Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Independent predictors and prognostic correlates of structural valve deterioration (SVD) after transcatheter aortic valve implantation (TAVI) have not been investigated beyond 5-year follow-up.
Aims: We aimed to investigate the association between the early residual mean postprocedural gradient (ERMPG) after TAVI and long-term SVD rates as well as the association of SVD with bioprosthetic valve failure (BVF) and 10-year mortality rates.
Methods: Patients with severe aortic valve stenosis enrolled in the Medtronic One Hospital Clinical Service at 10 Italian centres were included in the study. ERMPG was measured with echo-Doppler at hospital discharge or within 3 months from TAVI.
Results: Between September 2007 and December 2014, 1,291 patients undergoing TAVI with a CoreValve/Evolut valve met the enrolment criteria of the study. After a median follow-up of 59.4 months, there were 46 patients with SVD (cumulative incidence rate 3.6%). A significant stepwise increase in the risk of SVD was apparent across tertiles of ERMPG (p=0.009), and in the multivariable analysis, ERMPG was an independent predictor of SVD (adjusted subdistribution hazard ratio [sHR] 1.05, 95% confidence interval [CI]: 1.01-1.08; p=0.004). Among the 46 patients with SVD, 25 (54.3%) had or developed BVF. SVD was associated with increased 10-year rates of all-cause mortality (adjusted hazard ratio 2.12, 95% CI: 1.49-3.00; p<0.001) and cardiac mortality (adjusted sHR 5.78, 95% CI: 2.63-12.71; p<0.001) compared with no SVD.
Conclusions: Echo-Doppler-derived ERMPG measured within 90 days from TAVI is an independent predictor of SVD. SVD is associated with high rates of BVF, and it is an independent predictor of all-cause mortality and cardiovascular mortality.
Transcatheter aortic valve implantation (TAVI) has emerged as a breakthrough technology for the treatment of patients with severe aortic valve stenosis. Initially implemented in elderly patients at prohibitive or high risk for surgical aortic valve replacement (SAVR), TAVI has subsequently been extended to progressively younger and lower-risk patients, raising the issue of valve durability1234. A large observational study has in fact estimated that the median life expectancy in this category of patients is around 10-13 years after SAVR5. A key component of valve durability is structural valve deterioration (SVD), which implies intrinsic and structural changes of bioprosthetic leaflets including wear and tear, leaflet disruption, flail, fibrosis, calcification, or strut fracture or deformation in association with progressive haemodynamic valve deterioration (HVD)6. Understanding the mechanistic underpinnings of SVD is of the utmost importance for patient risk stratification, implementation of appropriate therapeutic strategies, and lifetime management of younger patients. Despite its clinical relevance, there are scant data on the predictors and prognostic correlates of SVD78.
SVD is a multifactorial process that shares some features with the progression of native aortic valve stenosis9. In this regard, animal studies have suggested an association between shear-induced, growth factor-mediated valve fibrosis and the progression of aortic valve stenosis10. However, the effect on the risk of subsequent SVD of increased shear rates across the bioprosthetic valve associated with high postprocedural gradients after TAVI has never been investigated. For this reason, we investigated the association between residual postprocedural gradients after TAVI and the risk of SVD, as well as the association of SVD with bioprosthetic valve failure (BVF), and with all-cause mortality and cardiovascular mortality at 10-year follow-up.
Methods
Patient population
The Medtronic One Hospital Clinical Service (OHCS) has already been described in detail11. Briefly, it is a clinical data repository and medical care quality improvement project, involving over 20 European hospitals, aimed at describing and improving the use of Medtronic TAVI implantable devices in real-world clinical practice. Prospective data collection began in 2007 and includes demographic, clinical, procedural, and outcome data of patients undergoing TAVI with the CoreValve/Evolut system (Medtronic). The indication for TAVI, valve type, and access route were determined at each participating centre according to local practice. Clinical, procedural, and echocardiographic data were prospectively collected within a dedicated dataset at each participating centre. Clinical follow-up was performed during clinical visits or by telephone contact at 1 month, 1 year, and yearly thereafter, or as per local practice. For the purpose of this study, we included all patients with severe aortic valve stenosis who had available echocardiographic data within 90 days from TAVI. Exclusion criteria were pure aortic regurgitation, procedural death, and unknown vital status at 5-year follow-up. To have a follow-up of at least 10 years, we included patients undergoing TAVI in the period between September 2007 and December 2014. The project was approved by each site’s institutional review board, and each patient signed an informed consent for data collection and analysis.
Endpoint and definitions
The objective of this study was to investigate the relationship between the early residual mean postprocedural gradient (ERMPG) after TAVI and the risk of SVD. We considered both moderate and severe SVD as previously defined8. Specifically, moderate SVD was defined as (1) HVD showing an increase in the mean aortic gradient ≥10 mmHg from discharge or 90-day echocardiography to the last available echocardiography with a final mean gradient ≥20 mmHg or (2) new occurrence or increase of 1 grade or more of intraprosthetic aortic regurgitation resulting in moderate or severe aortic regurgitation. Severe SVD was defined as (1) HVD showing an increase in the mean gradient ≥20 mmHg from discharge or 90-day echocardiography to the last available echocardiography with a final mean gradient ≥30 mmHg or (2) new occurrence or increase of 2 grades or more of intraprosthetic aortic regurgitation resulting in severe aortic regurgitation. Other clinical events were defined according to Valve Academic Research Consortium (VARC)-3 criteria6. SVD was also analysed in patients stratified into tertiles of ERMPG. The other main objective of the study was to analyse the association of SVD with BVF, all-cause mortality, and cardiovascular mortality at 10-year follow-up.
Echocardiographic assessment
Transthoracic echocardiography was performed as per standard practice. The mean gradients were calculated using the modified Bernoulli formula. The ERMPG was calculated at hospital discharge or within 90 days from TAVI as per local clinical practice. Echocardiographic follow-up at each centre was scheduled yearly thereafter. The absolute change in the mean gradient was calculated as the gradient at follow-up minus the ERMPG.
Statistical analysis
Continuous variables are reported as mean and standard deviation or median and interquartile range (IQR), as appropriate, and were compared with the Student’s t-test or the Wilcoxon rank-sum test, respectively. Categorical variables are reported as counts and percentages and were compared using the χ2 statistic or Fisher’s exact test, as appropriate.
Rates of SVD across tertiles of ERMPG were determined using cumulative incidence rates, and differences across groups were analysed with Gray’s test. The Bonferroni test was used for multiple comparisons. In all statistical tests, mortality was considered as a competing risk.
To further investigate the relationship between ERMPG and the risk of SVD, and to identify a potential threshold value, time-dependent receiver operating characteristic (ROC) curve analyses were performed. Inverse probability of censoring weighting (IPCW) was used to estimate ERMPG sensitivity and specificity for the yearly risk of SVD from 5 to 10 years after TAVI. The area under the curve (AUC) was computed at each timepoint, and the optimal ERMPG cutoff was derived by maximising Youden’s index. As a sensitivity analysis, we also performed a Fine-Gray subdistribution hazard ratio (sHR) analysis with restricted cubic splines (RCS), accounting for death as a competing event. Three internal knots were placed at the 10th, 50th, and 90th percentiles of the distribution of ERMPG; the reference value for splines (sHR 1) was set at the 50th percentile. Departure from linearity was tested using a likelihood ratio test, comparing the linear model against the model including linear and cubic spline terms.
To determine independent predictors of SVD at 10-year follow-up, unadjusted and adjusted sHR and 95% confidence intervals (CIs) were estimated with Fine-Gray models, accounting for death as a competing event. The following clinical variables were included in the model: diabetes, which differed between the SVD and non-SVD groups at baseline, and other potential confounders previously identified as independent predictors of SVD in previous studies (age, female sex, body surface area, hypertension)8. In these models, ERMPG was treated as a continuous variable.
The association between SVD and mortality was investigated by Simon-Makuch analysis instead of the Kaplan-Meier method to take into account the time-dependent nature of the survival analysis. Briefly, the two methods differ in that the number of subjects at risk within each of the covariate levels is fixed at time zero in the Kaplan-Meier method, but it is not in the Simon-Makuch method. Between-group comparisons were analysed by the Mantel-Byar test, which is a score test for a proportional hazards model with time-dependent covariates. Incidence rates of all-cause mortality and cardiovascular mortality in patients with and without SVD were determined by Poisson distribution. Cox and Fine-Gray multivariable analyses were performed to assess independent predictors of mortality and cardiovascular mortality at 10-year follow-up, respectively, considering SVD as a time-dependent covariate in both models. A stepwise selection was employed with an entry criterion of 0.3 and a stay criterion of 0.1. Variables included in the model are reported in Supplementary Table 1. For the analysis on cardiovascular mortality, non-cardiac mortality was considered as a competing risk. Sensitivity analyses were performed including patients whose vital status was not known at 5-year follow-up. All tests were two-sided, and a p-value<0.05 was considered statistically significant. SAS software, version 9.4 (SAS Institute) was used to perform all statistical analyses.
Results
Patients
In the period between September 2007 and December 2014, 1,291 patients met the study inclusion criteria and were considered for analyses. Of note, 264 patients were not included in the analyses because their vital status was unknown at 5 years. Overall, the clinical characteristics of these patients were similar to those of the study participants for most variables (Supplementary Table 2). During a median follow-up of 59.4 (IQR 27.3-91.5) months, 46 patients (3.6%) developed SVD, which was classified as bioprosthetic valve stenosis in 29 patients (63.7%), central insufficiency in 11 (24.0%), and mixed dysfunction in 6 (12.3%). The cumulative incidence function of SVD at 10-year follow-up is reported in Supplementary Figure 1. Baseline clinical and procedural characteristics of patients stratified by the occurrence of SVD are reported in Table 1 and Table 2, respectively, while baseline clinical and procedural characteristics of patients stratified by tertiles of ERMPG are reported in Supplementary Table 3 and Supplementary Table 4, respectively. Tertile I included patients with an ERMPG <6 mmHg, tertile II included patients with an ERMPG 6-9 mmHg, and tertile III included patients with an ERMPG >9 mmHg. The mean±standard deviation number of echocardiograms performed at follow-up was 7.3±4.1 in patients with SVD versus 4.0±3.1 in patients without SVD (p<0.001).
Table 1. Baseline clinical characteristics of patients stratified by structural valve degeneration.
| No SVD (n=1,245) | SVD (n=46) | p-value | |
|---|---|---|---|
| Age, years | 82.6±5.8 | 76.0±10.0 | <0.001 |
| Male | 548/1,245 (44.0) | 26/46 (56.5) | 0.09 |
| Hypertension | 993/1,245 (79.8) | 38/46 (82.6) | 0.64 |
| Body mass index, kg/m2 | 25.9±4.6 | 27.2±6.7 | 0.25 |
| Diabetes mellitus | 359/1,245 (28.8) | 7/46 (15.2) | 0.04 |
| Smoker | 96/1,245 (7.7) | 2/46 (4.3) | 0.57 |
| Prior myocardial infarction | 222/1,245 (17.8) | 9/46 (19.6) | 0.70 |
| Prior percutaneous coronary intervention | 347/1,245 (27.9) | 13/46 (28.3) | 0.99 |
| Prior coronary artery bypass grafting | 182/1,245 (14.6) | 12/46 (26.1) | 0.06 |
| Prior stroke | 85/1,245 (6.8) | 0/46 (0) | 0.07 |
| Chronic kidney disease* | 319/1,245 (25.6) | 8/46 (17.4) | 0.23 |
| COPD | 240/1,245 (19.3) | 8/46 (17.4) | 0.85 |
| Peripheral vascular disease | 378/1,245 (30.4) | 11/46 (23.9) | 0.42 |
| Left ventricular ejection fraction, % | 51.9±12.6 | 51.5±11.9 | 0.76 |
| NYHA Class III-IV | 917/1,245 (73.6) | 29/46 (63.0) | 0.11 |
| Pulmonary hypertension | 167/1,245 (13.4) | 4/46 (8.7) | 0.51 |
| Atrial fibrillation | 268/1,245 (21.5) | 3/46 (6.5) | 0.01 |
| Coronary artery disease | 552/1,245 (44.3) | 22/46 (47.8) | 0.64 |
| EuroSCORE II | 6.6±6.2 | 6.3±9.9 | 0.09 |
| Society of Thoracic Surgeons score | 6.2 (4.1-10.4) | 4.8 (2.5-11.3) | 0.12 |
| Preprocedural AVA, cm2 | 0.7±0.4 | 0.7±0.3 | 0.46 |
| Preprocedural MG, mmHg | 51.3±15.0 | 54.6±16.5 | 0.21 |
| Moderate or severe aortic regurgitation pre-TAVI | 357/1,245 (28.7) | 16/46 (34.8) | 0.41 |
| Moderate or severe mitral regurgitation pre-TAVI | 515/1,245 (41.4) | 17/46 (37.0) | 0.65 |
| Data are presented as n/N (%), mean±SD, or median (IQR). *Defined as glomerular filtration rate ≤30 mL/min/1.73 m². AVA: aortic valve area; COPD: chronic obstructive pulmonary disease; EuroSCORE: European System for Cardiac Operative Risk Evaluation; IQR: interquartile range; MG: mean aortic gradient; NYHA: New York Heart Association; SD: standard deviation; SVD: structural valve deterioration; TAVI: transcatheter aortic valve implantation | |||
Table 2. Baseline procedural characteristics stratified by structural valve degeneration.
| No SVD (n=1,245) | SVD (n=46) | p-value | |
|---|---|---|---|
| General anaesthesia | 359/1,245 (28.8) | 12/46 (26.1) | 0.69 |
| Access | 0.58 | ||
| Femoral | 963/1,216 (79.2) | 39/46 (84.8) | |
| Transaxillary | 170/1,216 (14.0) | 6/46 (13.0) | |
| Aortic | 83/1,216 (6.8) | 1/46 (2.2) | |
| Type of valve | 0.03 | ||
| CoreValve | 1,239/1,245 (99.5) | 44/46 (95.7) | |
| Evolut R | 6/1,245 (0.5) | 2/46 (4.3) | |
| Size of prosthesis | 0.03 | ||
| 23 mm | 22/1,244 (1.8) | 4/46 (8.7) | |
| 26 mm | 569/1,244 (45.7) | 17/46 (37.0) | |
| 29 mm | 555/1,244 (44.6) | 22/46 (47.8) | |
| 31 mm | 98/1,244 (7.9) | 3/46 (6.5) | |
| Predilation | 842/1,245 (67.6) | 34/46 (73.9) | 0.37 |
| Post-dilation | 269/1,245 (21.6) | 12/46 (26.1) | 0.47 |
| Valve-in-valve deployment | 34/1,211 (2.8) | 2/45 (4.4) | 0.37 |
| Device success | 1,181/1,245 (94.9) | 46/46 (100) | 0.12 |
| Procedural success | 1,195/1,245 (96.0) | 45/46 (97.8) | 0.53 |
| More than 1 valve implanted | 44/1,245 (3.5) | 1/46 (2.2) | 0.62 |
| Conversion to cardiac surgery | 2/1,238 (0.2) | 0/46 (0) | 0.79 |
| Data are presented as n/N (%). SVD: structural valve deterioration | |||
ERMPG and SVD
The median time from TAVI to SVD was 5.7±3.0 years. As shown in Figure 1, the median ERMPG was 10.0 mmHg (IQR 7.0-13.0 mmHg) in patients who developed SVD versus 8.0 mmHg (IQR 6.0-10.0 mmHg) in those who did not develop SVD (sHR 1.05, 95% CI: 1.02-1.07; p<0.001). A stepwise increase of SVD rates was apparent across tertiles of ERMPG, such that SVD 10-year cumulative incidence rates (95% CI) were 2.16% (1.06-3.94%) in tertile I, 3.02% (1.65-5.06%) in tertile II, and 5.06% (3.23-7.48%) in tertile III (overall p=0.009) (Figure 1). Patients in tertile III had a significantly higher risk of SVD compared with patients in tertile II (adjusted sHR 2.13, 95% CI: 1.05-4.35; p=0.034) and patients in tertile I (adjusted sHR 2.22, 95% CI: 1.00-5.00; p=0.049). By time-dependent ROC curves, the optimal ERMPG cutoff value for predicting the 5-year risk of SVD after TAVI was 10 mmHg (AUC=72%). Cutoff values beyond 5 years are reported in Supplementary Table 5. The same result was apparent in the RCS analysis. Specifically, the unadjusted sHR for SVD plotted against ERMPG showed an inflection point at 10 mmHg (non-linearity p=0.01) (Supplementary Figure 2). However, the non-linearity multivariable spline model test indicated no significant departure from linearity (p=0.20); thus, in the multivariable analysis investigating independent predictors of SVD, ERMPG was modelled as a linear term. After correcting for potential confounders, ERMPG was an independent predictor of SVD (adjusted sHR 1.05, 95% CI: 1.01-1.08; p=0.004). Other independent predictors of SVD are reported in Figure 2. Among the 264 patients not included in the main analysis because of unknown vital status at 5-year follow-up, there were 2 patients with SVD. Sensitivity analyses including these patients provided similar results as the main analysis (Supplementary Table 6). There was no significant association between ERMPG and either all-cause mortality (adjusted hazard ratio [HR] 1.00, 95% CI: 0.99-1.02) or cardiovascular mortality (HR 1.00, 95% CI: 0.97-1.04).
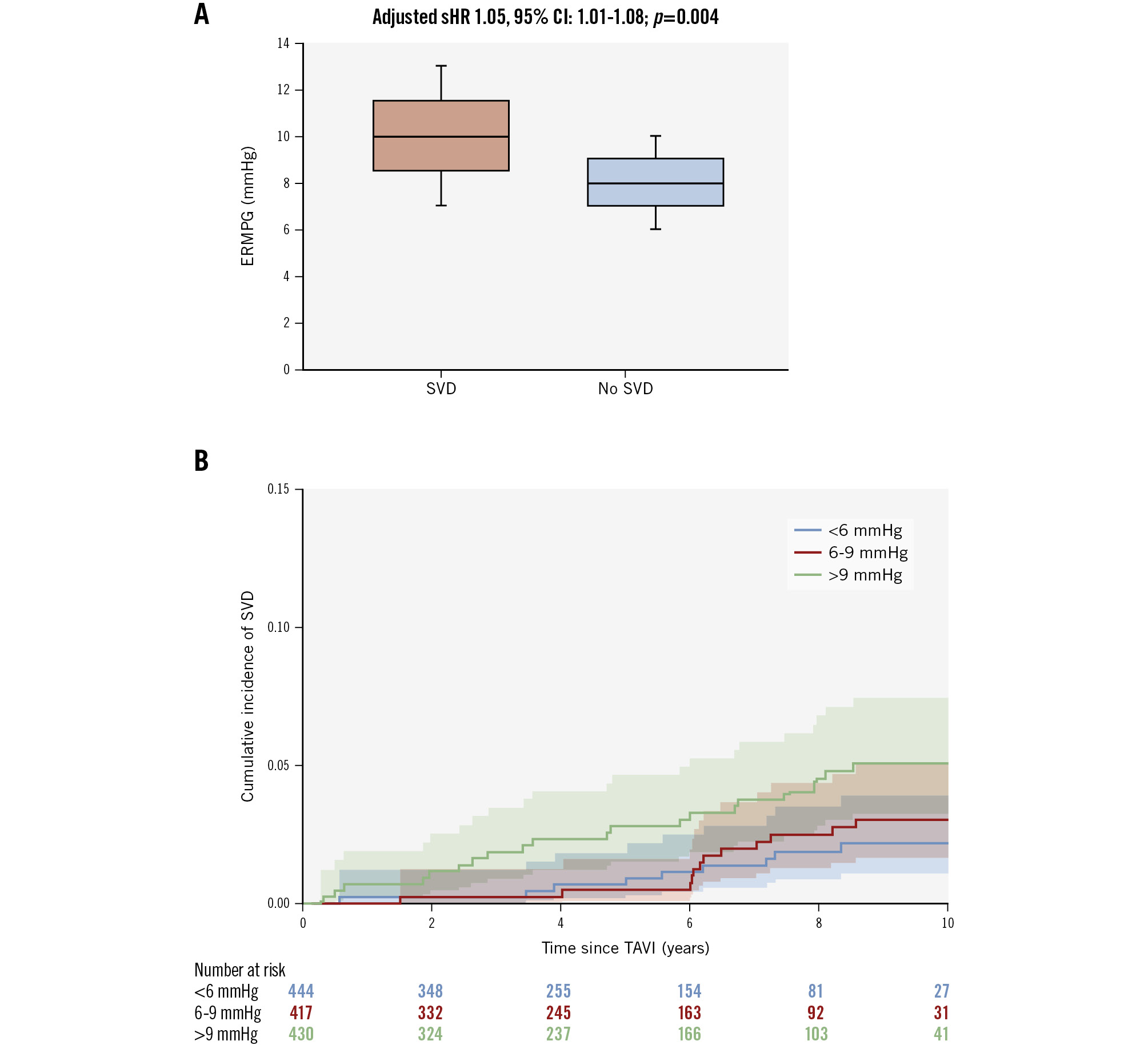
Figure 1. Mean postprocedural gradient and the risk of structural valve deterioration. A) Median values with interquartile range of early residual mean postprocedural gradient (ERMPG) in patients with structural valve deterioration (SVD) versus patients with no SVD. B) Risk of SVD in patients stratified by tertiles of ERMPG. Median values of ERMPG were significantly higher in patients with SVD versus patients with no SVD, and the risk of SVD significantly increased across tertiles of ERMPG. CI: confidence interval; sHR: subdistribution hazard ratio ; TAVI: transcatheter aortic valve implantation
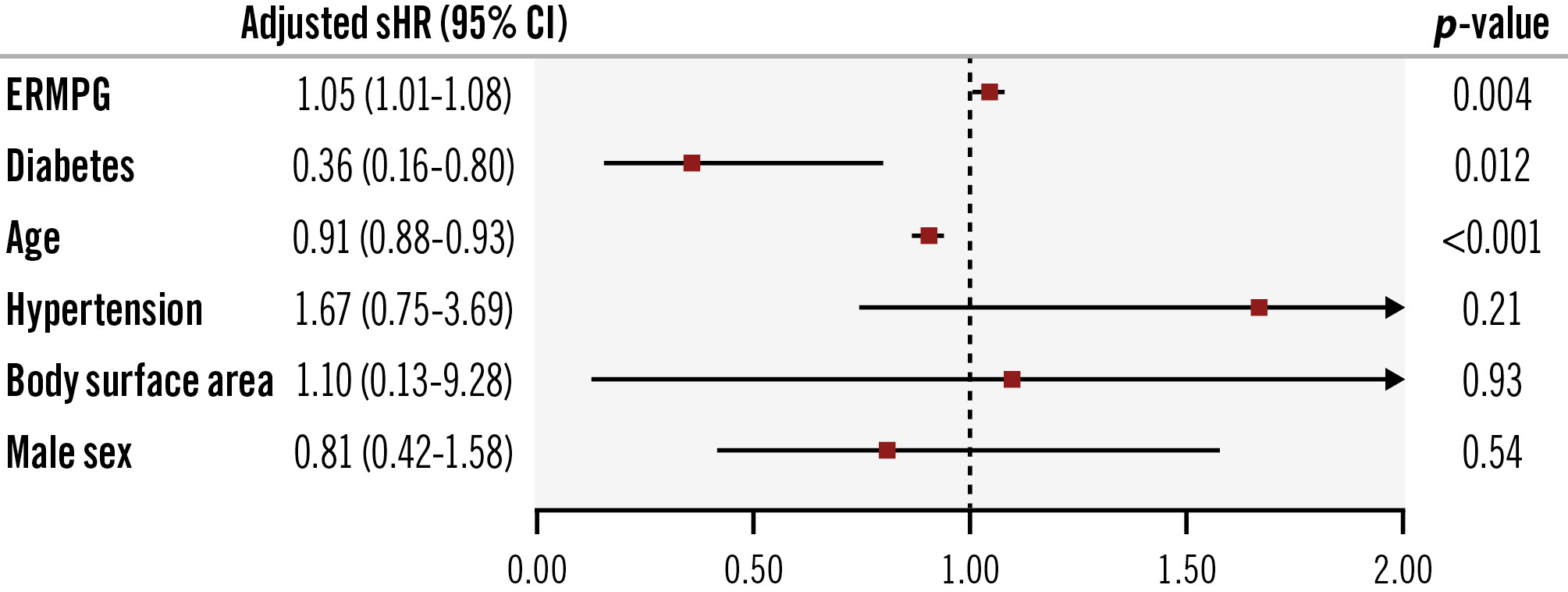
Figure 2. Independent predictors of structural valve deterioration. Early residual mean postprocedural gradient (ERMPG) measured at hospital discharge or within 90 days from transcatheter aortic valve implantation was an independent predictor of structural valve deterioration. Other independent predictors were diabetes and age. CI: confidence interval; sHR: subdistribution hazard ratio
Long-term mortality and SVD
Clinical outcomes at 10-year follow-up for the whole cohort of patients are shown in Supplementary Figure 3. Kaplan-Meier estimates of 10-year rates of all-cause death, cardiovascular death, and rehospitalisation for cardiac reasons were 88.1%, 26.1%, and 25.0%, respectively. Simon-Makuch survival analysis showed that SVD was associated with significantly higher rates of all-cause mortality (p<0.001; by the Mantel-Byar test) and cardiovascular mortality (p<0.001; also by the Mantel-Byar test) compared with no SVD (Figure 3). Specifically, 10-year rates for all-cause mortality were 39.5 per 100 patient-years with SVD versus 17.3 per 100 patient-years with no SVD (HR 1.73, 95% CI: 1.23-2.43; p=0.002). Similarly, 10-year rates for cardiovascular mortality were 12.1 per 100 patient-years with SVD versus 3.1 per 100 patient-years with no SVD (sHR 5.99, 95% CI: 3.00-11.97; p<0.001). The mean time from SVD to any cause of death was 1.5±1.4 years. After correcting for potential confounders, SVD was an independent predictor of all-cause mortality (adjusted HR 2.12, 95% CI: 1.49-3.00; p<0.001) and of cardiovascular mortality (adjusted sHR 5.78, 95% CI: 2.63-12.71; p<0.001). Other independent predictors of all-cause mortality and cardiovascular mortality are reported in Table 3 and Table 4, respectively. Sensitivity analyses including the 264 patients whose vital status was unknown at 5 years provided similar results (Supplementary Table 7, Supplementary Table 8, respectively).
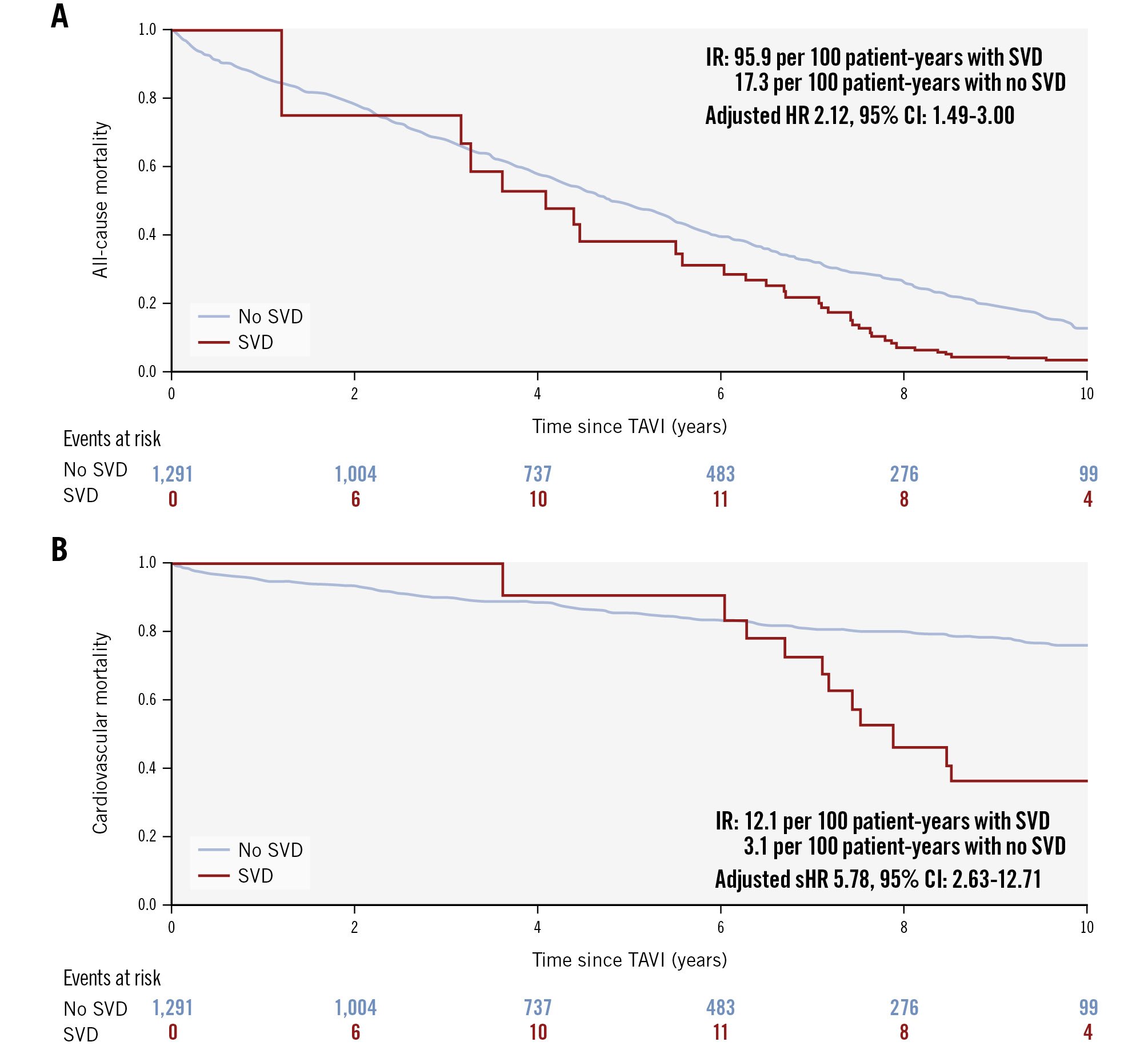
Figure 3. Simon-Makuch analyses of survival stratified by structural valve deterioration. A) All-cause mortality and (B) cardiovascular mortality. Patients with structural valve deterioration (SVD) had significantly higher rates of all-cause mortality and cardiovascular mortality. CI: confidence interval; HR: hazard ratio; IR: incidence rate; sHR: subdistribution hazard ratio ; TAVI: transcatheter aortic valve implantation
Table 3. Independent predictors of all-cause mortality.
| HR (95% CI) | p-value | |
|---|---|---|
| SVD | 2.12 (1.49-3.00) | <0.001 |
| Age | 1.03 (1.02-1.04) | <0.001 |
| CKD* | 1.39 (1.21-1.60) | <0.001 |
| Diabetes | 1.23 (1.08-1.41) | 0.002 |
| Pulmonary hypertension | 1.25 (1.05-1.49) | 0.012 |
| COPD | 1.51 (1.30-1.75) | <0.001 |
| LVEF per 5-unit increment | 0.98 (0.95-1.00) | 0.045 |
| *Defined as glomerular filtration rate ≤30 mL/min/1.73 m². CI: confidence interval; CKD: chronic kidney dysfunction; COPD: chronic obstructive pulmonary disease; HR: hazard ratio; LVEF: left ventricular ejection fraction; SVD: structural valve deterioration | ||
Table 4. Independent predictors of cardiovascular mortality.
| sHR (95% CI) | p-value | |
|---|---|---|
| SVD | 5.78 (2.63-12.71) | <0.001 |
| Diabetes | 1.43 (1.07-1.92) | 0.016 |
| Pulmonary hypertension | 1.82 (1.29-2.57) | <0.001 |
| COPD | 1.42 (1.03-1.97) | 0.033 |
| CI: confidence interval; COPD: chronic obstructive pulmonary disease; sHR: subdistribution hazard ratio; SVD: structural valve deterioration | ||
SVD and BVF
The relation between SVD and BVF is reported in Supplementary Figure 4. Among the 46 patients with SVD, 25 (54.3%) had BVF, 10 of whom had valve-related mortality, 9 underwent reintervention, and 6 had irreversible severe HVD. In total, reinterventions were performed in 11 patients, 4 of whom died thereafter from non-cardiovascular causes and 2 from cardiovascular causes. Reinterventions were redo-TAVI in 10 patients and surgical explant in 1 patient. There were 35 episodes of BVF, 25 (71.4%) of which were due to SVD, 7 (20.0%) due to non-SVD, and 3 (8.6%) due to endocarditis. ERMPG was significantly associated with BVF (sHR 1.04, 95% CI: 1.01-1.06; p=0.015). However, in the Fine-Gray multivariable model, the precision of the point estimate was reduced (adjusted sHR 1.03, 95% CI: 0.99-1.07), with age being the only independent predictor of BVF (adjusted sHR 0.92, 95% CI: 0.89-0.94).
Discussion
This is the first study to analyse the association between ERMPG, SVD, and the risk of long-term mortality. The main findings of this study are as follows: (1) ERMPG measured by Doppler echocardiography at hospital discharge or within 90 days from TAVI is an independent predictor of SVD; (2) when extending clinical surveillance at long-term follow-up (at least 10 years), SVD is the main modality of BVF; and (3) patients with SVD have higher 10-year rates of all-cause mortality and cardiac mortality compared with patients with no SVD.
The extension of TAVI to younger and lower-risk patients with severe aortic valve stenosis raises the problem of matching patient life expectancy with valve durability. A key component of valve durability is SVD, which is a chronic degenerative process of fibrocalcific bioprosthetic leaflet remodelling causing thickening and stiffening of the leaflets and/or leaflet tear, flail, or perforation. Although several factors have been associated with SVD, such as mechanical stress, glutaraldehyde fixation, systemic atherosclerosis, and humoral and cellular immune response, the exact mechanisms are unknown9.
Data on clinical and procedural predictors of SVD after TAVI are scant and inconsistent78. In addition, no plausible mechanistic association is apparent between most prior identified predictors and the risk of SVD. Specifically, in the study by O’Hair at al, age, sex, body surface area, prior percutaneous coronary intervention, hypertension, and prior atrial fibrillation were independent predictors of SVD8, whereas in the study by Del Trigo et al, absence of anticoagulation therapy at hospital discharge, a valve-in-valve procedure (TAVI in SAVR), the use of a 23 mm valve, and a greater body mass index were independent predictors of SVD7. Of note, a valve-in-valve procedure and the use of a 23 mm valve may be surrogates of increased postprocedural gradients.
Based on animal studies suggesting an association between shear-induced transforming growth factor-β1 activation and progression of aortic valve stenosis, we hypothesised that a higher ERMPG after TAVI could predispose patients to a vicious circle of fibrocalcific valve remodelling ultimately leading to SVD10. Consistent with this hypothesis, we found that patients with SVD had a significantly higher ERMPG compared with patients with no SVD, that patients in the upper tertile of ERMPG had significantly higher rates of SVD compared with patients in the intermediate and lower tertiles, and finally that ERMPG was an independent predictor of SVD.
This is the first study to show an association between ERMPG and SVD after TAVI. This finding is consistent with the observation that, in some randomised studies comparing TAVI versus SAVR, treatments associated with a lower ERMPG were also associated with lower SVD rates. Specifically, in the NOTION Trial, patients treated with the CoreValve/Evolut system (Medtronic) had both lower ERMPG (8.3 mmHg vs 12.2 mmHg; p<0.001) and lower 10-year rates of severe SVD (1.5% vs 10.0%, respectively; p=0.02) compared with SAVR12. Similarly, in a pooled dataset of two randomised trials including 2,099 patients, the CoreValve/Evolut system was again associated with lower ERMPG (9.1 mmHg vs 12.6 mmHg; p<0.001) and lower 5-year SVD rates (2.2% vs 4.3%; p=0.004) compared with SAVR8. In contrast, in the PARTNER 3 Trial, the SAPIEN 3 valve (Edwards Lifesciences) was associated with similar ERMPG (12.8 mmHg vs 11.2 mmHg) and similar 5-year SVD rates (4.2% vs 3.8%) compared with SAVR4. Finally, in the CHOICE Trial13 comparing CoreValve versus SAPIEN XT (Edwards Lifesciences), the former was associated with lower ERMPG (6.4 mmHg vs 8.4 mmHg; p<0.001) and lower 5-year SVD rates (0.0% vs 6.6%; p=0.018) compared with the latter. Whether these associations are mechanistic or the play of chance deserves further investigation.
Our findings may have practical implications, considering that several strategies can be implemented to reduce ERMPG after TAVI, such as the choice of supra-annular bioprostheses in small annuli14, a more liberal use of post-dilation after THV deployment, and the implementation of surgical valve fracturing in valve-in-valve procedures15. Whether these strategies may benefit patients undergoing TAVI deserves confirmation in randomised controlled trials. Of note, a signal of an increased risk of SVD was apparent for ERMPG values greater than 10 mmHg. However, given that the HR of ERMPG for the risk of SVD was 1.05, this risk would remain relatively small for ERMPG values just above 10 mmHg, becoming clinically significant only at higher ERMPG values.
Some studies have challenged the use of Doppler echocardiography to assess transcatheter heart valve function due to discordance with invasive measurements16. Abbas et al have reported lower mean postprocedural gradients after TAVI with self-expanding versus balloon-expandable valves with non-invasive measures and the opposite with invasive measures, suggesting that invasive and non-invasive measures are not interchangeable17. Furthermore, it is not known whether this discrepancy in the acute phase is maintained at long-term follow-up. Finally, the definition of SVD as serial changes in mean gradients with a concomitant reduction in effective orifice area measured by Doppler echocardiography is an arbitrary definition issued by a consortium of experts, but it has never received a prognostic validation in clinical studies at long-term follow-up6. Thus, studies investigating prognostic correlates of echocardiographic measures after TAVI are missing and eagerly needed to validate non-invasive measures as the appropriate tool to monitor valve dysfunction.
Importantly, we found that a Doppler-derived ERMPG was an independent predictor of SVD, and SVD defined with echocardiographic criteria was associated with all-cause mortality, cardiovascular mortality, and BVF (Central illustration). On the other hand, although pressure recovery is a real physical phenomenon based on sound fluid dynamics that may overestimate echo-Doppler gradients18, no study has ever investigated the association between invasive measures and SVD after TAVI, and therefore, the extent to which invasive versus non-invasive measures differ in predicting BVF or mortality at long-term follow-up remains undetermined. In addition, it would be unpractical to follow patients with TAVI using invasive measures. Our findings are consistent with the study by O’Hair et al, which reported increased 5-year rates of mortality in patients with SVD defined with the same echocardiographic criteria as in our study8. However, in that study, the relation between SVD and BVF was not explored, and follow-up was limited to 5 years. We extended follow-up to 10 years and found that SVD was responsible for the majority of cases (71.4%) of BVF, and therefore, it is a key element for lifetime management in young and low-risk patients.
Previous studies have reported lower rates for the association between SVD and BVF. Specifically, in the study by Pibarot et al, among the 15 patients with SVD, 33% had or developed BVF, whereas among the 16 patients with all-cause BVF, the rate of SVD was 32% with the SAPIEN 3 valve19. However, in that study, the length of follow-up was only 5 years, whereas in our study it was double that. It is therefore possible that to appraise the real incidence of BVF in patients with SVD, a follow-up longer than 5 years is needed.
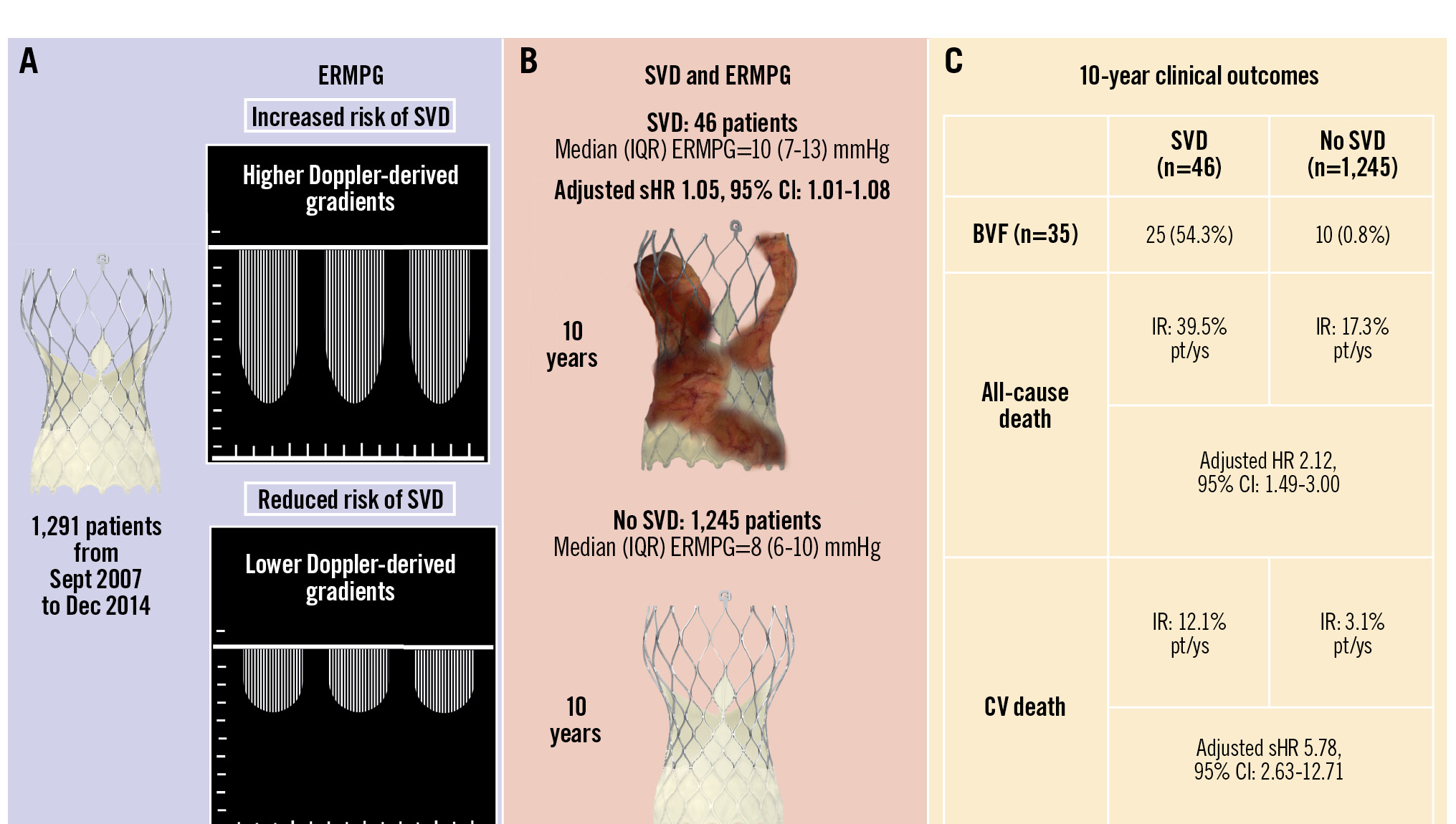
Central illustration. Postprocedural gradient, structural valve deterioration, and 10-year mortality after TAVI. A) Patients with higher early residual mean postprocedural gradient (ERMPG) measured by Doppler-echocardiography at hospital discharge or within 90 days from TAVI had increased rates of structural valve deterioration (SVD). B) Among the 46 patients with SVD, the median (interquartile range [IQR]) ERMPG was 10 (7-13) mmHg, whereas among the 1,245 patients with no SVD, the median (IQR) ERMPG was 8 (6-10) mmHg. C) Patients with SVD had increased rates of bioprosthetic valve failure (BVF) and increased incidence rates (IRs) of all-cause mortality and cardiovascular mortality compared with patients with no SVD. CI: confidence interval; CV: cardiovascular; HR: hazard ratio; pt/ys: patient-years; sHR: subdistribution hazard ratio; TAVI: transcatheter aortic valve implantation
Limitations
We used a definition of SVD which is slightly different from that provided by VARC-3 criteria in that we considered serial changes in mean gradients only, without considering the reduction in effective orifice area, which was not available in more than half of the patients6. However, the prognostic value of adding effective orifice area on top of the gradient assessment is unknown. In addition, a prior study reported a more robust prediction of clinical outcomes with the same definition used in our study compared with the complete VARC-3 SVD definition8. We cannot exclude that in some patients changes in gradients reflected changes in flow, but it is unlikely that this factor affected the main findings of the study. Echocardiographic assessment of bioprosthetic function was site-reported and not performed in a core laboratory. Finally, echocardiographic measures of flow velocities and gradients may be valve-specific and related to valve design, and therefore, whether these findings apply to other transcatheter heart valves deserves further investigation.
Conclusions
In an all-comer population of patients with severe aortic valve stenosis undergoing TAVI with the CoreValve/Evolut system, ERMPG was an independent predictor of SVD. Patients with SVD, defined as serial changes of Doppler-derived mean gradients or the presence of new aortic regurgitation, had a more than 50% risk of BVF. In addition, they had increased rates of mortality and cardiovascular mortality compared with patients without SVD.
Impact on daily practice
This is the first study suggesting an association between postprocedural gradient after transcatheter aortic valve implantation (TAVI) and the risk of structural valve deterioration (SVD). In addition, we found an increased risk of 10-year mortality in patients with SVD. These findings may have practical implications, since several strategies can be implemented to reduce the postprocedural gradient after TAVI, such as the choice of supra-annular bioprostheses in small annuli, a more liberal use of post-dilation after transcatheter heart valve deployment, and the implementation of surgical valve fracturing in valve-in-valve procedures. In addition, lower postprocedural gradients than those currently identified may be a better target to optimise clinical outcomes.
Conflict of interest statement
T. Palmerini has received speaker fees from Abbott, Biotronik, Edwards Lifesciences, and Medtronic; he is also a proctor for Evolut (Medtronic) implantation. F. Saia has received consultancy and lecture fees from Abbott, Edwards Lifesciences, and Medtronic. B. Bellini has received consultant fees from Medtronic. M. Montorfano has received consultant fees from Abbott, Boston Scientific, Kardia Medical, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

