Abstract
Background: Recent trials have shown that intravascular imaging (IVI)-guided percutaneous coronary intervention (PCI) improves clinical outcome, as compared to angiography-guided PCI, in complex coronary artery lesions. However, it is unclear whether this benefit is affected by overall lesion complexity in each patient.
Aims: The present study sought to investigate the impact of overall lesion complexity on the benefit of IVI-guided PCI.
Methods: A total of 4,611 patients with complex coronary artery lesions from the RENOVATE-COMPLEX-PCI trial (n=1,639) and the institutional registry of the Samsung Medical Center (n=2,972) were classified according to the number of complex lesion features found in each patient. The primary outcome was target vessel failure (TVF) at 3 years, a composite of cardiac death, target vessel myocardial infarction, or target vessel revascularisation.
Results: The cutoff value for the number of complex lesion features to predict TVF, determined using the maximally selected log-rank test, was 3. Patients with ≥3 complex lesion features had a higher risk of TVF than those with <3 complex lesion features (11.0% vs 7.2%, hazard ratio [HR] 1.59, 95% confidence interval [CI]: 1.28-1.96; p<0.001). IVI-guided PCI significantly reduced the risk of TVF compared with angiography-guided PCI in both groups (≥3 complex lesion features: 7.4% vs 14.4%, HR 0.49, 95% CI: 0.35-0.69; p<0.001; <3 complex lesion features: 5.7% vs 8.1%, HR 0.72, 95% CI: 0.53-0.98; p=0.039). The benefit of IVI-guided PCI tended to increase as the number of complex lesion features increased (absolute risk reduction for TVF: –0.012 vs –0.027 vs –0.055 vs –0.077, respectively, for 1 vs 2 vs 3 vs ≥4 complex lesion features; interaction p=0.048).
Conclusions: In patients with complex coronary artery lesions, IVI-guided PCI showed a lower risk of TVF across all degrees of lesion complexity. The prognostic benefit of IVI-guided PCI tended to increase as patients had more complex lesion features. (RENOVATE-COMPLEX-PCI [ClinicalTrials.gov: NCT03381872]; Institutional cardiovascular catheterisation database of the Samsung Medical Center [ClinicalTrials.gov: NCT03870815]).
Intravascular imaging (IVI), such as intravascular ultrasound (IVUS) or optical coherence tomography (OCT), is a useful tool for the assessment of lesion characteristics, preprocedural planning, and postprocedural optimisation of stented coronary artery segments12. Multiple randomised controlled trials (RCTs)34567 and large-scale registries8910 have demonstrated that IVI-guided percutaneous coronary intervention (PCI) is associated with better clinical outcomes compared with PCI guided by angiography alone. This would be particularly true for complex coronary artery lesions, for which the visual assessment of lesion severity, plaque morphology, and stented segment by angiography has clear limitations12. Indeed, recent RCTs have confirmed a significant benefit of IVI-guided PCI in patients with various types of complex coronary artery lesions regarding clinical outcomes34567 as well as stent expansion11.
Most RCTs included patients with one or more qualifying complex lesion features, such as unprotected left main, bifurcation lesion, long lesion, chronic total occlusion (CTO), or severely calcified lesion. However, not all patients included in those RCTs had a similar degree of overall lesion complexity. For example, some patients could have had one long lesion in the mid-to-distal segment of the target vessel, but others could have had a severely calcified left main bifurcation lesion carrying a higher risk of adverse outcomes after PCI12. Indeed, over 30% of patients in the RENOVATE-COMPLEX-PCI trial had 3 or more qualifying complex lesion features3. One can infer that the more complex the lesions that patients have, the more benefits IVI can provide regarding clinical outcome after PCI.
In this regard, the current study sought to evaluate the prognostic benefits of IVI-guided PCI compared to angiography-guided PCI according to overall lesion complexity in each patient.
Methods
Study population and data collection
The current study was a patient-level pooled analysis that combined two separate cohorts. The first dataset came from the institutional registry of the Samsung Medical Center, which enrolled patients undergoing PCI for coronary artery disease from February 2008 to December 2015. Previous reports were published using part of this institutional registry813. The second dataset came from the RENOVATE-COMPLEX-PCI trial, a prospective, multicentre, randomised controlled trial enrolling patients with complex coronary artery lesions from May 2018 to May 20213.
To match the institutional registry datasets to that of RENOVATE-COMPLEX-PCI, patients with non-complex coronary artery lesions, those treated with balloon angioplasty alone, or those who had undergone stent implantation with either bare metal stents or 1st-generation drug-eluting stents were excluded from the institutional registry. The exclusion criteria that were applied to both datasets were as follows: coronary lesions that were not amenable for performing PCI according to the operators’ discretion; cardiogenic shock (Killip class IV) at presentation; known hypersensitivity or contraindication to aspirin, clopidogrel, prasugrel, ticagrelor, heparin, everolimus, or contrast media; and patients who were pregnant or breastfeeding. As a result, a total of 4,611 patients (2,972 patients from the institutional registry and 1,639 patients from the RENOVATE-COMPLEX-PCI trial) who had complex coronary artery lesions and had undergone IVI-guided or angiography-guided PCI with 2nd-generation drug-eluting stents were included in this study (Figure 1).
This study was approved by the institutional review board of the Samsung Medical Center. For patients in the institutional registry, informed consent was waived by the institutional review board of the Samsung Medical Center. Patients from the RENOVATE-COMPLEX-PCI trial provided informed consent prior to enrolment. Both studies were registered at ClinicalTrials.gov (RENOVATE-COMPLEX-PCI: NCT03381872; Institutional cardiovascular catheterisation database of Samsung Medical Center: NCT03870815). The present study was conducted according to the principles of the Declaration of Helsinki.
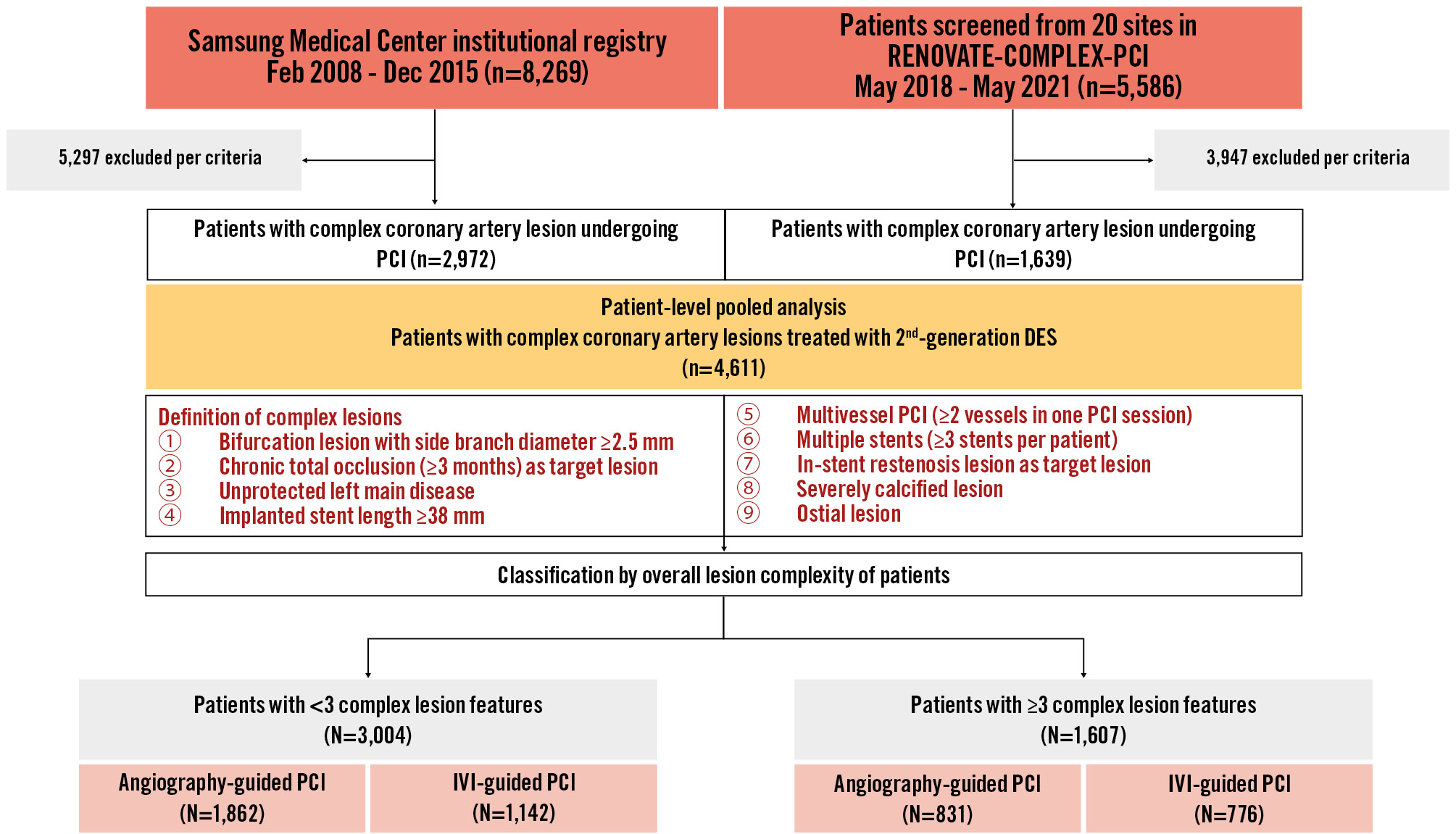
Figure 1. Study flowchart. A total of 4,611 patients (2,972 patients from the Samsung Medical Center institutional registry and 1,639 patients from the RENOVATE-COMPLEX-PCI trial) who had complex coronary artery lesions undergoing IVI-guided or angiography-guided PCI with 2nd-generation drug-eluting stents were included in this study. DES: drug-eluting stent; IVI: intravascular imaging; PCI: percutaneous coronary intervention
Assessment of overall lesion complexity
Overall lesion complexity was assessed by the total number of complex lesion features in each patient. Complex lesion features included (1) true bifurcation lesion with a side branch diameter ≥2.5 mm, (2) CTO with a duration ≥3 months, (3) unprotected left main disease, (4) long coronary artery lesion with a stent length of at least 38 mm, (5) multivessel PCI involving at least 2 major epicardial coronary arteries treated at the same time, (6) lesion requiring multiple stents (at least 3 stents), (7) in-stent restenosis lesion, (8) severely calcified lesion (encircling calcium on angiogram), and (9) ostial lesions of a major epicardial coronary artery38. If a target lesion simultaneously exhibited 2 or more complex features from the criteria listed above, duplication in numbering was permitted to designate the overall complexity of the lesion.
Treatments
Both IVI- and angiography-guided PCI for the respective lesion complexity group were performed based on current practice guidelines using 2nd-generation drug-eluting stents1415. In the institutional registry, the decision to use IVI devices, the type of IVI device (either IVUS or OCT), and the timing of IVI use (pre-, post-PCI or both) were left to the operators’ discretion. There were no mandated procedural optimisation protocols for IVI. In RENOVATE-COMPLEX-PCI, on the other hand, the use of IVI or angiography guidance was randomly allocated with a 2:1 ratio. The type of IVI device and timing of IVI use was at the operators’ discretion, but post-PCI IVI was mandated for stent optimisation under standardised optimisation protocols. The standardised protocols for IVI acquisition and procedural optimisation were defined according to the up-to-date expert consensus1.
All participants were prescribed a loading dose of aspirin (300 mg) and P2Y12 inhibitors (clopidogrel 300-600 mg, ticagrelor 180 mg, or prasugrel 60 mg) before PCI unless they had previously received these antiplatelet medications. Anticoagulation with low-molecular-weight heparin or unfractionated heparin was performed to achieve an activated clotting time of 250 to 300 seconds. The revascularisation strategy, use of glycoprotein IIb/IIIa receptor inhibitors, duration of dual antiplatelet therapy, and any pharmacological treatment after PCI was at the discretion of the operators. Regardless of PCI being guided by IVI or angiography, current guideline-directed medical treatment was provided for all patients according to the current ACC/AHA/SCAI or ESC/EACTS guidelines1415.
Clinical outcomes and definitions
The primary outcome was target vessel failure (TVF), a composite of cardiac death, target vessel-related myocardial infarction (MI), or target vessel revascularisation. Secondary outcomes were a composite of cardiac death or MI, all-cause death, cardiac death, MI, target vessel revascularisation, target lesion revascularisation, and definite stent thrombosis. Death from an unknown origin was defined as cardiac death, and other endpoints were defined according to the definition of the Academic Research Consortium16. Spontaneous MI was defined based on the third universal definition of MI17. Procedure-related MI was excluded from the present analysis. Both target vessel revascularisation and target lesion revascularisation were clinically driven, which was defined as revascularisation at the previously treated segment from 5 mm proximal to the stent to 5 mm distal to the stent with ≥70% diameter stenosis and at least one of the following: (1) recurrence of angina, (2) positive non-invasive test, or (3) positive invasive physiological test. Definite stent thrombosis was defined according to the Academic Research Consortium16.
Clinical follow-up was conducted during outpatient clinic visits scheduled at 1, 6, and 12 months, and yearly thereafter. Patients unable to attend outpatient clinical visits were contacted by telephone. Cross-validation of survival status was performed using the Korean National Health Insurance database. All clinical events and follow-up data were truncated at 3 years from the index procedure.
Statistical analysis
Continuous variables are presented as means±standard deviations or median with interquartile range according to the data distribution assessed by the Kolmogorov-Smirnov test, and were analysed by Welch’s t-tests or the Mann-Whitney U test, respectively. Categorical variables are presented as numbers and relative frequencies (%) and were analysed using the chi-square test. The Kaplan-Meier method was used to calculate the cumulative incidence of the primary and secondary outcomes at 3 years, and the significance of differences between groups was assessed by the log-rank test. The hazard ratio (HR) and 95% confidence intervals (CIs) were calculated by the Cox proportional hazards regression model. Multivariable models for Cox regression included variables that were significant in univariate analysis or clinically relevant: age, sex, body mass index, class of acute coronary syndrome, diabetes mellitus, hypertension, current smoking, history of stroke, history of peripheral vascular disease, left ventricular ejection fraction <40%, mean pre- and post-PCI diameter stenosis, transradial approach, volume of contrast used, use of an intravascular imaging device, and postadjunctive balloon dilatation. The proportional hazards assumption was evaluated with a 2-sided score test of the scaled Schoenfeld residuals over time at the 0.05 level. The cutoff value for the number of complex lesion features which were associated with a higher risk of TVF was calculated by maximally selected log-rank statistics. All probability values were 2-sided, and p-values<0.05 were considered statistically significant. Statistical analyses were performed using statistical software R, version 4.2.1 (R Foundation for Statistical Computing).
Results
Baseline characteristics
A total of 4,611 patients were analysed in the present study. The median follow-up duration was 2.7 years (interquartile range: 1.6-3.0 years). By maximally selected log-rank statistics, the cutoff value for the number of complex lesion features associated with a significantly higher risk of TVF was 3. As such, patients were classified into two groups: complex lesion features numbering <3 (n=3,004) or ≥3 (n=1,607) (Figure 1).
Between these two groups, patients with ≥3 complex lesion features had a higher proportion of cardiovascular risk factors and more severe target lesion characteristics than those with <3 complex lesion features, resulting in more frequent device usage and higher contrast volumes (Table 1, Table 2). In patients with ≥3 complex lesion features, the proportions of left main disease and ostial lesions were comparatively higher than other complex lesion features (Supplementary Figure 1). When comparing baseline patient and lesion characteristics between IVI- and angiography-guided PCI, significant differences were present for both patients with <3 and ≥3 complex lesion features; these differences were included in the adjusted analysis regarding clinical outcomes (Supplementary Table 1, Supplementary Table 2).
Table 1. Baseline characteristics of patients according to overall lesion complexity.
| Parameters | Patients with <3 complex lesion features (N=3,004) | Patients with ≥3 complex lesion features (N=1,607) | p-value |
|---|---|---|---|
| Age, years | 64.4±10.9 | 65.0±10.6 | 0.059 |
| Age >70 years | 1,032 (34.4) | 580 (36.1) | 0.251 |
| Male | 2,290 (76.2) | 1,267 (78.8) | 0.048 |
| Body mass index, kg/m2 | 24.7±3.2 | 24.5±3.0 | 0.006 |
| Initial presentation | 0.049 | ||
| Stable ischaemic heart disease | 1,697 (56.5) | 957 (59.6) | |
| Acute coronary syndrome | 1,307 (43.5) | 650 (40.4) | |
| Unstable angina | 744 (24.8) | 389 (24.2) | |
| Non-ST-elevation myocardial infarction | 413 (13.7) | 224 (13.9) | |
| ST-elevation myocardial infarction | 150 (5.0) | 37 (2.3) | |
| Medical history | |||
| Diabetes mellitus | 1,376 (45.8) | 827 (51.5) | <0.001 |
| Hypertension | 1,837 (61.2) | 1,047 (65.2) | 0.008 |
| Current smoking | 635 (21.1) | 299 (18.6) | 0.045 |
| Dyslipidaemia | 1,231 (41.0) | 628 (39.1) | 0.222 |
| Chronic kidney disease | 193 (6.4) | 128 (8.0) | 0.058 |
| History of previous PCI | 623 (20.7) | 302 (18.8) | 0.125 |
| History of previous CABG | 78 (2.6) | 45 (2.8) | 0.754 |
| History of stroke | 184 (6.1) | 127 (7.9) | 0.026 |
| Peripheral vascular disease | 72 (2.4) | 60 (3.7) | 0.012 |
| Family history of coronary artery disease | 275 (9.2) | 159 (9.9) | 0.443 |
| Left ventricular ejection fraction, % | 59.2±11.3 | 58.0±12.3 | 0.003 |
| Left ventricular ejection fraction <40% | 168 (7.0) | 122 (9.4) | 0.012 |
| Baseline laboratory findings | |||
| Haemoglobin, g/dL | 13.6±1.9 | 13.4±2.2 | 0.001 |
| LDL cholesterol, mg/dL | 100.7±38.2 | 97.8±37.9 | 0.019 |
| Peak CK-MB, ng/mL | 3.2 [1.7, 8.4] | 4.7 [2.5, 12.3] | <0.001 |
| Peak troponin T or I, ng/mL | 0.1 [0.0, 0.8] | 0.3 [0.1, 1.7] | <0.001 |
| NT-proBNP, pg/mL | 177.5 [59.0, 878.3] | 290.5 [75.0, 1,397.0] | <0.001 |
| Medication at discharge | |||
| Aspirin | 2,829 (94.2) | 1,516 (94.3) | 0.873 |
| P2Y12 inhibitor | 2,887 (96.1) | 1,531 (95.3) | 0.204 |
| Clopidogrel | 2,596 (86.4) | 1,389 (86.4) | 0.438 |
| Prasugrel | 127 (4.2) | 59 (3.7) | |
| Ticagrelor | 164 (5.5) | 83 (5.2) | |
| Beta blocker | 1,316 (43.8) | 765 (47.6) | 0.015 |
| RAS blockade | 1,544 (51.4) | 857 (53.3) | 0.223 |
| Statin | 2,803 (93.3) | 1,492 (92.8) | 0.593 |
| Values are presented as either mean±standard deviation, median [1st quartile, 3rd quartile], or number (%). CABG: coronary artery bypass graft; CK-MB: creatinine kinase-myoglobin band; LDL: low density lipoprotein; NT-proBNP: N-terminal pro-brain natriuretic peptide; PCI: percutaneous coronary intervention; RAS: renin-angiotensin system | |||
Table 2. Baseline angiographic and procedural characteristics of patients according to overall lesion complexity.
| Parameters | Patients with <3 complex lesion features (N=3,004) | Patients with ≥3 complex lesion features (N=1,607) | p-value |
|---|---|---|---|
| Target lesion characteristics | |||
| Number of diseased vessels | <0.001 | ||
| 1-vessel disease | 1,109 (36.9) | 151 (9.4) | |
| 2-vessel disease | 1,229 (40.9) | 743 (46.2) | |
| 3-vessel disease | 666 (22.2) | 713 (44.4) | |
| Location of lesion | |||
| Left anterior descending | 2,102 (70.0) | 1,129 (70.3) | 0.869 |
| Left circumflex | 1,107 (36.9) | 840 (52.3) | <0.001 |
| Right coronary | 1,255 (41.8) | 859 (53.5) | <0.001 |
| Left main | 149 (5.0) | 426 (26.5) | <0.001 |
| Complex coronary lesion | |||
| Bifurcation lesion | 838 (27.9) | 801 (49.8) | <0.001 |
| Chronic total occlusion | 441 (14.7) | 497 (30.9) | <0.001 |
| Left main lesion | 149 (5.0) | 426 (26.5) | <0.001 |
| Long lesion (≥38 mm) | 1,305 (43.4) | 1,264 (78.7) | <0.001 |
| Multivessel PCI (≥2 vessels treated in one PCI session) | 946 (31.5) | 1,293 (80.5) | <0.001 |
| Multiple stents (≥3 stents per patient) | 97 (3.2) | 786 (48.9) | <0.001 |
| In-stent restenosis lesion | 269 (9.0) | 151 (9.4) | 0.658 |
| Severely calcified lesion | 148 (4.9) | 180 (11.2) | <0.001 |
| Ostial lesion | 251 (8.4) | 410 (25.5) | <0.001 |
| Total length of lesions, mm | 28.3±15.5 | 46.0±28.5 | <0.001 |
| Mean pre-PCI diameter stenosis, % | 86.9±10.3 | 86.2±9.8 | 0.024 |
| Mean post-PCI diameter stenosis, % | 5.6±9.4 | 6.3±10.3 | 0.018 |
| Procedural characteristics | |||
| Transradial approach | 2,404 (80.0) | 1,173 (73.0) | <0.001 |
| Volume of contrast used, mL | 195.5±89.4 | 243.1±98.0 | <0.001 |
| Use of intravascular imaging device | |||
| Intravascular ultrasound | 915 (30.5) | 721 (44.9) | <0.001 |
| Optical coherence tomography | 227 (7.6) | 55 (3.4) | <0.001 |
| Number of stents used | 1.0 [1.0, 2.0] | 2.0 [2.0, 3.0] | <0.001 |
| Mean diameter of stents, mm | 3.1±0.9 | 3.1±0.4 | 0.691 |
| Total length of stents, mm | 39.3±18.4 | 68.9±33.1 | <0.001 |
| Use of rotablation | 59 (2.0) | 92 (5.7) | <0.001 |
| Use of postadjunctive dilatation | 1,211 (40.3) | 823 (51.2) | <0.001 |
| Procedural complication | 41 (1.4) | 32 (2.0) | 0.134 |
| Values are presented as either mean±standard deviation, median [1st quartile, 3rd quartile], or number (%). PCI: percutaneous coronary intervention | |||
Clinical outcomes according to overall lesion complexity
A comparison of clinical outcomes between patients with <3 versus ≥3 complex lesion features is demonstrated in Table 3 and Figure 2. Patients with ≥3 complex lesion features had a significantly higher risk of TVF at 3 years compared with patients with <3 complex lesion features (11.0% vs 7.2%, HR 1.59, 95% CI: 1.28-1.96; p<0.001). A significantly higher risk of clinical events was consistently observed in patients with ≥3 complex lesion features regarding the risk of the composite of cardiac death or MI, all-cause death, cardiac death, and definite stent thrombosis in both univariate and multivariate analyses (Table 3).
Table 3. Comparison of clinical outcomes according to overall lesion complexity.
| Clinical outcomes | Patients with <3 complex lesion features (N=3,004) | Patients with ≥3 complex lesion features (N=1,607) | Univariable HR (95% CI) | Multivariable HR (95% CI)* | p-value† |
|---|---|---|---|---|---|
| Target vessel failure§ | 186 (7.2) | 154 (11.0) | 1.59 (1.28-1.96) | 1.34 (1.04-1.72) | 0.021 |
| Cardiac death or MI | 100 (3.9) | 94 (6.8) | 1.78 (1.35-2.37) | 1.63 (1.18-2.26) | 0.003 |
| All-cause death | 127 (5.0) | 115 (8.5) | 1.72 (1.34-2.21) | 1.59 (1.19-2.13) | 0.002 |
| Cardiac death | 62 (2.3) | 76 (5.5) | 2.32 (1.66-3.25) | 1.98 (1.33-2.94) | 0.001 |
| Spontaneous MI | 53 (2.1) | 35 (2.4) | 1.25 (0.82-1.92) | 1.37 (0.85-2.24) | 0.199 |
| Target vessel MI | 38 (1.4) | 29 (2.0) | 1.44 (0.89-2.34) | 1.67 (0.95-2.96) | 0.077 |
| Non-target vessel MI | 15 (0.6) | 5 (0.4) | 0.63 (0.23-1.74) | 0.67 (0.23-1.89) | 0.445 |
| Target vessel revascularisation | 131 (5.4) | 85 (6.4) | 1.25 (0.95-1.64) | 1.12 (0.81-1.54) | 0.493 |
| Target lesion revascularisation | 81 (3.2) | 55 (4.1) | 1.30 (0.92-1.83) | 1.16 (0.79-1.72) | 0.443 |
| Definite stent thrombosis | 33 (1.2) | 33 (2.1) | 1.89 (1.16-3.05) | 2.29 (1.23-4.26) | 0.009 |
| Data are given as n (%) unless stated otherwise. Percentages are 3-year Kaplan-Meier estimates. *Multivariate adjustment using age, male sex, body mass index, class of acute coronary syndrome, diabetes mellitus, hypertension, current smoking, history of stroke, history of peripheral vascular disease, left ventricular ejection fraction <40%, mean pre-/post-PCI diameter stenosis, transradial approach, volume of contrast used, use of intravascular device, and postadjunctive dilatation. †P-values are calculated from multivariate-adjusted analysis. §Target vessel failure is a composite of cardiac death, target vessel MI, or target vessel revascularisation. CI: confidence interval; HR: hazard ratio; MI: myocardial infarction; PCI: percutaneous coronary intervention | |||||
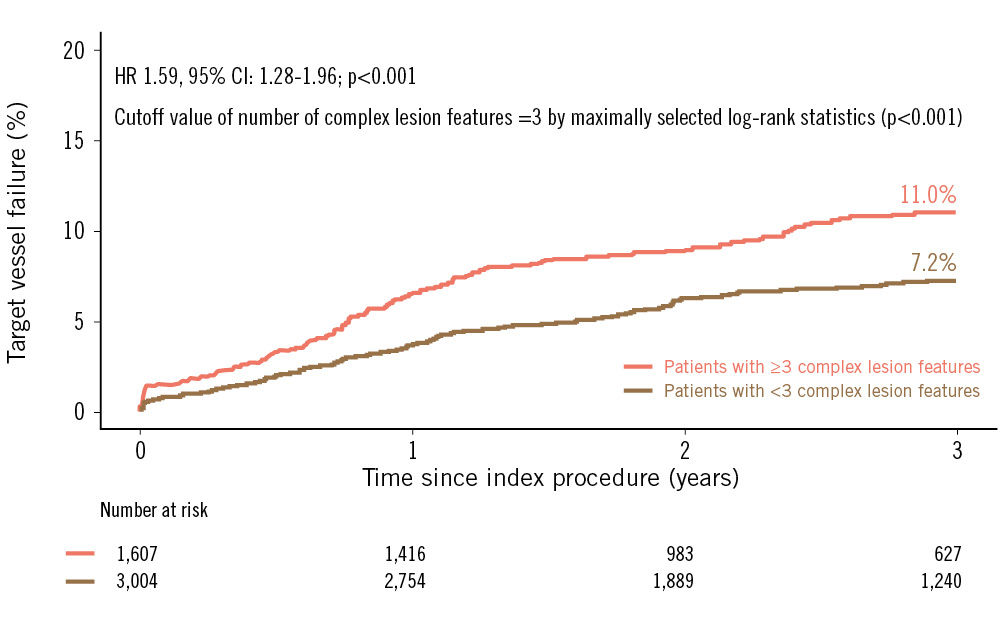
Figure 2. Target vessel failure according to overall lesion complexity. The Kaplan-Meier survival curve is shown for the comparison of target vessel failure between patients with ≥3 complex lesion features (pink line) and patients with <3 complex lesion features (brown line). CI: confidence interval; HR: hazard ratio
Clinical outcomes between IVI-guided PCI and angiography-guided PCI according to overall lesion complexity
The cumulative incidence of TVF was significantly lower after IVI-guided PCI compared with angiography-guided PCI in both patients with <3 complex lesion features (5.7% vs 8.1%, HR 0.72, 95% CI: 0.53-0.98; p=0.039) and ≥3 complex lesion features (7.4% vs 14.4%, HR 0.49, 95% CI: 0.35-0.69; p<0.001) (Figure 3). IVI-guided PCI showed a lower risk of cardiac death and target vessel MI than angiography-guided PCI without significant interaction, but it did not demonstrate a significant reduction in risk for other secondary endpoints, including target vessel revascularisation, target lesion revascularisation and non-target vessel MI. Even after multivariate adjustment, IVI-guided PCI showed a similar trend of lower TVF risk, compared to angiography-guided PCI, in both patients with <3 and ≥3 complex lesion features (Table 4).
The prognostic benefits of IVI-guided PCI over angiography-guided PCI increased as the number of complex lesion features increased (Figure 4). The absolute risk reduction of TVF following IVI-guided PCI tended to increase as the number of complex lesion features increased (absolute risk reduction for TVF: –1.2% vs –2.7% vs –5.5% vs –7.7%, respectively, for 1 vs 2 vs 3 vs ≥4 complex lesion features), with significant interaction (interaction p=0.048) (Central illustration).
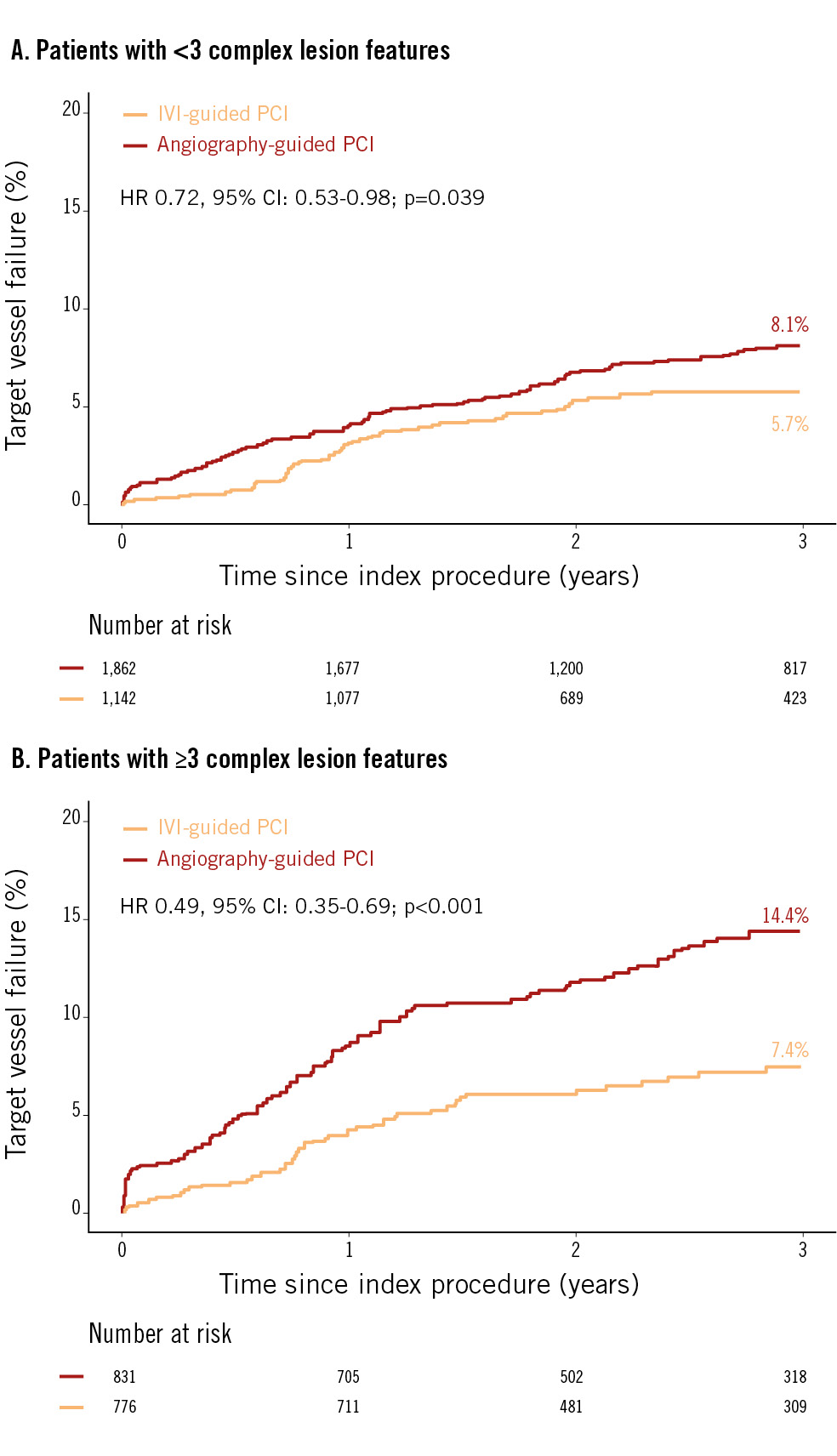
Figure 3. Target vessel failure between IVI-guided and angiography-guided PCI. The Kaplan-Meier survival curve is shown for comparison of target vessel failure between IVI-guided PCI (orange line) and angiography-guided PCI (red line) in patients with <3 complex lesion features (A) and patients with ≥3 complex lesion features (B). CI: confidence interval; HR: hazard ratio; IVI: intravascular imaging; PCI: percutaneous coronary intervention
Table 4. Comparison of clinical outcomes between intravascular imaging-guided and angiography-guided PCI according to overall lesion complexity.
| Outcome | Number of complex lesion features | IVI | Angiography | Univariable HR (95% CI) | Multivariable HR (95% CI)* | p-value† | Interaction p-value |
|---|---|---|---|---|---|---|---|
| Target vessel failure§ | Less (<3) | 57 (5.7) | 129 (8.1) | 0.72 (0.53-0.98) | 0.77 (0.53-1.12) | 0.177 | 0.108 |
| More (≥3) | 50 (7.4) | 104 (14.4) | 0.49 (0.35-0.69) | 0.63 (0.41-0.94) | 0.026 | ||
| Cardiac death or MI | Less (<3) | 22 (2.2) | 78 (4.9) | 0.46 (0.29-0.74) | 0.46 (0.26-0.82) | 0.008 | 0.662 |
| More (≥3) | 26 (3.9) | 68 (9.5) | 0.40 (0.25-0.63) | 0.59 (0.34-1.01) | 0.056 | ||
| All-cause death | Less (<3) | 32 (3.4) | 95 (6.0) | 0.55 (0.37-0.83) | 0.52 (0.31-0.87) | 0.012 | 0.497 |
| More (≥3) | 35 (5.4) | 80 (11.3) | 0.46 (0.31-0.68) | 0.53 (0.32-0.86) | 0.011 | ||
| Cardiac death | Less (<3) | 11 (1.0) | 51 (3.1) | 0.35 (0.18-0.67) | 0.35 (0.16-0.77) | 0.009 | 0.844 |
| More (≥3) | 18 (2.7) | 58 (8.1) | 0.32 (0.19-0.55) | 0.50 (0.26-0.94) | 0.031 | ||
| Spontaneous MI | Less (<3) | 12 (1.2) | 41 (2.6) | 0.48 (0.25-0.91) | 0.50 (0.23-1.07) | 0.074 | 0.996 |
| More (≥3) | 11 (1.6) | 24 (3.1) | 0.48 (0.24-0.98) | 0.66 (0.28-1.57) | 0.352 | ||
| Target vessel MI | Less (<3) | 8 (0.9) | 30 (1.8) | 0.44 (0.20-0.95) | 0.45 (0.17-1.16) | 0.098 | 0.878 |
| More (≥3) | 8 (1.2) | 21 (2.7) | 0.40 (0.18-0.90) | 0.60 (0.21-1.70) | 0.338 | ||
| Non-target vessel MI | Less (<3) | 4 (0.4) | 11 (0.8) | 0.60 (0.19-1.89) | 0.56 (0.16-2.04) | 0.380 | 0.578 |
| More (≥3) | 3 (0.4) | 3 (0.5) | 1.06 (0.21-5.23) | 1.24 (0.21-7.33) | 0.812 | ||
| TVR | Less (<3) | 47 (4.9) | 84 (5.7) | 0.92 (0.64-1.31) | 0.96 (0.62-1.49) | 0.872 | 0.389 |
| More (≥3) | 35 (5.6) | 50 (7.1) | 0.71 (0.46-1.10) | 0.79 (0.46-1.34) | 0.377 | ||
| TLR | Less (<3) | 34 (3.5) | 47 (3.0) | 1.18 (0.76-1.84) | 1.08 (0.64-1.83) | 0.770 | 0.265 |
| More (≥3) | 24 (3.8) | 31 (4.3) | 0.79 (0.47-1.36) | 0.78 (0.41-1.47) | 0.439 | ||
| Definite stent thrombosis | Less (<3) | 5 (0.5) | 28 (1.6) | 0.29 (0.11-0.75) | 0.63 (0.20-1.99) | 0.428 | 0.528 |
| More (≥3) | 5 (0.7) | 28 (3.4) | 0.19 (0.07-0.49) | 0.25 (0.07-0.88) | 0.031 | ||
| Data are presented as n (%) unless otherwise stated. Percentages are 3-year Kaplan-Meier estimates. *Multivariate adjustment using age, male sex, body mass index, class of acute coronary syndrome, diabetes mellitus, hypertension, current smoking, history of stroke, history of peripheral vascular disease, left ventricular ejection fraction <40%, mean pre-/post-PCI diameter stenosis, transradial approach, volume of contrast used, and postadjunctive dilatation. †P-values are calculated from multivariate-adjusted analysis. §Target vessel failure is a composite of cardiac death, target vessel MI, or target vessel revascularisation. CI: confidence interval; HR: hazard ratio; IVI: intravascular imaging; MI: myocardial infarction; PCI: percutaneous coronary intervention; TLR: target lesion revascularisation; TVR: target vessel revascularisation | |||||||
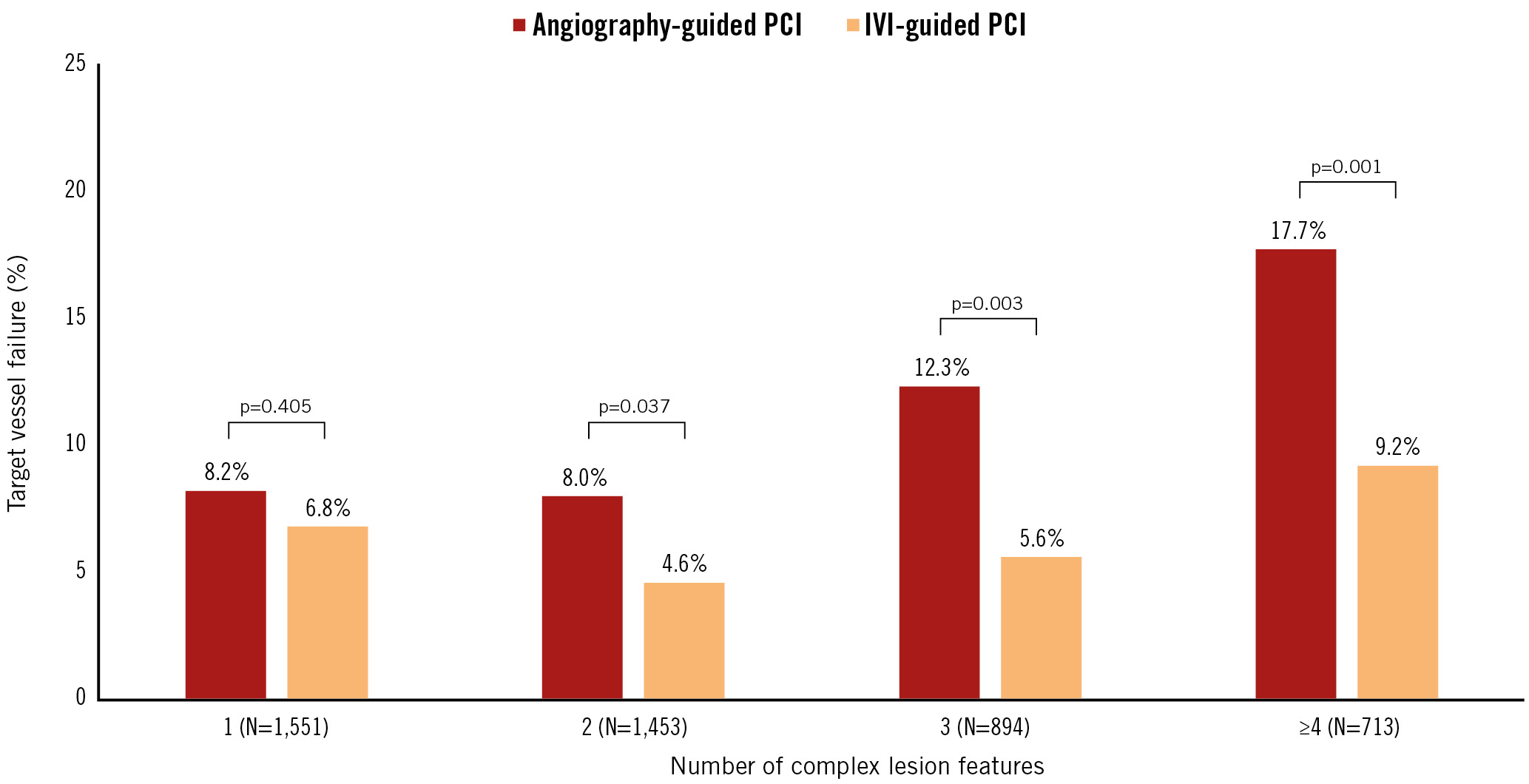
Figure 4. Target vessel failure between IVI-guided and angiography-guided PCI according to overall lesion complexity. The bar chart shows the cumulative incidence of target vessel failure according to the number of complex lesion features between IVI-guided PCI (orange bar) and angiography-guided PCI (red bar). IVI: intravascular imaging; PCI: percutaneous coronary intervention
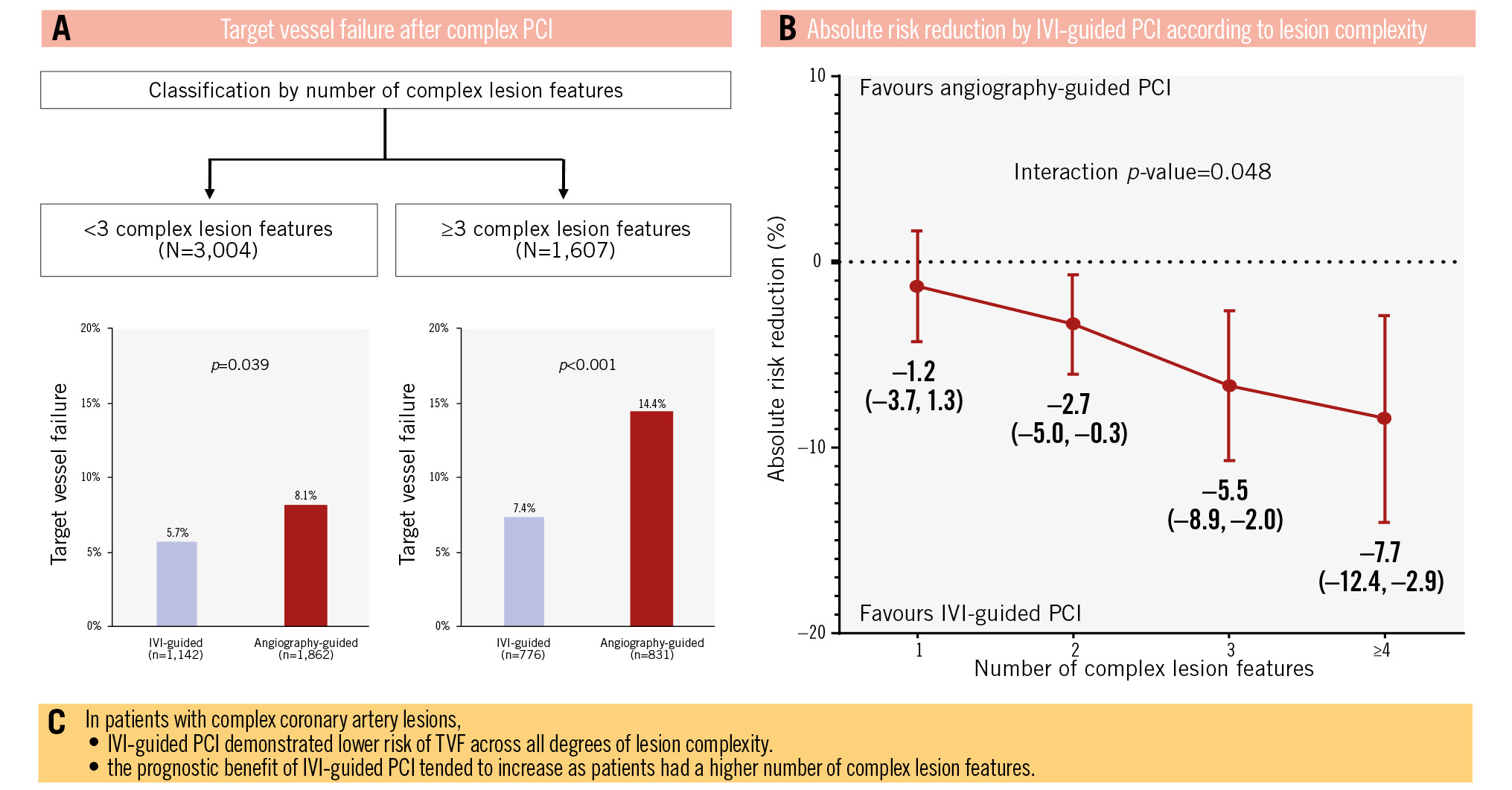
Central illustration. A pooled analysis from the RENOVATE-COMPLEX-PCI trial and the Samsung Medical Center institutional registry. The current study evaluated the prognostic benefit of IVI-guided PCI compared with angiography-guided PCI according to overall lesion complexity in patients with complex coronary artery lesions. Complex lesion features included (1) true bifurcation lesion with a side branch diameter ≥2.5 mm, (2) CTO with a duration ≥3 months, (3) unprotected left main disease, (4) long coronary artery lesion with a stent length of at least 38 mm, (5) multivessel PCI involving at least 2 major epicardial coronary arteries treated at the same time, (6) lesion requiring multiple stents (at least 3 stents), (7) in-stent restenosis lesion, (8) severely calcified lesion (encircling calcium on angiogram), and (9) ostial lesion of a major epicardial coronary artery. From our pooled analysis, the frequency of target vessel failure after complex PCI is detailed in (A), the absolute risk reduction according to lesion complexity is provided in (B), and (C) provides the conclusions of the analysis. CTO: chronic total occlusion; IVI: intravascular imaging; PCI: percutaneous coronary intervention; TVF: target vessel failure
Discussion
The current study evaluated the prognostic benefit of IVI-guided PCI compared with angiography-guided PCI according to overall lesion complexity in patients with complex coronary artery lesions. The main study findings are as follows. First, patients with ≥3 complex lesion features demonstrated a significantly higher risk of TVF than those with <3 complex lesion features. Second, in patients with complex coronary artery lesions undergoing PCI, IVI-guided PCI was associated with a significantly lower risk of TVF compared with angiography-guided PCI, regardless of whether patients had ≥3 or <3 complex lesion features. Third, the absolute risk reduction in TVF by IVI-guided PCI increased as the number of complex lesion features increased. These findings suggest that the prognostic benefit of IVI-guided PCI increases with the number of complex lesion features.
Previous RCTs have consistently shown a prognostic benefit of IVUS-guided PCI compared with angiography-guided PCI5671819. Recently, the RENOVATE-COMPLEX-PCI trial demonstrated superior clinical outcomes following IVI-guided PCI in patients with complex coronary artery lesions3. Similar results were shown in the OCTOBER trial, in which patients with complex coronary bifurcation lesions were included4. However, the patients included in these studies had varying degrees of overall lesion complexity. Some may have had one qualifying complex lesion feature, whereas others could have had multiple coexisting features which might have increased adverse procedural and clinical outcomes12. As expected, patients with ≥3 complex lesion features showed a significantly higher risk of TVF after PCI than those with <3 complex lesion features. This could, in part, be explained by the fact that the number of complex lesion features in individual patients may reflect the severity of their systemic atherosclerotic disease process.
Nevertheless, the presence of more complex lesion features may also increase procedural ambiguity and complexity, for which IVI-guided PCI could particularly be helpful. The current study sought to evaluate this hypothesis. IVI-guided PCI significantly reduced the risk of TVF in both patients with <3 complex lesion features and those with ≥3 complex lesion features. Indeed, the cumulative incidence of TVF in patients with ≥3 complex lesion features after IVI-guided PCI was numerically lower than in patients with <3 complex lesion features treated by angiography-guided PCI. More importantly, the prognostic benefits of IVI-guided PCI compared with angiography-guided PCI tended to increase as the number of complex lesion features increased. Furthermore, significant interaction was observed between the number of complex lesion features and the treatment effect of IVI-guided PCI compared with angiography-guided PCI.
This finding could be explained by the following reasons. First, the presence of multiple complex lesion features could make the potential limitations of angiography-guided PCI more prominent by increasing the ambiguity of target lesions12. It is well known that coronary angiographic images are often foreshortened and limited in their evaluation of tortuous, overlapping, heavily calcified, or long lesions1220. In contrast, IVI could reduce the ambiguity of these angiographic images and enable a more accurate assessment of lesion severity, plaque characteristics, and hidden calcifications, which are crucial for pre-PCI planning. Second, the presence of multiple complex lesion features may increase procedural complications and suboptimal results requiring post-PCI optimisation which can be better guided by IVI than angiography alone. Third, the increased number of complex lesion features may imply higher atherosclerotic disease burden in target vessels or greater systemic atherosclerotic burden. This can lead to a higher chance of landing a stent in the diseased reference segment when guided only by angiography, causing subsequent edge restenosis202122.
The present study also provides a possible clue to explain the conflicting results among recent trials311. Among 24 RCTs to date23, RESET, AIR-CTO, and the most recent ILUMIEN IV trial showed no significant difference in their primary endpoint between IVI-guided and angiography-guided PCI112425. This might be attributed to the varying severity of complex lesion features in those trials. Indeed, long lesions were defined as lesion length ≥28 mm in the RESET and the ILUMIEN IV trials1125, which was shorter than the definition used in the RENOVATE-COMPLEX-PCI trial3. Furthermore, the ILUMIEN IV trial excluded unprotected left main disease and ostial lesions of major coronary arteries and included patients with diabetes mellitus and acute coronary syndrome, even in the absence of complex lesion features. As such, the proportions of each complex lesion subset in ILUMIEN IV were relatively lower than those in RENOVATE-COMPLEX-PCI. For example, the proportions of upfront 2-stenting in bifurcation lesions (3.3% vs 13.0%), CTO (7.0% vs 19.5%), in-stent restenosis (10.8% vs 14.4%), and severely calcified lesions (11.5% vs 14.1%) were lower in ILUMIEN IV than in RENOVATE-COMPLEX-PCI311. Considering the higher absolute risk reduction following IVI-guided PCI in patients with more complex lesion features, the inclusion of relatively less complex coronary artery lesions could have affected the results of the ILUMIEN IV trial11. Indeed, similar trends were observed in the current study. In patients with <3 complex lesion features, the benefits of IVI-guided PCI were mitigated after multivariable adjustment. However, in patients with ≥3 complex lesion features, the benefits of IVI-guided PCI over angiography-guided PCI remained significant even after multivariable adjustment. Consequently, it can be inferred that IVI-guided PCI would be more effective to reduce the risk of TVF in patients with more complex lesion features, as shown by the recent substudy of the ILUMIEN IV trial26. These results align with the updated European Society of Cardiology (ESC) guidelines, which promote the use of IVI for anatomically complex lesions, particularly left main, true bifurcation, or long lesions, as a Class I, Level of Evidence A recommendation27. Studies are warranted to further define the appropriate criteria identifying optimal candidates for IVI-guided PCI to maximise the prognostic benefits and cost-effectiveness of IVI-guided PCI28.
Limitations
Several limitations should be acknowledged. First, although one of the included datasets was a prospective registry, selection bias affecting the independent variables is a fundamental limitation compared to RCTs. Furthermore, the heterogeneity of enrolment and follow-up periods between these two included datasets could be a confounding factor. However, it should be noted that the overall trends of the results were not changed even after exclusion of the institutional data as a sensitivity analysis (Supplementary Figure 2). Second, the severity of individual complex lesion features was not considered in this study. For example, the current study could not assess detailed lesion characteristics in CTO or unprotected left main lesions, such as morphological characteristics that could influence procedural difficulty. Furthermore, it was not feasible to include an analysis based on the SYNTAX score, which is a widely used method for assessing lesion complexity. Third, procedural optimisation criteria for IVI-guided PCI were predefined in RENOVATE-COMPLEX-PCI, but there was no mandated procedural optimisation criteria in the institutional registry29. In RENOVATE-COMPLEX-PCI, although patients with ≥3 complex lesion features (TVF in angiography-guided vs non-optimised IVI-guided vs optimised IVI-guided: 11.3% vs 5.9% vs 4.7%; log-rank p=0.17) tended to demonstrate better clinical outcomes after optimised IVI-guided PCI than patients with <3 complex lesion features (TVF in angiography-guided vs non-optimised IVI-guided vs optimised IVI-guided: 7.8% vs 6.0% vs 3.9%; log-rank p=0.12), statistical significance was not achieved because of the relatively small sample size. Fourth, we could not present the detailed analysis of intravascular imaging parameters in the current study. However, a detailed analysis of intravascular imaging parameters would be beyond the scope of the current analysis.
Conclusions
In patients with complex coronary artery lesions, IVI-guided PCI showed a lower risk of TVF across all degrees of lesion complexity. The prognostic benefit of IVI-guided PCI tended to increase as patients had a higher number of complex lesion features.
Impact on daily practice
The current study demonstrated that intravascular imaging (IVI)-guided percutaneous coronary intervention (PCI) conferred a lower risk of target vessel failure (TVF) than angiography-guided PCI, regardless of the number of complex lesion features. However, the absolute risk reduction in TVF by IVI-guided PCI increased as the number of complex lesion features increased, with significant interaction. Considering the higher absolute risk reduction following IVI-guided PCI in patients with more complex lesion features, the current results underscore the importance of patient selection, rather than universal application of IVI, to maximise the prognostic benefits and cost-effectiveness of IVI-guided PCI. Studies are warranted to further define the appropriate criteria identifying optimal candidates for IVI-guided PCI.
Funding
The RENOVATE-COMPLEX-PCI trial is investigator initiated with grant support from Abbott and Boston Scientific. Other than providing financial support, the sponsors were not involved with protocol development or the study process, including site selection, study management, data collection, and analysis of the results.
Conflict of interest statement
J.M. Lee received institutional research grants from Abbott, Boston Scientific, Philips Volcano, Terumo Corporation, Zoll Medical, MicroPort, Dong-A ST, and Yuhan Pharmaceutical. J.-Y. Hahn has received institutional research grants from the National Evidence-based Healthcare Collaborating Agency, Ministry of Health & Welfare, Korea, as well as Abbott, Biosensors, Boston Scientific, Daiichi Sankyo, Dong-A ST, Hanmi Pharmaceutical, and Medtronic. H.-C. Gwon received institutional research grants from Boston Scientific, Genoss, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

