Cory:
Unlock Your AI Assistant Now!
The recently updated European Society of Cardiology/European Association for Cardio-Thoracic Surgery (ESC/EACTS) Guidelines for the management of valvular heart disease recommend transcatheter aortic valve implantation (TAVI) as the preferred treatment strategy for patients aged ≥70 years with tricuspid aortic valve stenosis across the entire operative risk spectrum1. In this context, lifetime management has become a key aspect, as the life expectancy of most patients likely now exceeds valve durability, mandating long-term planning for potential future valve reinterventions. Due to the lack of solid evidence, however, the long-term durability of transcatheter heart valves (THVs) remains a matter of debate. Available studies with up to ten years of follow-up provide reassuring results with low rates of structural valve deterioration (SVD) and bioprosthetic valve failure (BVF) after TAVI2. However, as TAVI expands to younger patients, a deeper understanding of THV durability and its determinants becomes critical to inform individualised lifetime management strategies.
In this issue of EuroIntervention, Palmerini and colleagues present an important study exploring the association between early residual mean postprocedural gradients (ERMPGs) and long-term SVD rates, as well as the prognostic implications of SVD for BVF and mortality at 10 years, in a multicentre registry of 1,291 patients treated with a self-expanding, supra-annular CoreValve/Evolut prosthesis (Medtronic)3. The cumulative incidence of SVD was 3.6% (n=46) during extended follow-up, with a median time to SVD of 5.7±3.0 years. Notably, ERMPG emerged as an independent predictor of SVD, with a stepwise increase in SVD risk across the tertiles of ERMPG. An ERMPG threshold of 9 mmHg best predicted the 5-year risk of SVD. More than half of the patients with SVD (54%) developed BVF, and SVD was strongly associated with higher all-cause (adjusted hazard ratio [HR] 2.12; p<0.001) and cardiovascular mortality (adjusted HR 5.78; p<0.001) (Figure 1).
The authors should be commended for providing these valuable long-term data, which extend our understanding of SVD after TAVI, an area where evidence remains scant and heterogeneous45. The hypothesis proposed by the authors seems convincing: higher residual postprocedural gradients may trigger a vicious circle of shear-dependent, fibrocalcific remodelling leading to progressive valve degeneration6. Consistent with this, they demonstrated that residual postprocedural gradients, even when modest in magnitude, independently predict SVD, which, in turn, is associated with higher long-term mortality.
Several methodological aspects merit attention when interpreting these findings. The authors defined SVD primarily by an increase in mean gradients over time (or new intraprosthetic regurgitation), without including a concomitant decrease in effective orifice area or Doppler velocity index, as recommended by current Valve Academic Research Consortium-3 criteria and reflected in current ESC/EACTS guidelines17. The gradients might be influenced by different flow characteristics, and furthermore, the absence of confirmatory imaging, such as four-dimensional computed tomography (CT), transoesophageal echocardiography, or positron emission tomography-CT, limits the possibility to exclude non-structural valve dysfunction (i.e., prosthesis-patient mismatch), THV thrombosis, or endocarditis. These assessments are essential to elucidate the underlying pathophysiology and intrinsic morphological changes related to SVD, such as leaflet fibrosis, calcification, or strut deformation, and to determine whether the process leading to SVD might be modifiable.
CT-derived preprocedural anatomical data, such as annulus size and extent, and distribution of calcification, are not reported in the study by Palmerini et al. Interestingly, the proportion of smaller prosthesis implantation was higher among patients with SVD (p=0.03), whereas pre- (68% vs 74%; p=0.37) and post-dilatation rates (22% vs 26%; p=0.47) were similar between the two groups, without and with SVD. The significantly lower prevalence of atrial fibrillation among patients with SVD (6.5% vs 21.5%) is noteworthy, given that previous studies have suggested that oral anticoagulation may reduce the incidence of haemodynamic valve deterioration58, albeit at the cost of higher long-term mortality in this elderly population9.
The role of echocardiography in assessing THV function also warrants careful consideration. Discrepancies between invasive and echocardiography-derived transprosthetic gradients are well recognised and largely explained by the pressure recovery phenomenon following TAVI1011. Consequently, these two modalities are not interchangeable. Recent data published in EuroIntervention indicate that invasive, but not echocardiographic, gradients predict all-cause mortality at two years, with a mean invasive gradient >10 mmHg identifying patients at risk for adverse outcomes across both supra- and intra-annular THVs12. Several dedicated ongoing prospective studies will provide further insights in this regard (e.g., IVEGA-TAVI; ClinicalTrials.gov: NCT06753227). The current findings by Palmerini and colleagues should therefore be interpreted within this broader context.
Finally, these data are exclusively limited to the self-expanding supra-annular CoreValve/Evolut prostheses. Whether similar associations between early postprocedural gradients after TAVI and valve deterioration exist for balloon-expandable intra-annular THVs remains an open question. Supra-annular valves generally yield lower echocardiography-derived gradients but comparable invasive gradients to intra-annular platforms1213. Future research should therefore integrate multimodal imaging and both invasive and non-invasive haemodynamic assessment to better delineate true predictors of SVD across THV platforms.
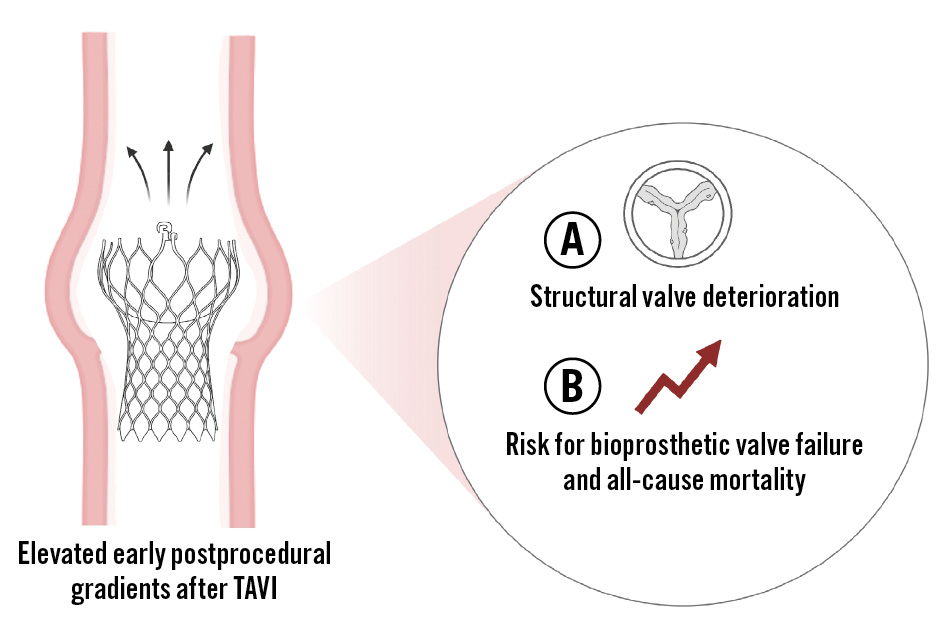
Figure 1. Impact of early residual mean postprocedural gradients after transcatheter aortic valve implantation using a self-expanding, supra-annular transcatheter heart valve on structural valve deterioration, bioprosthetic valve failure, and mortality. TAVI: transcatheter aortic valve implantation
Conflict of interest statement
T. Rheude discloses honoraria from AstraZeneca, Boston Scientific, Edwards Lifesciences, Lilly, SIS Medical AG, Terumo, and Translumina; and support from Boehringer Ingelheim, Edwards Lifesciences, LifeTech Scientific, and SIS Medical AG. H.A. Alvarez Covarrubias discloses consulting fees from LifeTech Scientific; honoraria from SIS Medical AG, Terumo, Edwards Lifesciences, Translumina, and LifeTech Scientific; and support from Abbott, SIS Medical AG, and LifeTech Scientific.

