86 (55.1) | 241 (66.4) | 0.015* |
| BMI, kg/m2 | 26.5 (23.7-29.7) | 26.6 (23.7-30.1) | 26.4 (23.7-29.4) | 0.432 |
| Hypertension | 449 (86.5) | 136 (87.2) | 313 (86.2) | 0.771 |
| Diabetes | 161 (31) | 43 (27.5) | 118 (32.5) | 0.264 |
| Smoking history | 199 (38.3) | 59 (37.4) | 140 (38.6) | 0.811 |
| Family history of CAD | 50 (9.5) | 19 (12) | 31 (8.5) | 0.283 |
| CKD | 209 (40.3) | 60 (38.5) | 149 (41.2) | 0.566 |
| AF | 125 (24.1) | 28 (18) | 97 (26.7) | 0.035* |
| PAD | 122 (23.5) | 39 (25) | 83 (22.9) | 0.599 |
| Previous PPM implantation | 43 (8.3) | 13 (8.3) | 30 (8.3) | 0.979 |
| Previous MI | 140 (27) | 44 (28.2) | 96 (26.5) | 0.679 |
| Previous PCI | 252 (48.5) | 45 (28.5) | 207 (57) | <0.001* |
| Previous CABG | 47 (9) | 13 (8.3) | 34 (9.4) | 0.707 |
| Previous stroke | 63 (12.2) | 14 (9) | 49 (13.5) | 0.174 |
| NYHA Class >II | 279 (54.2) | 85 (55) | 194 (53.5) | 0.785 |
| CCS class >2 | 111 (21.4) | 31 (19.5) | 80 (22.2) | 0.559 |
| Syncope | 40 (7.9) | 8 (5.2) | 32 (8.8) | 0.223 |
| EuroSCORE II, % | 4.4 (2.4-9.7) | 6.2 (3.2-12.4) | 3.8 (2.3-7.4) | <0.001* |
| STS score, % | 3.9 (2.4-6.2) | 4 (2.4-5.8) | 3.8 (2.5-6.2) | 0.806 |
| Medical therapy at admission | ||||
| Beta blockers | 264 (50.8) | 87 (55.6) | 177 (48.7) | 0.155 |
| SAPT | 188 (36.2) | 56 (35.9) | 132 (36.4) | 0.115 |
| DAPT | 140 (27) | 19 (12.2) | 121 (33.3) | <0.001* |
| OAC-based | 129 (24.8) | 35 (22.4) | 94 (25.9) | 0.538 |
| LVEF, % | 55 (45-60) | 56 (49-62) | 55 (45-60) | 0.245 |
| LVEF <35% | 75 (14.5) | 18 (11.8) | 57 (15.7) | 0.249 |
| Mean gradient, mmHg | 42 (33-50) | 40 (33-49) | 42 (33-50) | 0.434 |
| AVA, cm2 | 0.75 (0.6-0.9) | 0.8 (0.65-0.9) | 0.7 (0.6-0.9) | 0.256 |
| Moderate-severe AR | 83 (16) | 28 (18) | 55 (15) | 0.408 |
| Creatinine, mg/dl | 1 (0.9-1.3) | 1.1 (0.9-1.3) | 1 (0.9-1.3) | 0.612 |
| Haemoglobin, g/dl | 12.5 (11-13.4) | 12.5 (11.1-13.4) | 12.4 (11-13.5) | 0.817 |
| Platelets x 109/L | 208 (165-248) | 212 (167-235) | 207.5 (164.5-255.5) | 0.542 |
| Data are presented as n (%) or median (25th-75th percentiles). *Indicates statistical significance. AF: atrial fibrillation; AR: aortic regurgitation; AVA: aortic valve area; BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CCS: Canadian Cardiovascular Society; CKD: chronic kidney disease; DAPT: dual antiplatelet therapy; EuroSCORE: European System for Cardiac Operative Risk Evaluation; LVEF: left ventricular ejection fraction; MI: myocardial infarction; NYHA: New York Heart Association; OAC: oral anticoagulant; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; PPM: permanent pacemaker; SAPT: single antiplatelet therapy; STS: Society of Thoracic Surgeons | ||||
Procedural characteristics
Criteria to define complex/high-risk PCI are shown in Table 2; over 60% of patients presented with more than one feature of procedural complexity/risk. Of note, 32.5% of patients underwent a left main PCI, 37.4% a bifurcation PCI, and 39.7% underwent extensive stenting (≥30 mm), while 24.9% needed a calcium debulking strategy, most commonly rotational atherectomy (n=73; 56.6%). From a coronary anatomy standpoint, 886 lesions were treated in total (Supplementary Table 2). Of them, 102 lesions were “true” bifurcation (Medina class 1,1,1; 1,0,1; and 0,1,1), and 669 were American College of Cardiology/American Heart Association (ACC/AHA) B or C lesions. The median length of implanted drug-eluting stent was 46 (IQR 28-66) mm, and intracoronary imaging was used in 31.4% of cases, most commonly intravascular ultrasound (n=153; 93.8%). Only 79 (15.2%) patients presented with an acute coronary syndrome. Device-based haemodynamic support was deemed necessary in 3.5% of cases, with Impella (Abiomed) being most commonly used (n=16; 88.8%). Our population represents a wide variety of bioprosthetic valve types (Table 2), including 159 (30.7%) of the SAPIEN family (Edwards Lifesciences), 168 (32.4%) from the CoreValve/Evolut series (Medtronic) and 106 (20.4%) ACURATE neo/neo2 (Boston Scientific). Supra-annular platforms were used more frequently in the concomitant procedure group, while the intra-annular ones were more frequently used in the staged group. Need of predilation was common (46.8% of all cases).
Table 2. Procedural characteristics.
| Entête ajoutée | ||||
|---|---|---|---|---|
| Overall (n=519) | Concomitant (n=156) | Staged (n=363) | p-value | |
| PCI procedure | ||||
| ASCoP indication | ||||
| Left main PCI | 169 (32.5) | 49 (31.4) | 120 (33) | 0.713 |
| Three-vessel PCI | 138 (26.6) | 33 (21.1) | 105 (28.9) | 0.066 |
| Last remaining vessel | 3 (0.6) | 1 (0.6) | 2 (0.6) | 0.907 |
| Bifurcation PCI | 194 (37.4) | 32 (20.5) | 162 (44.6) | <0.001* |
| Calcium debulking | 129 (24.9) | 31 (19.9) | 98 (27) | 0.085 |
| Lesion length ≥30 mm | 206 (39.7) | 53 (34) | 153 (42.1) | 0.081 |
| Need of haemodynamic support# | 18 (3.5) | 5 (3.2) | 13 (3.6) | 0.830 |
| ≥2 characteristics | 322 (62) | 85 (54.5) | 237 (65.3) | 0.020* |
| Radial access | 293 (56.6) | 41 (25.7) | 252 (69.4) | <0.001* |
| PCI target vessel | ||||
| Left main | 169 (32.5) | 49 (31.4) | 120 (33) | 0.713 |
| Left anterior descending | 256 (49.3) | 58 (37.2) | 198 (54.5) | <0.001* |
| Left circumflex | 130 (25) | 39 (25) | 91 (25) | 0.987 |
| Right coronary artery | 188 (36.2) | 54 (34.6) | 134 (36.9) | 0.617 |
| Bypass graft conduit | 2 (0.4) | 1 (0.6) | 1 (0.3) | 0.538 |
| Multivessel PCI | 123 (23.7) | 24 (15.4) | 99 (27.3) | 0.003* |
| DES, n | 2 (1-3) | 2 (1-2) | 2 (1-3) | <0.001* |
| DES length, mm | 46 (28-66) | 38 (22-60) | 48 (33-66) | 0.002* |
| Intracoronary imaging | 163 (31.4) | 31 (19.9) | 132 (36.4) | <0.001* |
| IVUS | 153 (29.5) | 30 (19.2) | 123 (33.9) | 0.001* |
| OCT | 10 (1.9) | 1 (0.6) | 9 (2.5) | 0.162 |
| Calcium debulking | 129 (24.9) | 31 (19.9) | 98 (27) | 0.085 |
| Rotational atherectomy | 73 (14) | 17 (10.9) | 56 (15.4) | 0.174 |
| Orbital atherectomy | 6 (1.2) | 4 (2.6) | 2 (0.6) | 0.049* |
| Intravascular lithotripsy | 19 (3.7) | 8 (5.1) | 11 (3) | 0.243 |
| Scoring/cutting/OPNa balloon | 36 (6.9) | 3 (1.9) | 33 (9.1) | 0.003* |
| External mechanical circulatory support | 18 (3.5) | 5 (3.2) | 13 (3.6) | 0.830 |
| Impellab | 16 (3.1) | 5 (3.2) | 11 (3) | 0.916 |
| ECMO | 1 (0.2) | 1 (0.6) | 0 (0) | 0.127 |
| IABP | 1 (0.2) | 0 (0) | 1 (0.3) | 0.512 |
| TandemHeartc | 1 (0.2) | 0 (0) | 1 (0.3) | 0.512 |
| Clinical presentation | 0.206 | |||
| CCS | 440 (84.8) | 137 (87.8) | 303 (83.5) | |
| ACS | 79 (15.2) | 19 (12.2) | 60 (16.5) | |
| TAVI procedure | ||||
| TAVI model | 0.004* | |||
| S3/S3 Ultra/S XTd | 159 (30.7) | 51 (32.7) | 108 (29.7) | |
| Evolut R/PRO/PRO+/FXe | 148 (28.5) | 45 (28.8) | 103 (28.4) | |
| CoreValvee | 20 (3.9) | 6 (3.9) | 14 (3.9) | |
| Porticof | 45 (8.7) | 6 (3.9) | 39 (10.7) | |
| Navitorf | 19 (3.6) | 1 (0.6) | 18 (4.9) | |
| ACURATE neo/neo2g | 106 (20.4) | 42 (26.9) | 64 (17.6) | |
| Lotusg | 9 (1.7) | 0 (0) | 9 (2.5) | |
| Others | 13 (2.5) | 5 (3.2) | 8 (2.2) | |
| Supra-annular | 285 (54.9) | 98 (62.8) | 187 (51.5) | 0.018* |
| Intra-annular | 234 (45.1) | 58 (37.2) | 176 (48.5) | 0.018* |
| Self-expanding | 350 (67.4) | 105 (67.3) | 245 (67.5) | 0.968 |
| Balloon-expandable | 159 (30.6) | 51 (32.7) | 108 (29.8) | 0.505 |
| Valve-in-valve | 12 (2.3) | 6 (3.9) | 6 (1.6) | 0.127 |
| Predilation | 243 (46.8) | 73 (46.8) | 170 (46.8) | 0.997 |
| Post-dilation | 124 (24) | 31 (20.1) | 93 (25.7) | 0.345 |
| Data are presented as n (%) or median (25th-75th percentiles). *Indicates statistical significance. #ECMO and Impella. aBy SIS Medical; bby Abiomed; cby LivaNova; dby Edwards Lifesciences; eby Medtronic; fby Abbott; gby Boston Scientific. ACS: acute coronary syndrome; ASCoP: Aortic Stenosis with COmplex PCI; CCS: chronic coronary syndrome; DES: drug-eluting stent; ECMO: extracorporeal membrane oxygenation; IABP: intra-aortic balloon pump; IVUS: intravascular ultrasound; OCT: optical coherence tomography; PCI: percutaneous coronary intervention; S: SAPIEN; TAVI: transcatheter aortic valve implantation | ||||
In-hospital events
Out of our cohort, 513 (98.8%) patients were discharged alive; medical therapy at discharge is shown in Supplementary Table 3. In-hospital events included 36 (6.9%) acute kidney injuries, 60 (11.6%) vascular complications and 53 (10.2%) bleeding events. Of note, major vascular (4.5% vs 1.9%) and major bleeding (10.9% vs 3.9%) complications were higher in the concomitant procedure group than in the staged procedure group (Table 3). Bailout surgery was not needed in any case. Technical success9 was achieved in 95.2% of our cohort.
Table 3. Events at follow-up.
| Entête ajoutée | ||||
|---|---|---|---|---|
| Overall (n=519) | Concomitant (n=156) | Staged (n=363) | p-valueb | |
| VARC-3 endpoints | ||||
| Technical success | 494 (95.2) | 147 (94.2) | 347 (95.6) | 0.507 |
| Device success | 388 (74.8) | 114 (73) | 274 (75.5) | 0.563 |
| Early safety | 290 (55.9) | 81 (51.9) | 209 (57.6) | 0.234 |
| In-hospital events | ||||
| Death | 6 (1.2) | 2 (1.3) | 4 (1.1) | 0.860 |
| Acute kidney injury | 36 (6.9) | 12 (7.7) | 24 (6.6) | 0.657 |
| Vascular complications | 60 (11.6) | 26 (16.7) | 34 (9.4) | 0.017* |
| Minor | 46 (8.9) | 19 (12.2) | 27 (7.4) | 0.081 |
| Major | 14 (2.7) | 7 (4.5) | 7 (1.9) | 0.099 |
| Bleeding | 53 (10.2) | 21 (13.5) | 32 (8.8) | 0.109 |
| Minor | 22 (4.2) | 4 (2.6) | 18 (5) | 0.214 |
| Major | 31 (6) | 17 (10.9) | 14 (3.9) | 0.002* |
| Stroke | 9 (1.7) | 4 (2.6) | 5 (1.4) | 0.342 |
| TIA | 4 (0.8) | 0 (0) | 4 (1.1) | 0.188 |
| 1-year follow-upa | ||||
| Primary endpoint | 151 (36.5) | 41 (36.1) | 110 (36.7) | 0.980 |
| MACCE | 93 (19.8) | 36 (25.8) | 57 (17.4) | 0.014* |
| All-cause death | 37 (8.8) | 10 (8.8) | 27 (8.8) | 0.960 |
| CV death | 15 (3.6) | 3 (2.9) | 12 (3.9) | 0.523 |
| All-cause rehospitalisation | 129 (32) | 34 (30.7) | 95 (32.5) | 0.923 |
| CV rehospitalisation | 68 (18.1) | 15 (14.6) | 53 (19.3) | 0.370 |
| Stroke | 14 (2.9) | 7 (5) | 7 (2.1) | 0.134 |
| Major bleedings | 33 (6.5) | 18 (11.8) | 15 (4.4) | 0.001* |
| Major vascular complications | 16 (3.4) | 8 (5.7) | 8 (2.4) | 0.046* |
| Myocardial infarction | 12 (2.9) | 5 (4.9) | 7 (2.2) | 0.162 |
| Repeat PCI | 13 (3.2) | 5 (4.3) | 8 (2.8) | 0.356 |
| Data are presented as n (%). *Indicates statistical significance. a1-year follow-up: events are reported as absolute numbers (Kaplan-Meier estimate rates); bp-values from the log-rank test. CV: cardiovascular; MACCE: major adverse cardiac and cerebrovascular events; PCI: percutaneous coronary intervention; TIA: transient ischaemic attack; VARC: Valve Academic Research Consortium | ||||
Follow-up
The median follow-up time after the index procedure was 441 (IQR 182-824) days. At 1-year follow-up, 37 patients had died, with a similar incidence of events in the concomitant and staged procedure groups (8.8% vs 8.8%; log-rank p=0.960) (Table 3). The primary endpoint occurred in 151 (36.5%) cases, without any significant difference in our group of interest (36.1% vs 36.7%; log-rank p=0.980) (Figure 1A). The MACCE endpoint occurred in 93 (19.8%) cases, including 36 (25.8%) in the concomitant procedure group and 57 (17.4%) in the staged procedure group (log-rank p=0.014) (Figure 1B).
The landmark analysis showed a similar rate of incidence of the primary endpoint between the concomitant and staged groups both from 0 to 30 days (3.7% vs 3.7%; log-rank p=0.994) and from 31 to 365 days (32.4% vs 33.3%; log-rank p=0.963) (Supplementary Table 4, Figure 2A). Occurrence of MACCE was significantly higher in the first 30 days in the concomitant group (15.8% vs 6.3%; log-rank p<0.001), mainly due to the higher rate of major vascular and bleeding complications, with no difference considering the follow-up period (11.1% vs 11.9%; log-rank p=0.801) (Supplementary Table 4, Figure 2B) .
Univariate predictors for both our endpoints of interest are presented in Supplementary Table 5. After adjusting for baseline characteristics and known predictors of adverse outcome, independent predictors of our primary endpoint were platelet count (HR 1.02, 95% CI: 1.01-1.05; p=0.037) and left ventricular ejection fraction (HR 0.98, 95% CI: 0.97-0.99; p=0.034), while the timing of the procedure did not independently influence this endpoint (HR 0.90, 95% CI: 0.55-1.45; p=0.672) (Table 4). On the contrary, when the MACCE endpoint was analysed, a significantly higher number of events was observed with a concomitant strategy, independent of other covariates (HR 1.85, 95% CI: 1.09-3.14; p=0.021). Other independent predictors of MACCE were creatinine (HR 1.27, 95% CI: 1.08-1.52; p=0.005) and platelet count (HR 1.03, 95% CI: 1.01-1.06; p=0.029).
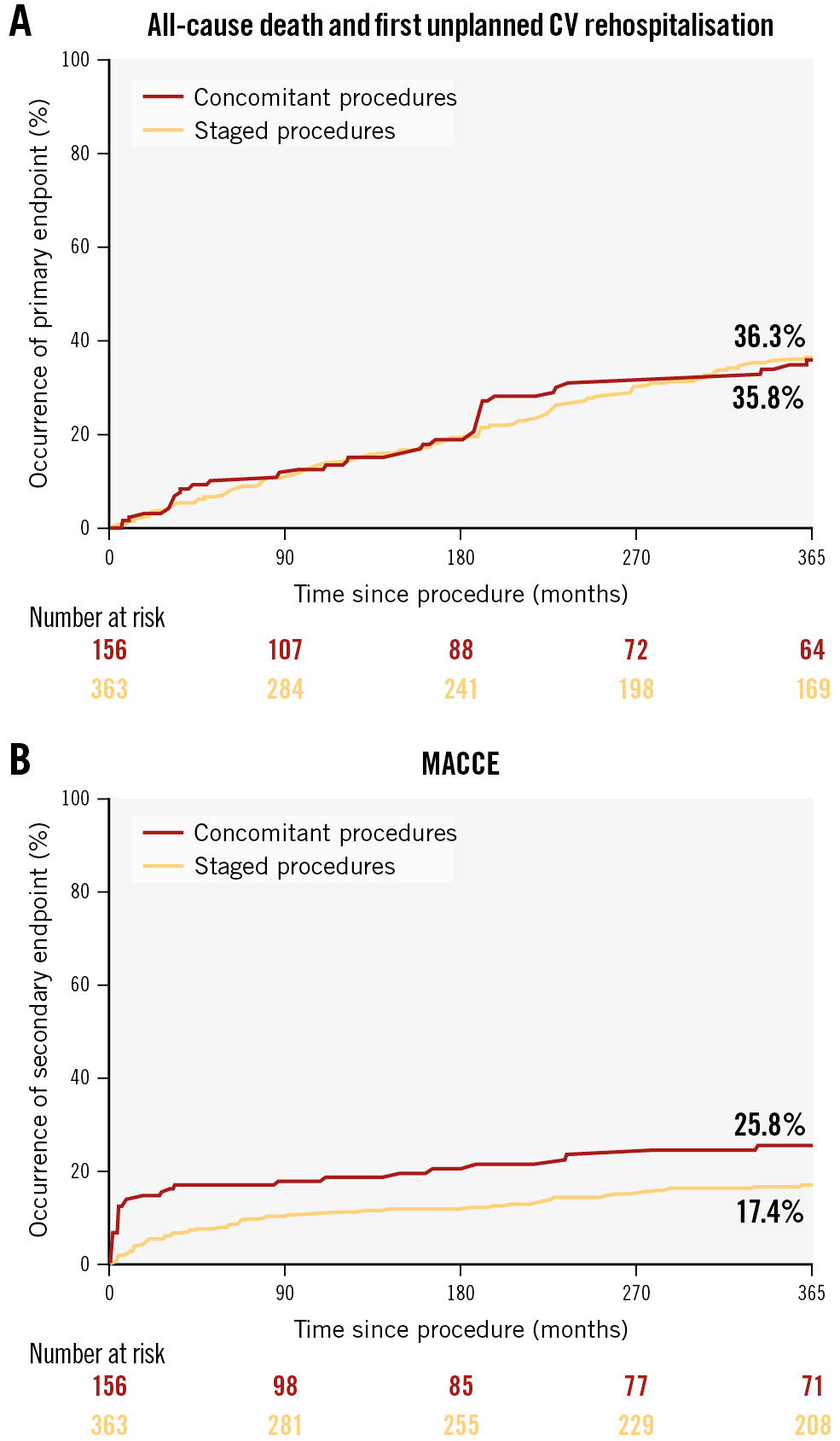
Figure 1. Survival curves for concomitant versus staged TAVI and PCI in ASCoP patients. The primary endpoint (A) is a composite of all-cause death and first unplanned cardiovascular rehospitalisation; the secondary endpoint (B) is a composite of all-cause death, stroke, acute myocardial infarction, major bleeding, major vascular complication and unplanned revascularisation. ASCoP: Aortic Stenosis with Complex PCI; CV: cardiovascular; MACCE: major adverse cardiac and cerebrovascular events; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation
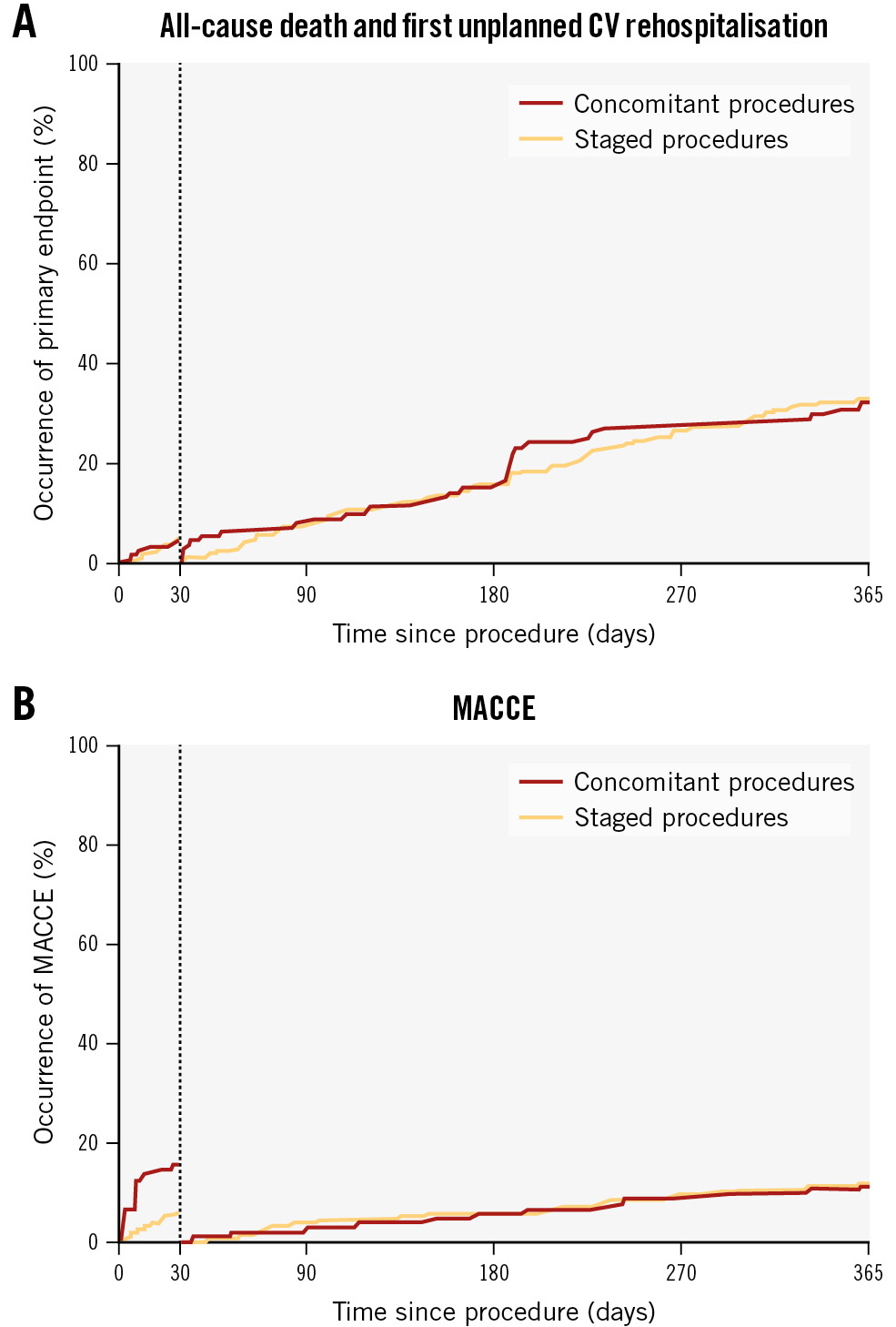
Figure 2. Landmark analysis for the primary and secondary endpoints in the 2-way analysis. A) Primary endpoint analysis; (B) MACCE endpoint analysis. CV: cardiovascular; MACCE: major adverse cardiac and cerebrovascular events
Table 4. Multivariable Cox regression.
| Entête ajoutée | |||
|---|---|---|---|
| HR | 95% CI | p-value | |
| Primary endpoint | |||
| Age, years | 0.99 | 0.96-1.02 | 0.540 |
| Male | 1.10 | 0.69-1.76 | 0.677 |
| NYHA Class >II | 1.15 | 0.77-1.75 | 0.487 |
| Creatinine, mg/dl | 1.16 | 0.99-1.36 | 0.052 |
| Platelets x 109/L | 1.02 | 1.01-1.05 | 0.037 |
| LVEF, % | 0.98 | 0.97-0.99 | 0.034 |
| TR ≥2 | 1.55 | 0.87-2.75 | 0.131 |
| Multivessel PCI | 0.66 | 0.40-1.09 | 0.110 |
| LM PCI | 0.77 | 0.49-1.22 | 0.273 |
| Lesion length ≥30 mm | 1.16 | 0.75-1.81 | 0.495 |
| Concomitant procedures | 0.90 | 0.55-1.45 | 0.672 |
| Secondary endpoint | |||
| Age, years | 0.97 | 0.94-1.01 | 0.168 |
| Male | 0.90 | 0.54-1.52 | 0.703 |
| Diabetes | 0.58 | 0.32-1.06 | 0.078 |
| Creatinine, mg/dl | 1.27 | 1.08-1.52 | 0.005 |
| Platelets x 109/L | 1.03 | 1.01-1.06 | 0.029 |
| Need of haemodynamic support | 1.63 | 0.39-6.74 | 0.501 |
| Concomitant procedures | 1.85 | 1.09-3.14 | 0.021 |
| CAD: coronary artery disease; CI: confidence interval; HR: hazard ratio; LM: left main; LVEF: left ventricular ejection fraction; NYHA: New York Heart Association Class; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation; TR: tricuspid regurgitation | |||
Sensitivity analysis
A small proportion (n=30, 5.7%) of our patients were treated with PCI after TAVI. A sensitivity analysis excluding this group is shown in Supplementary Table 6, Supplementary Table 7, Supplementary Figure 2, and Supplementary Figure 3. In brief, no change in the occurrence of adverse events was observed when excluding this cohort from the analysis.
E-value analysis revealed that an unmeasured confounder should have an association with both treatment allocation and MACCE by an HR of at least 2.439 (95% CI: 1.337) to explain away our findings, but weaker confounders could not do so. The E-value for our primary endpoint was 1.357 (CI: 1.00).
Discussion
The main results of our study are as follows:
1. In high-volume centres, the occurrence of coexisting severe aortic stenosis and CAD with high-risk/complex features is rare (2.83%) but has shown an increasing trend over the years.
2. Concomitant TAVI and high-risk PCI is associated with an overall high rate of adverse events, especially in the in-hospital phase.
To the best of our knowledge, the ASCoP registry is the largest report dedicated to the management of TAVI with concomitant CAD and high-risk/complex PCI features, including 14 large-volume centres and 18,333 TAVI procedures screened for inclusion. Despite an increasing scientific interest in recent years, the management of concomitant CAD and TAVI is still a matter of debate, and, as a result, international guidelines give recommendations with only a lower level of evidence. In particular, the European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) guidelines1 give a Class IIa, Level of Evidence (LoE) C recommendation for PCI only in cases of severe stenosis (>70%) affecting the proximal coronary vessel, while the ACC/AHA guidelines2 give a Class 2a, LoE C (limited data) for PCI of the LM or proximal segment before TAVI. Notably, only the ACC/AHA document expresses a recommendation regarding the optimal timing of PCI, stating that it should be performed pre-TAVI in order to minimise procedural risk and diminish PCI procedural complexity. However, there is also an argument for staging PCI after TAVI, as performing PCI in the setting of ongoing, severe AS can augment procedural risk. This holds especially true in cases of complex/high-risk CAD, which is notably associated with increased procedural risk even in the absence of severe AS6. It should further be noted that the widespread adoption of the commissural alignment technique during valve implantation13 and the availability of newer-generation valve models that allow easier coronary cannulation after TAVI have increased the feasibility of PCI post-TAVI over the years, along with the overall rate of cannulation post-TAVI.
To this end, while no difference was detected between the different treatment strategies (concomitant vs PCI before TAVI vs PCI after TAVI) in an older registry14, the more recent REVASC-TAVI study5 showed better outcomes for patients treated with staged PCI after TAVI. Nonetheless, both these registries featured all-comers PCI patients with an overall lower risk compared to our registry. For comparison, the REVASC-TAVI registry featured only 11.4% LM PCI (vs 32.5% in our cohort), use of debulking in 4.9% of cases (vs 24.9% in our cohort) and a median total stent length of 23.0 mm (vs 46.0 mm in our cohort). The ACTIVATION and NOTION-3 trials featured even lower-risk CAD patients (LM PCI in 3.8% and 0% of cases, respectively; median lesion length=17.4 mm in the ACTIVATION trial; mean number of stents implanted=1 in NOTION-3), at least partially as per exclusion criteria1516. Therefore, we believe that our data are informative of a truly neglected cohort of high-risk subjects that is currently investigated only by case reports and a smaller registry1718, despite representing up to 2.8% of TAVI subjects in high-volume centres. Moreover, in our registry we observed an increasing trend over the years, with ASCoP patients representing up to 3.99% of patients treated in the years 2021-2022 (vs 1.48% in 2013-2014) (Supplementary Figure 1). This likely reflects a trend of wider adoption of TAVI, including patients with concomitant CAD who would traditionally be referred for surgery, and of increased confidence of operators in treating ever more complex scenarios. This further reinforces the importance of having some level of evidence to orient clinical decision-making, and our data, albeit observational, represent a first cornerstone.
From a clinical standpoint, performing TAVI and complex/high-risk PCI in the same procedure has a rationale to minimise procedural risk. In fact, complex/high-risk PCI and TAVI can be performed from the same large-bore arterial access, and bailout balloon-aortic valvuloplasty could be performed if needed. Moreover, especially during complex PCI cases, ongoing full platelet inhibition is preferable to minimise periprocedural ischaemic risk. This can be achieved with intravenous P2Y12 agents, such as cangrelor, to be started only once the large-bore access is safely in place19. On the other hand, staged procedures make it possible to address the clinically most relevant conditions first, albeit with a theoretically increased risk.
Our data suggest that the latter strategy is most commonly used (69.9% of cases) and is associated with a similar occurrence of all-cause death and unplanned hospitalisation (36.7% vs 36.1%; p=0.98) compared to concomitant TAVI and PCI, and with a lower occurrence of MACCE (17.4% vs 25.8%; p=0.014). Most of the adverse events in the concomitant group occurred in the first days after the index procedure, with a significantly higher number of in-hospital vascular complications (16.7% vs 9.4%; p=0.017) and major bleedings (10.9% vs 3.9%; p=0.02), while events at 1-year follow-up, including MI and revascularisation, were similar (Table 3, Supplementary Table 3). It should be noted that most of the patients in the staged group were treated with PCI before TAVI (91.7%), and our sensitivity analysis excluding the small proportion of patients treated with PCI after TAVI confirmed these results (Supplementary Table 5, Supplementary Figure 2, Supplementary Figure 3). Therefore, the staged group should be interpreted as PCI before TAVI and, grossly, the comparison should be interpreted as a comparison of a concomitant strategy versus PCI before TAVI. In this light, intrahospital data should be interpreted with caution: when PCI is the index procedure, it is reasonable that vascular and bleeding complications, even in cases of complex/high-risk PCI, are lower than after TAVI. Nonetheless, the occurrence of major vascular (5.7% vs 2.4%; p=0.046) and bleeding (11.8% vs 4.4%; p=0.001) complications is significantly higher in the concomitant strategy group at 1 year, which is only partly explained by the higher baseline procedural risk (European System for Cardiac Operative Risk Evaluation [EuroSCORE] II: 6.2% vs 3.8%; p<0.001) since we observed similar rates of in-hospital (1.3% vs 1.1%) and 1-year mortality (both 8.8%). In summary, a concomitant strategy appears to be associated with an overall increased procedural risk; these data seem to reject the arguments in favour of concomitant TAVI and complex/high-risk PCI.
Even when a staged procedure is preferred, it is not straightforward to decide whether TAVI or complex/high-risk PCI should be carried out first. It could be reasonable to address the clinically most relevant condition first (e.g., CAD in cases admitted for an acute coronary syndrome or severe AS if exertional dyspnoea is most prominent), but in clinical practice, it is often difficult to distinguish a clear clinical culprit. Moreover, periprocedural higher ischaemic risk is to be expected if TAVI, which can include a period of rapid pacing and transitory hypotension, is performed with ongoing severe/high-risk CAD. In the context of complex/high-risk PCI, when the need for increased support is anticipated, it is even more important to plan TAVI in order to minimise the risk of difficult coronary cannulation. This holds especially true in such complex and advanced patients as those included in our registry, where severe AS and complex CAD often intermingle. Our registry showed that only a small minority of patients underwent staged PCI after TAVI (5.7%), which is a similar finding to that of another observational registry in this area5. It is reasonable that one of the main reasons for this finding is the anticipated difficulty of coronary cannulation after TAVI20 that could augment procedural complexity and/or mandate femoral access (vs radial), which is associated with worse outcomes after PCI21. Scientific interest in this area has increased significantly over the years, and the advancement of newer valve generations has proven that easy coronary reaccess can be achieved if planned beforehand. This is of particular importance considering that the REVASC-TAVI registry showed that staged PCI post-TAVI is associated with better outcomes5. In summary, we believe that operators should not fear per se to defer complex/high-risk PCI after TAVI, but the need for coronary access should be systematically and carefully assessed during preprocedural planning, valve models that allow for easy coronary access should be preferred, and commissural alignment techniques should be used13. Nonetheless, it should be noted that, even when commissural alignment is systematically employed, it appears that the Evolut platform is associated more commonly with unsuccessful coronary cannulation post-TAVI22. Although this finding could be challenged by the newest iteration of the valve model23, it should prompt tailored decision-making regarding the timing of complex/high-risk PCI. Moreover, considering significant, ongoing technological improvements in this area, it is reasonable to expect that this strategy will grow in use over the years. In spite of this, the small number of patients treated with deferred complex/high-risk PCI in our registry does not allow definite conclusions to be drawn in this respect from our study.
Results from ongoing trials in the field of PCI in patients undergoing TAVI will shed light on the prognostic impact of PCI (COMPLETE TAVR; ClinicalTrials.gov: NCT04634240). Nonetheless, observational data are encouraged in this area, as it is unlikely that randomised controlled trials will investigate such high-risk scenarios in the near future, and our observational study is important to collect evidence in this understudied and rare population.
Limitations
There are several limitations to our study. First, our study is retrospective and, therefore, has all the usual limitations associated with its design. In particular, we recognise a potential selection bias towards the operators’ preference over procedural timing, and this limits the generalisability of our findings. A sensitivity analysis with computation of the E-value was performed to confirm the robustness of our results, showing that only a strong (HR >2.439) unmeasured predictor of adverse events could neutralise these findings, which is unlikely as known major predictors of death were accounted for. Second, a relatively small number of patients were treated with staged PCI post-TAVI; this could carry an inclusion bias as intrinsically higher PCI complexity could influence the operators’ preference. However, a sensitivity analysis was performed by excluding this group from the analysis, and it revealed no significant bias. Third, since patients had to undergo both TAVI and PCI to be included in the registry, an underestimation of early fatal events is possible. Fourth, despite all those involved being high-volume centres, the long timeframe of observation (2013-2023) might include disparate learning curves, changing clinical practice and evolving technologies. In particular, earlier valve generations, as well as less experience overall, could account for a possibly higher rate of complications in earlier cohorts. This could account for an overestimation of events compared to contemporary cohorts.
Conclusions
Patients with severe AS and CAD with a clinical indication for TAVI and complex/high-risk PCI are rare but have an increasing prevalence in large-volume centres. In this context, a concomitant strategy was associated with a higher rate of adverse events and increased procedural risk.
Impact on daily practice
In patients undergoing transcatheter aortic valve implantation (TAVI), the occurrence of coexisting severe aortic stenosis and coronary artery disease with high-risk/complex features is rare (2.83%) but has demonstrated an increasing trend over the years. Performing high-risk percutaneous coronary intervention (PCI) concomitantly to TAVI is associated with an increased risk of in-hospital vascular and bleeding complications. In conclusion, in this subset of high-risk patients, a strategy of staged PCI before TAVI appears associated with the best outcomes.
Acknowledgments
In loving memory of Maria Franco (Bari, 1937-2023).
Conflict of interest statement
K. Arslani has received research grants from the Swiss Academy of Medical Sciences, the Gottfried and Julia Bangerter-Rhyner-Foundation, and the Swiss National Science Foundation (P500PM_202963), Switzerland, outside the submitted work. The other authors have no conflicts of interest relevant to the present work to declare.
Supplementary data
To read the full content of this article, please download the PDF.

