Cory:
Unlock Your AI Assistant Now!
High-risk percutaneous coronary intervention (PCI) is increasingly performed because of an ageing population with a high incidence of comorbidities and high surgical risk scores1. Veno-arterial extracorporeal membrane oxygenation (VA-ECMO) is an effective method to prevent haemodynamic deterioration during high-risk PCI. VA-ECMO is mainly surgically deployed under general anaesthesia. New developments have facilitated a completely percutaneous insertion of VA-ECMO with local anaesthesia, reducing operating team sizes and enabling early mobilisation after PCI without the need for intensive care unit (ICU) admission.
This study is a single-centre registry that included all patients undergoing high-risk PCI with VA-ECMO support between January 2020 and March 2024 at the Radboud University Medical Center in Nijmegen, the Netherlands. The study design has been previously described in detail2. Preprocedural angiographic computed tomography (CT) scans were performed in all cases to assess peripheral access. The procedural set-up for VA-ECMO (de)cannulation was similar for all cases (Supplementary Figure 1, Moving image 1).
Procedural success was defined as successful revascularisation (final residual stenosis <50% with a Thrombolysis in Myocardial Infarction flow grade 3, achieved in at least one target vessel) without the occurrence of periprocedural myocardial infarction (MI) or death. Major adverse cardiac events (MACE) were defined as a composite endpoint of all-cause death, MI, target vessel revascularisation or clinical coronary bleeding requiring covered stent deployment or surgical treatment. The study endpoints were in-hospital MACE and MACE at 90 days, assessed after discharge. VA-ECMO-related access or access site complications were assessed using the Valve Academic Research Consortium (VARC)-2 consensus document, classifying major and minor complications.
Elective percutaneous VA-ECMO-assisted high-risk PCI, indicated according to expert consensus on the use of mechanical circulatory support (MCS) devices for high-risk PCI3, was performed in 35 patients. The mean age of the population was 71.4±8.7 years, and 28 (80.0%) patients were male. Previous PCI and coronary artery bypass graft (CABG) had been performed in 8 (22.9%) and 3 (8.6%) patients, respectively. Peripheral artery vessel disease was present in 34.3% of the population. Three-vessel coronary artery disease and left main stenosis were present in 23 (65.7%) and 22 (62.9%) patients, respectively. The mean left ventricular ejection fraction was 28.5±10.9%, and complexity indices were high, including the Society of Thoracic Surgeons mortality score: 3.6 (interquartile range [IQR] 1.8-5.8), SYNTAX score I: 31.8±9.3, SYNTAX score II (PCI): 54.6±10.7 and SYNTAX score II (CABG): 41.8±11.4. Two (5.7%) patients were admitted to the ICU before the PCI procedure. Baseline and procedural characteristics are described in Supplementary Table 1 and Supplementary Table 2.
VA-ECMO cannulation was performed in the femoral artery in 30 (85.7%) patients. Local anaesthesia with preprocedural oral benzodiazepine was the preferred choice of anaesthesia (85.7%). Closure was predominantly performed with a percutaneous closure device (97.1%), mostly a suture-based closure device (Perclose ProGlide System [Abbott]), which was generally sufficient after using two Proglides (77.4%). VA-ECMO-related access or access site complications occurred in 6 (17.1%) patients. All were minor VARC-2 complications that occurred after using suture-based closure devices (Supplementary Table 3).
Procedural success was achieved in all patients. Four (11.4%) patients were admitted to the ICU after PCI. The median time until discharge was 1 (IQR 1-3) day. In-hospital MACE occurred in 2 (5.7%) patients. MACE occurred in 5 patients (17.2%) within 90 days after discharge (Central illustration).
Our study investigated whether VA-ECMO-assisted high-risk PCI procedures could be performed completely percutaneously and with local anaesthesia, and, as a result, with a small care team, admitting patients to the cardiac care unit for recovery afterwards and mobilising them within 6 hours after PCI. To date, this concept has not been thoroughly evaluated. The present study results are excellent and comparable to other studies4. Almost 90% of the procedures were performed with only local anaesthesia, without the presence of an anaesthesiologist. Procedures were predominantly performed with percutaneous deployment of VA-ECMO and closure with the Perclose ProGlide System. No cardiothoracic surgeon was present in the catheterisation laboratory. Outcomes of percutaneous closure using the ProGlide in this study were comparable to percutaneous closure as well as surgical closure in a previous study5. Almost all patients were admitted to the cardiac care unit afterwards and discharged to their referring hospital or home shortly after the PCI procedure. This study − investigating the largest population using completely percutaneous VA-ECMO with local anaesthesia − demonstrates the safety and feasibility of this simplified use of VA-ECMO during high-risk PCI. It provides new options for hospitals regarding the use of VA-ECMO. Future research should focus on studies comparing this concept with surgical techniques with respect to clinical outcomes.
Due to the retrospective and observational design of this study, some procedural and ECMO-related data are missing. This was also a highly selected population, increasing the risk of selection bias. Furthermore, no control group was included in the study. It is, therefore, not possible to make definite conclusions about a preferable treatment.
Completely percutaneous VA-ECMO-assisted high-risk PCI with local anaesthesia is a novel concept. Our results are excellent with respect to successful revascularisation and adverse events, subsequently, providing a suitable alternative to the standard surgical use of VA-ECMO with general anaesthesia.
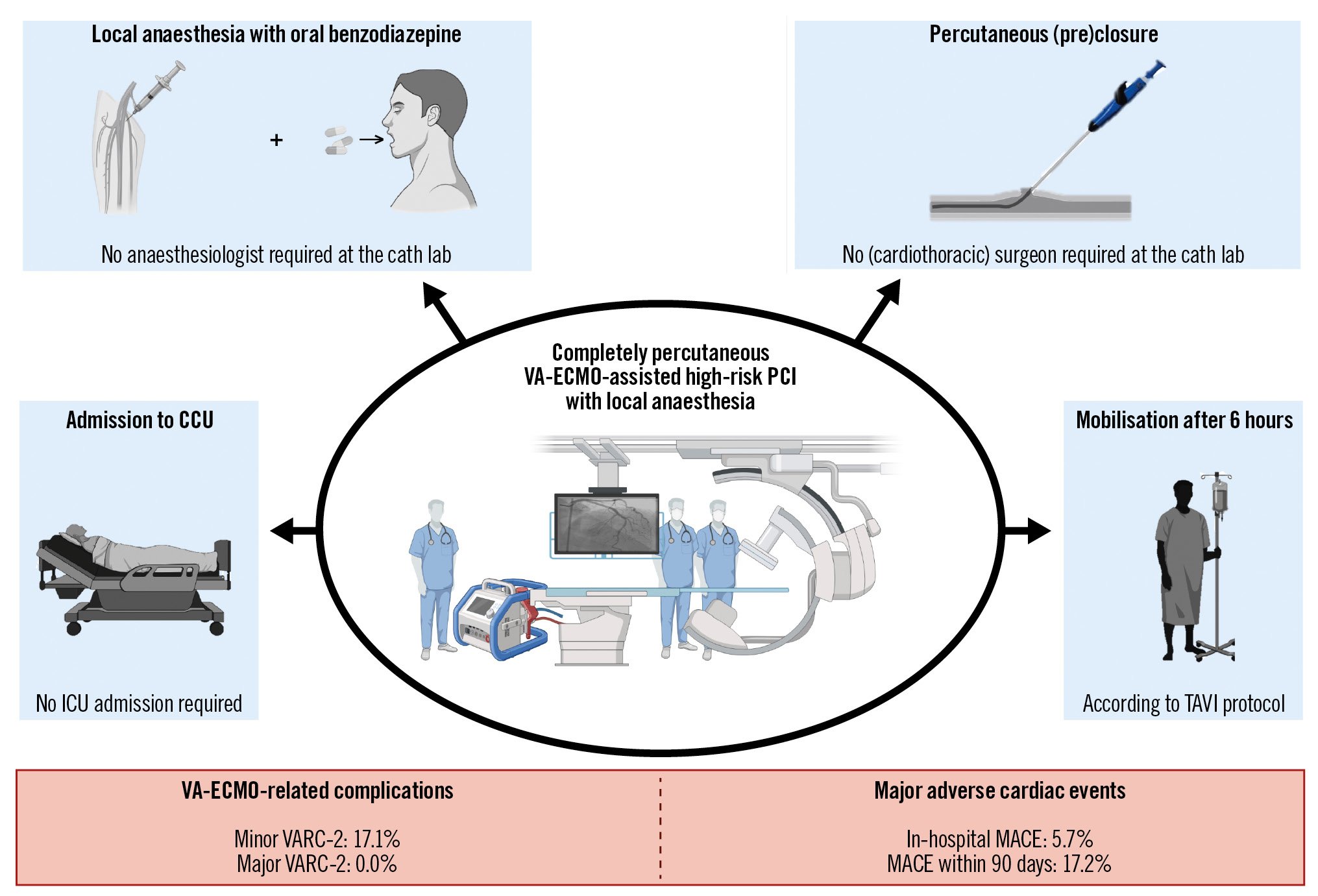
Central illustration. Key features of completely percutaneous VA-ECMO-assisted high-risk PCI with local anaesthesia along with adverse outcomes. The key features are illustrated in the blue boxes, and adverse outcomes are detailed in the red boxes. CCU: cardiac care unit; ICU: intensive care unit; MACE: major adverse cardiac events; PCI: percutaneous coronary intervention; TAVI: transcatheter aortic valve implantation; VA-ECMO: veno-arterial extracorporeal membrane oxygenation; VARC: Valve Academic Research Consortium
Conflict of interest statement
P. Damman reported grants, speaker fees and consultancy fees from Philips; grants and speaker fees from Abbott; and grants from AstraZeneca. N. van Royen reported grants and speaker fees from Abbott; and grants from Medtronic, Biotronik, and Philips. R.J.M. van Geuns reported grants and personal fees from Boston Scientific, Abbott, AstraZeneca, and Amgen; and grants from InfraRedx. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Procedural set-up for VA-ECMO (de) cannulation.

