Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Subclinical leaflet thrombosis, as indicated by hypoattenuated leaflet thickening (HALT) on computed tomography (CT) imaging, remains a major concern owing to its potential impact on valve function and patient outcomes.
Aims: We aimed to evaluate the association between HALT and clinical outcomes in patients undergoing valve-in-valve (ViV) transcatheter aortic valve implantation (TAVI) with balloon-expandable valves and to identify predictors of leaflet thrombosis.
Methods: Consecutive patients who underwent ViV TAVI with balloon-expandable valves at the Cedars-Sinai Medical Center were retrospectively analysed. We analysed both pre- and postprocedural CT scans to identify predictors of HALT at 1 month after ViV TAVI and the association of HALT with clinical outcomes. The primary outcome was a composite of all-cause mortality, hospitalisation for heart failure (HF), or stroke at 3 years.
Results: Among the 117 patients analysed, HALT was detected in 37 (31.6%). In the multivariable analysis, anticoagulation therapy (odds ratio [OR] 0.28, 95% confidence interval [CI]: 0.08-0.92; p=0.037) and greater transcatheter heart valve (THV) expansion at the minimum area level (OR 0.95, 95% CI: 0.91-0.99; p=0.026) were significant predictors of reduced HALT following ViV TAVI. While there was no significant difference in all-cause mortality between patients with and without HALT (OR 1.13, 95% CI: 0.42-3.02; p=0.8), those with HALT had a significantly higher incidence of the composite primary outcome (OR 2.31, 95% CI: 1.04-5.15; p=0.04).
Conclusions: HALT was frequently observed in patients who underwent ViV TAVI. Additionally, the presence of HALT correlated with a higher incidence of composite outcomes, including all-cause mortality, hospitalisation for HF, and stroke. Assessment of TRanscathetEr and Surgical Aortic BiOprosthetic VaLVe Dysfunction and Its TrEatment with Anticoagulation (RESOLVE; ClinicalTrials.gov: NCT02318342).
Subclinical leaflet thrombosis remains a significant concern, with a higher incidence following transcatheter aortic valve implantation (TAVI) than following surgical aortic valve replacement (SAVR)12. The prognostic implications of leaflet thrombosis, detected as hypoattenuated leaflet thickening (HALT) and hypoattenuation affecting motion (HAM) on computed tomography (CT), remain a subject of debate despite its association with altered valve haemodynamics and increased risk of stroke or transient ischaemic attack (TIA)134. It is also hypothesised that subclinical thrombosis may contribute to accelerated haemodynamic valve degeneration, thereby playing a crucial role in valve durability5. The identification and therapeutic management of leaflet thrombosis are crucial for optimising patient outcomes post-TAVI.
Bioprosthetic valve use for SAVR has expanded even among younger patients6. Consequently, the prevalence of valve-in-valve (ViV) TAVI procedures, serving as a crucial strategy in the lifelong management of structural valve degeneration (SVD), is anticipated to increase significantly. Although prior studies have suggested that ViV TAVI may offer improved short-term outcomes compared to performing a second SAVR789, neither the impact of leaflet thrombosis after ViV TAVI on clinical outcomes nor its potential predictors have been sufficiently examined. This study aimed to explore the prevalence of HALT in patients undergoing ViV TAVI with balloon-expandable valves, assess clinical outcomes associated with leaflet thrombosis, and identify contributing risk factors through detailed peri- and postprocedural CT analysis.
Methods
Study population
This retrospective observational study was conducted at the Cedars-Sinai Medical Center and included consecutive patients who underwent ViV TAVI between March 2015 and September 2021. Among them, 139 patients underwent electrocardiogram (ECG)-gated, contrast-enhanced cardiac CT to detect HALT and assess transcatheter heart valve (THV) expansion at 1 month post-TAVI as part of the Assessment of Transcatheter and Surgical Aortic Bioprosthetic Valve Thrombosis and Its Treatment with Anticoagulation (RESOLVE) registry (ClinicalTrials.gov: NCT02318342). Patients who underwent ViV TAVI using self-expanding prosthetic valves were excluded. The study protocol adhered to the ethical standards of the Declaration of Helsinki (1975) and was approved by the Institutional Review Board of the Cedars-Sinai Medical Center. Informed consent was obtained from all the participants.
Contrast-enhanced multidetector CT image acquisition
A detailed description of the contrast-enhanced multidetector CT (MDCT) image acquisition is provided in Supplementary Appendix 1.
Peri- and postprocedural CT analysis
Periprocedural CT assessment
To define the geometric parameters relevant to THV placement, we established a reference line connecting the central points of the proximal ascending aorta, aortic valve annulus, and left ventricular outflow tract (LVOT). The basal inflow plane was defined as the plane perpendicular to the aforementioned line at the lowest point of the THV ring on the three leaflets. Various crucial data points, such as the orthogonal major and minor dimensions, area, and perimeter, were obtained through segment tracing. LVOT was measured from a perpendicular plane within the left ventricle positioned 4 mm below the basal inflow plane. The distance between the virtual THV and the coronary ostia (VTC) was assessed using a linear calliper with a cylinder approximating the diameter of the THV superimposed on the tip of the previous valve column. Aortic angulation was calculated from the coronal view at the annular level and defined as the angle subtended by the annular plane and horizontal reference.
Postprocedural CT assessment
A four-dimensional MDCT scan was performed at 1 month post-ViV TAVI to evaluate prosthetic valve geometry and detect leaflet thrombosis. HALT was defined as a discernible increase in leaflet thickness in the diastolic phase (Figure 1A). HALT at 30 days was identified based on a systematic CT methodology as described in previous studies10. When HALT was present, motion reduction of each leaflet was evaluated using a multiphase volume-rendered en face cine projection. A leaflet with motion reduction by >50% relative to the radius of the bioprosthetic frame was termed “HAM” (Figure 1B). To quantify the geometry of the THV post-ViV TAVI, we included measurements of the external stent frame area, as well as maximum and minimum diameters at the following anatomical levels: frame inflow, native annulus, leaflet inflow, prosthesis waist (the tip of the outer skirt), leaflet outflow, frame outflow, and the level with the minimum area (Figure 1C). The minimum area was determined by sorting the CT images to determine the smallest area within the THV frame. THV expansion was assessed at seven anatomical levels, and the percentage of expansion was calculated by comparing the measured external THV area at each level with the nominal area of the valve. THV eccentricity was assessed as the percentage ratio of the minimum to the maximum diameters at each level (Figure 1D). After identifying all three commissure angles of the THV, we measured the intercommissural angles as the angles formed between the commissures of each cusp (Supplementary Figure 1A). The angle of coronary overlap was determined by measuring the angular distance between the coronary ostia and the nearest commissures of the THV. Severe coronary overlap was defined as an angle of less than 15 degrees from any commissural post to the coronary ostium, following a previous study (Supplementary Figure 1B, Supplementary Figure 1C)11. The horizontal distances from the frame of the THV to each coronary ostium and each cusp of the sinus of Valsalva were measured (Supplementary Figure 1D-Supplementary Figure 1E-Supplementary Figure 1F). The implantation depth of the THV was defined as the mean of the distances from the THV inflow to the basal plane at each native aortic valve cusp (Supplementary Figure 1G-Supplementary Figure 1H-Supplementary Figure 1I).
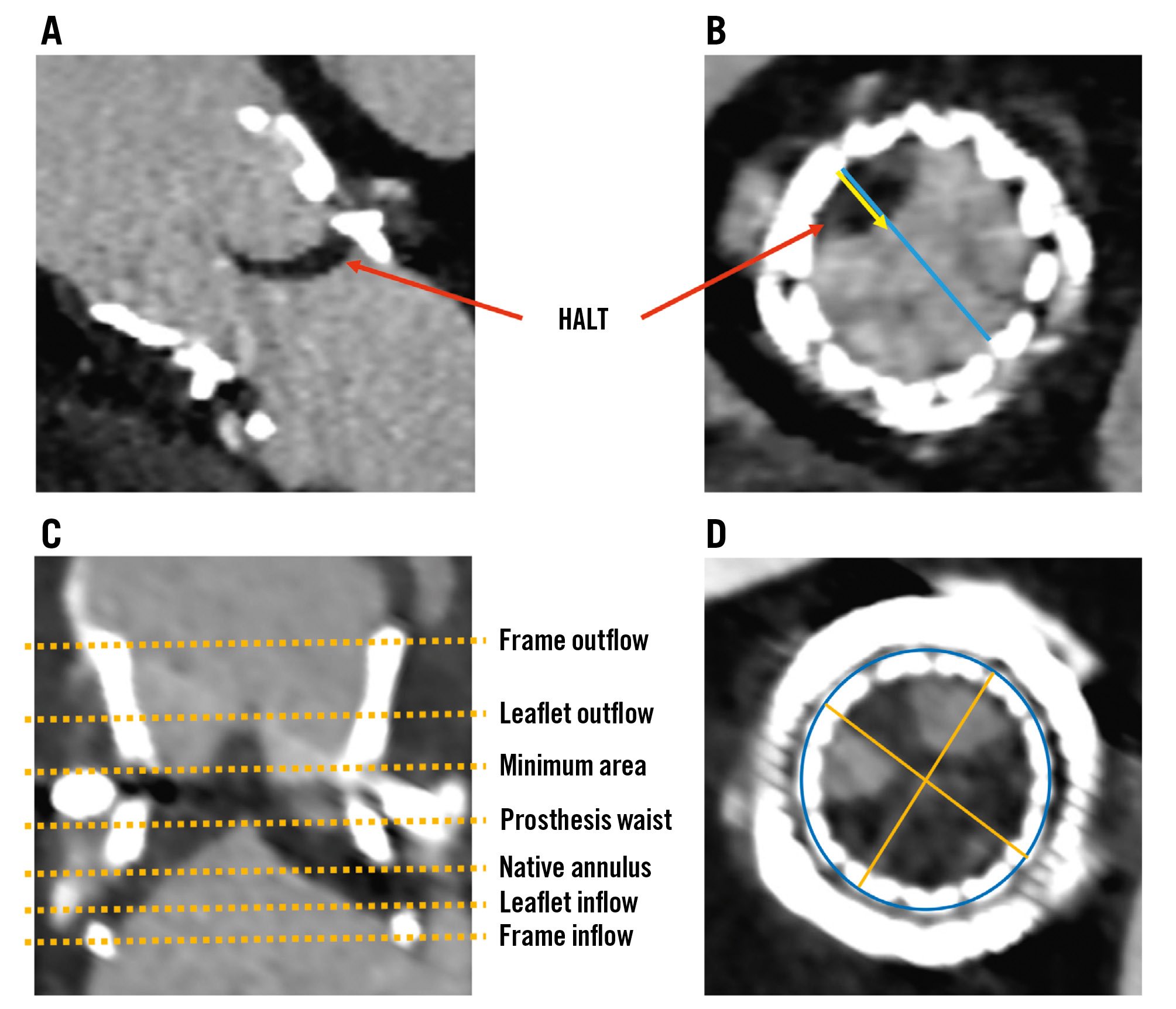
Figure 1. Detection of HALT and HAM, and assessment of THV expansion and eccentricity. Four-dimensional multidetector computed tomography scans obtained at 1 month post-ViV TAVI displaying a longitudinal cut demonstrating leaflet thickness (A) and a cross-sectional view of the prosthetic valve in diastole (B). These images illustrate the detection of HALT and HAM, with HALT identified by increased leaflet thickness during the diastolic phase (red arrow) and HAM evidenced by a reduction in leaflet motion exceeding 50% relative to the radius of the bioprosthetic frame. The blue line represents the diameter of the bioprosthetic frame, whereas the yellow arrow represents the reduction in leaflet motion, indicating the extent of HALT. Longitudinal (C) and axial (D) cross-sectional views after ViV TAVI. The stent frame area and diameters are measured at specified anatomical levels, with yellow dashed lines indicating the levels: frame inflow, leaflet inflow, native annulus, prosthesis waist, minimum area, leaflet outflow, and frame outflow (C). The minimum area, as well as maximum and minimum diameters are represented by the blue circle and yellow lines across all levels (D). These measurements are used to calculate the expansion and eccentricity of the THV at each level. The stent expansion percentage formula: (stent frame area/manufacturer’s nominal stent frame area)×100%. The formula for eccentricity: 1–(minimum external stent diameter/maximum external stent diameter)×100%. HALT: hypoattenuated leaflet thickening; HAM: hypoattenuation affecting motion; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve; ViV: valve-in-valve
Echocardiographic follow-up
Postoperative transthoracic echocardiography (TTE) was conducted at 30 days, 1 year, and 2 years to monitor haemodynamic outcomes and assess valve performance. The severity of the paravalvular leak was categorised based on the recommended echocardiography criteria: none, trace, mild, moderate, or severe12.
TAVI procedures
The TAVI procedures were performed under general anaesthesia using fluoroscopic and echocardiographic guidance. Weight-adjusted unfractionated heparin was administered during TAVI to maintain an activated clotting time of ≥250 s. Balloon-expandable prostheses, specifically SAPIEN XT, SAPIEN 3, and SAPIEN Ultra (all Edwards Lifesciences), were used for all TAVI procedures. The selection of THV size and vascular access was determined through CT imaging.
Clinical outcomes and follow-up
The primary outcome was a composite of all-cause mortality, hospitalisation for heart failure (HF) or stroke within 3 years after ViV TAVI. Secondary outcomes included the following individual component endpoints: all-cause mortality, hospitalisation for HF, and stroke within 3 years after ViV TAVI. To evaluate procedural outcomes and functional status after ViV TAVI, in-hospital events were analysed. Adverse events were assessed according to Valve Academic Research Consortium 3 criteria13. Functional status, as measured by New York Heart Association (NYHA) Functional Class, was assessed at baseline, 30 days, 1 year, and 3 years after ViV TAVI. Severe patient-prosthesis mismatch (PPM) was defined as an indexed effective orifice area (EOA) ≤0.65 cm²/m² for non-obese patients (body mass index <30 kg/m²) and an indexed EOA ≤0.55 cm²/m² for obese patients (body mass index ≥30 kg/m²)13. Data on patient demographics, CT scans, echocardiography findings, medical history, procedural details, and follow-up were retrospectively collected from the interventional cardiology laboratory database of our institution. Supplementary data were obtained during outpatient clinic visits and telephone follow-ups.
Statistical analyses
Continuous variables are presented as mean±standard deviation or median and interquartile range. Categorical variables are presented as frequencies and percentages. The Student’s t-test or the Mann-Whitney U test were used to compare continuous variables between the two groups, as appropriate. Categorical variables were assessed using the chi-square test. Cumulative event rates were estimated using Kaplan-Meier survival analysis, and the log-rank test was used to compare the groups. Logistic regression analysis was used to identify predictors of HALT 1 month after ViV TAVI. The presented model encompassed the following variables: age, sex, anticoagulation status, minimum area expansion, and severe overlap between the THV commissure and the right coronary artery (RCA), with their respective odds ratios (ORs) and 95% confidence intervals (CIs). Cox regression analysis was utilised to compute hazard ratios (HRs) and 95% CIs. Statistical analyses were performed using SPSS, version 26 (IBM), with a p-value of ≤0.05 considered statistically significant.
Results
Patient characteristics
This study included 139 consecutive patients who underwent MDCT 1 month after ViV TAVI (Supplementary Figure 2). Twenty-two patients were excluded due to a lack of follow-up data, suboptimal CT image quality, or received self-expanding valves, and the included 117 patients were divided based on postprocedural HALT, with 37 (31.6%) demonstrating HALT and 80 (68.4%) not. Table 1 summarises the baseline characteristics of the entire cohort and of each group. The overall mean age was 76.1 years, with males accounting for 56.4%. The Society of Thoracic Surgeons risk scores showed no significant differences between patients with and without HALT. Although trends were observed in the use of anticoagulation therapy between the groups, the differences were not statistically significant. Echocardiographic parameters, such as aortic valve area, left ventricular ejection fraction, and mean aortic valve gradients, were comparable between the groups. There were no procedural differences between patients who underwent post-TAVI CT scans and those who did not (Supplementary Table 1).
Table 1. Baseline patient characteristics.
| Overall N=117 | HALT (+) N=37 | HALT (−) N=80 | p-value | |
|---|---|---|---|---|
| Age, years | 76.1±11.2 | 77.8±12.5 | 75.4±10.5 | 0.31 |
| Sex, male | 66 (56.4) | 22 (59.5) | 44 (55.0) | 0.65 |
| BMI, kg/m2 | 26.8±5.9 | 26.7±4.9 | 27.1±6.4 | 0.52 |
| Hypertension | 101 (86.3) | 32 (86.5) | 69 (86.3) | 0.97 |
| Hyperlipidaemia | 93 (79.5) | 31 (83.8) | 62 (77.5) | 0.43 |
| Prior CVA/TIA | 25 (21.4) | 7 (18.9) | 18 (22.5) | 0.66 |
| Porcelain aorta | 15 (12.8) | 6 (16.2) | 9 (11.3) | 0.46 |
| Smoker | 41 (35.0) | 15 (40.5) | 26 (32.5) | 0.4 |
| Diabetes | 25 (21.4) | 5 (13.5) | 20 (25.0) | 0.16 |
| Current dialysis | 2 (1.7) | 1 (2.7) | 1 (1.3) | 0.57 |
| Chronic lung disease | 16 (13.7) | 7 (18.9) | 9 (11.3) | 0.26 |
| AFib | 39 (33.3) | 9 (24.3) | 30 (37.5) | 0.16 |
| Prior PAD | 4 (3.4) | 2 (5.4) | 2 (2.5) | 0.42 |
| Previous PPI | 20 (17.1) | 7 (18.9) | 13 (16.3) | 0.72 |
| CAD | 49 (41.9) | 12 (32.4) | 37 (46.3) | 0.16 |
| Prior MI | 13 (11.1) | 3 (16.2) | 7 (8.8) | 0.23 |
| CKD, stage ≥3 | 15 (12.8) | 5 (13.5) | 10 (12.5) | 0.88 |
| Prior CABG | 32 (27.4) | 5 (24.3) | 23 (28.8) | 0.62 |
| Prior PCI | 12 (10.3) | 3 (8.1) | 9 (11.3) | 0.6 |
| NYHA Class | 0.59 | |||
| I | 0 (0) | 0 (0) | 0 (0) | |
| II | 9 (7.7) | 4 (10.8) | 5 (6.3) | |
| III | 60 (51.3) | 17 (45.9) | 43 (53.8) | |
| IV | 48 (41.0) | 16 (43.2) | 16 (43.2) | |
| STS-PROM risk score* | 5.61±3.37 | 6.00±3.9 | 5.42±3.01 | 0.43 |
| Antithrombotic therapy | ||||
| Aspirin (any) | 81 (69.2) | 28 (75.7) | 53 (66.3) | 0.31 |
| P2Y12 (any) | 33 (28.2) | 11 (29.7) | 22 (27.5) | 0.8 |
| Anticoagulation therapy | 32 (27.4) | 6 (16.2) | 26 (32.5) | 0.066 |
| NOACs | 15 (12.8) | 3 (8.1) | 12 (15.0) | 0.3 |
| Warfarin | 17 (14.5) | 3 (8.1) | 14 (17.5) | 0.18 |
| LVEF, % | 58.1±13.6 | 58.4±14.4 | 58.0±13.2 | 0.88 |
| Mean PG, mmHg | 34.4±15.0 | 35.5±12.9 | 33.8±16.0 | 0.53 |
| Peak PG, mmHg | 58.7±24.1 | 60.5±21.0 | 57.9±25.5 | 0.57 |
| Preprocedural AVA, cm² | 0.84±0.33 | 0.76±0.36 | 0.87±0.32 | 0.11 |
| Bicuspid aortic valve | 40 (34.2) | 12 (32.4) | 28 (35.0) | 0.79 |
| Aortic insufficiency | 57 (48.7) | 16 (43.2) | 41 (51.3) | 0.21 |
| Moderate or severe MR | 8 (6.8) | 1 (2.7) | 7 (8.7) | 0.11 |
| Values are mean±SD or n (%). *The Society of Thoracic Surgeons Predicted Risk of Mortality (STS-PROM) score estimates patient risk at the time of cardiovascular surgery. AFib: atrial fibrillation; AVA: aortic valve area; BMI: body mass index; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CKD: chronic kidney disease; CVA: cerebrovascular accident; HALT: hypoattenuated leaflet thickening; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MR: mitral regurgitation; NYHA: New York Heart Association; PAD: peripheral artery disease; PCI: percutaneous coronary intervention; PG: pressure gradient; PPI: permanent pacemaker implantation; SD: standard deviation; TIA: transient ischaemic attack | ||||
Procedural data
The interval since the initial SAVR/TAVI procedure was comparable between the groups, with no statistically significant differences in the type or size of the TAVI device employed (Supplementary Table 2). The use of adjunctive procedures such as cerebral embolic protection, Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA), and percutaneous coronary intervention (PCI) was similar.
In terms of in-hospital outcomes, the frequency of adverse events − including in-hospital death, cardiac arrest, cerebrovascular accident, TIA, acute coronary obstruction, major bleeding, major vascular complications, acute kidney injury, and the necessity for permanent pacemaker implantation − did not significantly differ between patients with and without HALT. Cumulative in-hospital complications and duration of hospital stay also showed no significant differences between the groups. The changes in antithrombotic therapy in patients with HALT after CT are shown in Figure 2.
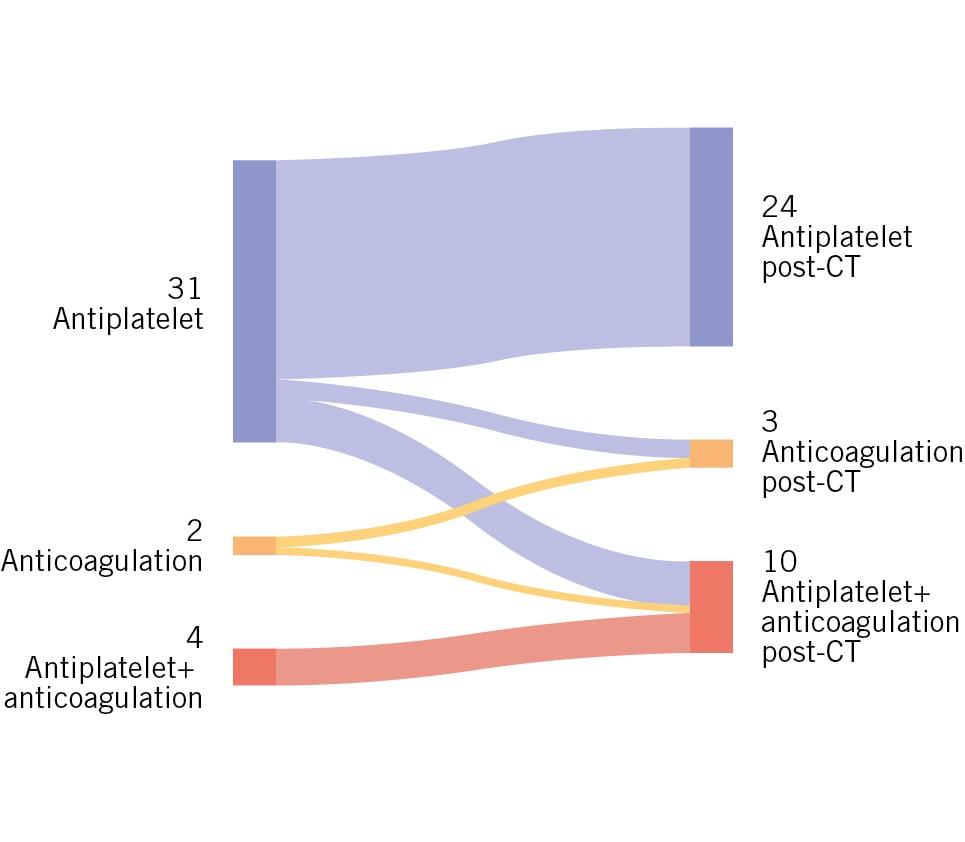
Figure 2. Sankey diagram of antithrombotic therapy transitions pre- and post-CT in the HALT group. The left side represents the initial therapy categories: antiplatelet (31 patients), anticoagulation (2 patients), and antiplatelet+anticoagulation (4 patients). The right side shows the corresponding therapies post-CT: antiplatelet (24 patients), anticoagulation (3 patients), and antiplatelet+anticoagulation (10 patients). The flow lines between the categories depict the number of patients transitioning between different therapies following CT assessment. CT: computed tomography; HALT: hypoattenuated leaflet thickening
Peri- and postprocedural CT analysis
Supplementary Table 3 details peri- and postprocedural CT measurements, comparing patients with HALT against those without HALT. Anatomical features, including annulus area, sinus of Valsalva diameter, sinotubular junction area, and heights of the coronary arteries, showed no significant differences between groups. The VTC distances and angulation were also comparable.
In the post-TAVI CT evaluation, significant differences were observed in the expansion of the prosthesis at the waist level (p=0.047) and the minimum area of the THV (p=0.019). Conversely, no significant differences were noted in the expansion at other THV levels. The eccentricity of the THV was not significantly different between patients with or without HALT. Overlap with the RCA was observed more frequently in patients with HALT than in those without HALT (p=0.041). Measurements from the THV frame to both the left coronary artery and RCA, as well as the sinus of Valsalva, were consistent across the groups. Similarly, implantation depth relative to the native annulus showed no significant differences.
Predictors of HALT after ViV TAVI
Multivariable analysis identified anticoagulation therapy (OR 0.28, 95% CI: 0.08-0.92; p=0.037) and THV expansion at the minimum area level (OR 0.95, 95% CI: 0.91-0.99; p=0.026) as significant predictors of HALT (Table 2). Commissural misalignment with the RCA did not show a significant association with HALT (OR 2.46, 95% CI: 0.66-9.12; p=0.18).
Table 2. Univariable and multivariable analyses for predictors of HALT.
| Univariable model | Multivariable model | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age, per 1 year increase | 1.02 (0.98-1.06) | 0.28 | 1.02 (0.97-1.08) | 0.38 |
| Male | 1.2 (0.54-2.65) | 0.65 | 1.82 (0.64-5.18) | 0.27 |
| Atrial fibrillation | 0.51 (0.22-12.9) | 0.16 | ||
| THV size for ViV TAVI, per 1 mm increase | 1.05 (0.90-1.22) | 0.56 | ||
| Aspirin | 1.59 (0.66-3.83) | 0.31 | ||
| P2Y12 inhibitor | 1.12 (0.47-2.63) | 0.8 | ||
| Anticoagulation | 0.40 (0.15-1.08) | 0.072 | 0.28 (0.08-0.92) | 0.037 |
| Predilatation | 0.92 (0.22-3.78) | 0.91 | ||
| Post-dilatation | 0.80 (0.30-2.13) | 0.66 | ||
| VTC distance (LCA), per 1 mm increase | 0.88 (0.72-1.08) | 0.21 | ||
| VTC distance (RCA), per 1 mm increase | 1.02 (0.85-1.22) | 0.86 | ||
| THV expansion at prosthetic waist level, per 1% increase | 0.97 (0.93-1.01) | 0.082 | ||
| THV expansion at minimum area level, per 1% increase | 0.95 (0.91-0.99) | 0.021 | 0.95 (0.91-0.99) | 0.026 |
| THV eccentricity at prosthetic waist level, per 1% increase | 0.84 (0.65-1.10) | 0.84 | ||
| THV eccentricity at minimum area level, per 1% increase | 0.88 (0.7-10.7) | 0.87 | ||
| Commissure misalignment RCA | 2.89 (1.01-8.25) | 0.047 | 2.46 (0.66-9.12) | 0.18 |
| Commissure misalignment LCA | 2.02 (0.75-5.40) | 0.16 | ||
| Implantation depth, per 1 mm increase | 0.99 (0.81-1.20) | 0.9 | ||
| CI: confidence interval; HALT: hypoattenuated leaflet thickening; LCA; left coronary artery; OR: odds ratio; RCA; right coronary artery; TAVI; transcatheter aortic valve implantation; THV: transcatheter heart valve; ViV: valve-in-valve; VTC: virtual THV to the coronary ostia | ||||
Clinical and haemodynamic outcomes at follow-up
Figure 3 and Central illustration B show the 3-year clinical outcomes of ViV TAVI. The composite outcomes, which included all-cause mortality, stroke, and hospitalisation for HF, were significantly higher in patients with HALT (OR 2.31, 95% CI: 1.04-5.15; p=0.04). No significant differences were observed in all-cause mortality, stroke/TIA, hospitalisation for HF, valve reintervention, major bleeding events, or myocardial infarction between patients with and without HALT (Table 3).
No significant differences were noted in all-cause mortality, stroke/TIA, or hospitalisation for HF following ViV TAVI when stratified by the presence of HAM (Supplementary Table 4).
No significant differences were observed in NYHA Functional Class between groups throughout the follow-up period (Supplementary Figure 3).
Over the 3-year post-ViV TAVI period, the aortic valve area and left ventricular ejection fraction showed no significant differences between patients with and without HALT (Figure 4A, Figure 4B). Although the mean gradients were comparable across both groups from baseline to 1 year, a non-significant trend towards higher mean gradients was observed in patients with HALT at 2-year follow-up (Figure 4C). Hospitalisation for HF was evaluated with respect to the presence of severe PPM; however, no statistically significant difference was observed (log-rank p=0.107) (Supplementary Figure 4).
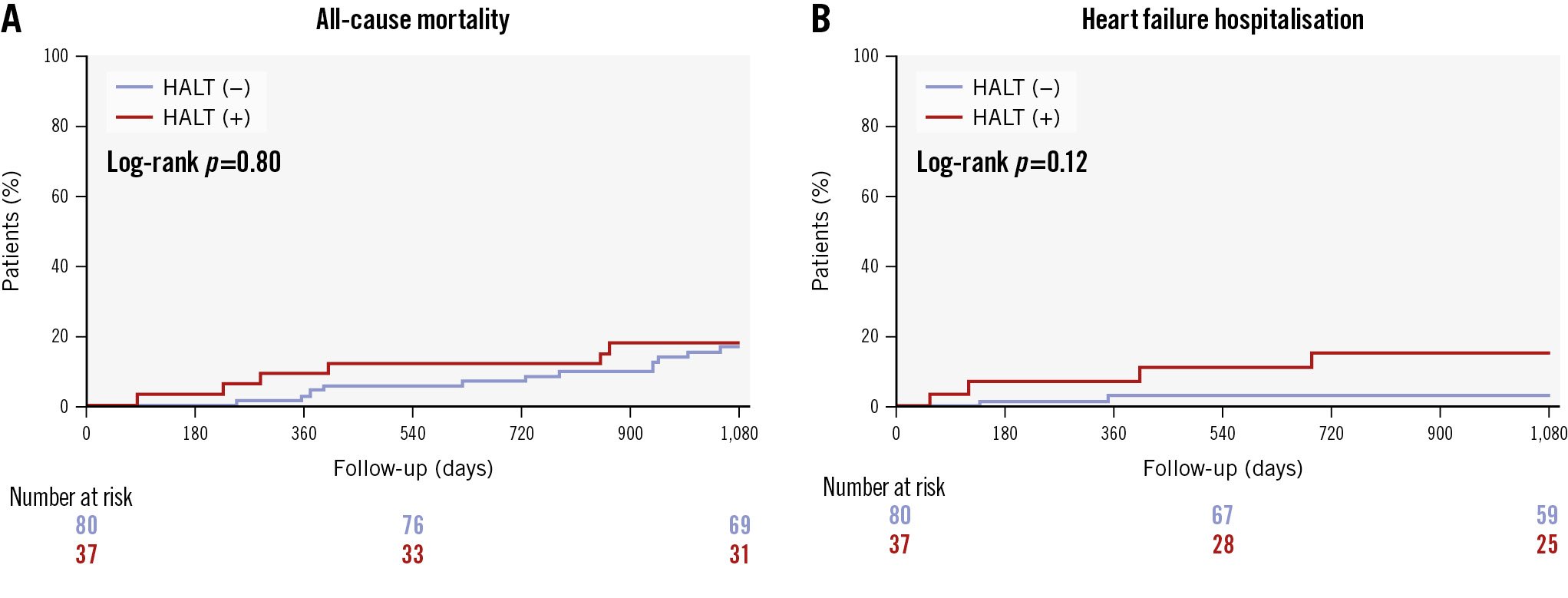
Figure 3. Kaplan-Meier curves of clinical outcomes in patients with and without HALT post-ViV TAVI. A) All-cause mortality. B) HFH. HALT: hypoattenuated leaflet thickening; HFH: heart failure hospitalisation; TAVI: transcatheter aortic valve implantation; ViV: valve-in-valve
Table 3. Three-year clinical outcomes after ViV TAVI.
| Overall N=117 | HALT (+) N=37 | HALT (-) N=80 | HR (95% CI) | p-value | |
|---|---|---|---|---|---|
| Composite outcome of all-cause mortality, stroke, or HF hospitalisation |
24 (20.5) | 12 (32.4) | 12 (15.0) | 2.31 (10.4-5.15) | 0.04 |
| All-cause mortality | 18 (15.4) | 6 (16.2) | 12 (15.0) | 1.13 (0.42-3.02) | 0.8 |
| Stroke/TIA | 5 (4.3) | 3 (8.1) | 2 (2.5) | 3.47 (0.58-20.8) | 0.17 |
| Stroke | 5 (4.3) | 3 (8.1) | 2 (2.5) | 3.47 (0.58-20.8) | 0.17 |
| TIA | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| HF hospitalisation | 7 (6.0) | 4 (10.8) | 3 (3.8) | 3.07 (0.69-13.7) | 0.14 |
| Valve reintervention | 4 (3.4) | 1 (2.7) | 3 (3.8) | 0.79 (0.08-7.56) | 0.79 |
| Major bleeding events | 7 (6.0) | 1 (2.7) | 6 (7.5) | 0.38 (0.05-3.2) | 0.39 |
| Myocardial infarction | 1 (0.9) | 1 (2.7) | 0 (0) | NA | 0.7 |
| Severe paravalvular leak | 0 (0) | 0 (0) | 0 (0) | NA | NA |
| CI: confidence interval; HALT: hypoattenuated leaflet thickening; HF: heart failure; HR: hazard ratio; NA: not applicable; TAVI: transcatheter aortic valve implantation; TIA: transient ischaemic attack; ViV: valve-in-valve | |||||
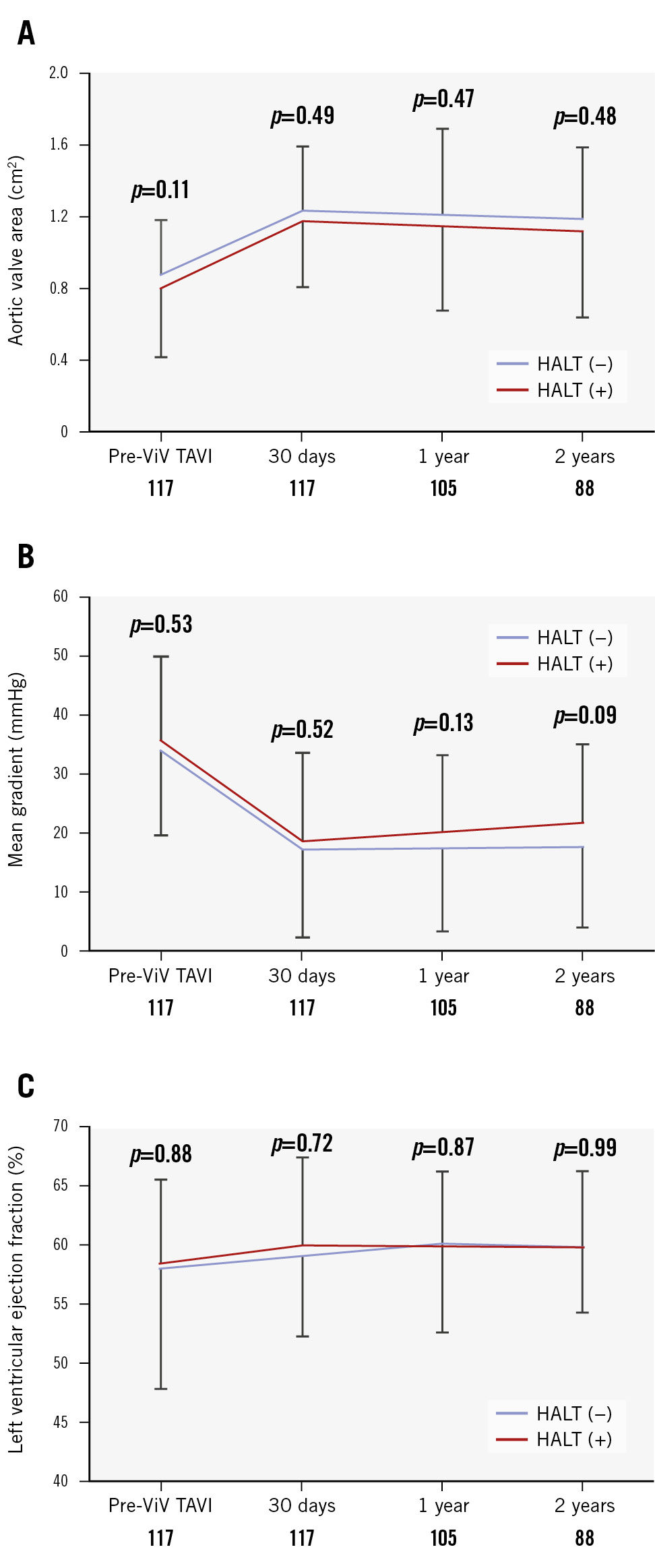
Figure 4. Echocardiographic outcomes up to 2 years. A) Mean aortic valve area pre-ViV TAVI and at 30 days, 1 year, and 2 years post-procedure. B) Mean transaortic gradient. C) Mean left ventricular ejection fraction. Error bars represent the standard deviation at each timepoint, illustrating the variability within each group. HALT: hypoattenuated leaflet thickening; TAVI: transcatheter aortic valve implantation; ViV: valve-in-valve
Discussion
This study investigated clinical outcomes of ViV TAVI, with a focus on HALT detected using CT imaging. To the best of our knowledge, this study is the first to systematically analyse the prognosis and outcomes in patients with and without HALT undergoing ViV TAVI and to identify potential predictors of leaflet thrombosis. This study has several notable findings. First, it included 117 patients, with 37 (31.6%) demonstrating HALT (Central illustration). Second, we noted significant disparities in the expansion of the prosthetic waist and minimal area levels of the THV, along with variations in the rate of overlap between the THV commissure and RCA between the groups. Anticoagulation therapy and minimum area expansion emerged as significant predictors of HALT levels after ViV TAVI. Third, there were no significant differences in all-cause mortality or stroke/TIA between patients with and without HALT during the 3-year follow-up period. However, the composite outcomes of all-cause mortality, hospitalisation for HF, and stroke were significantly higher in the HALT group than those in the control group. Fourth, post-ViV TAVI echocardiographic assessments showed no significant differences in the aortic valve area or left ventricular ejection fraction between the groups, with a non-significant trend towards higher mean gradients in patients with HALT at the 2-year follow-up.
Previous research has demonstrated that the frequency of leaflet thrombosis after TAVI in patients with native aortic stenosis (AS) varies from 7% to 23%14151617. Our findings indicate a higher proportion of HALT cases following ViV TAVI than that in those reports. Although ViV TAVI has previously been identified as a potential risk factor for THV thrombosis18, the evidence to date remains limited. Our study provides additional evidence supporting this relationship. Several mechanisms have been considered to explain the role of ViV TAVI as a risk factor for leaflet thrombosis. The placement of two THVs within a confined space is likely to disrupt the normal haemodynamic patterns, leading to turbulence and flow stagnation. Additionally, pre-existing thrombotic and degenerative conditions in the previous bioprosthetic valve may serve as a foundation for subsequent thrombus development after implantation. The use of balloon-expandable valves in our study may have contributed to the higher incidence of HALT, as previous studies have shown a higher occurrence of HALT with balloon-expandable valves than with self-expanding valves192021. This could be due to the specific deployment dynamics and mechanical stresses imparted by balloon-expandable valves, which may have exacerbated the conditions conducive to thrombosis. Consequently, these factors may have accounted for the higher incidence of HALT observed in the present study. However, several studies have reported no significant difference in all-cause mortality between balloon-expandable and self-expanding valves in the context of ViV TAVI192223. Further research is warranted to elucidate whether the use of balloon-expandable valves in ViV TAVI, as compared to self-expanding valves, influences the incidence of HALT and subsequent adverse outcomes.
Preoperative CT measurements were comparable between the groups. As noted previously, the degeneration of the previous valve may influence the progression of fibrosis or thrombosis following ViV TAVI. However, detecting such features preoperatively using CT is challenging, suggesting that such evaluation may have inherent limitations in predicting HALT after ViV TAVI. Additionally, while previous studies showed that larger THVs were associated with an increased risk of THV thrombosis after TAVI1524, the THV size used for ViV TAVI demonstrated no significant differences between the groups in our study. THV size was meticulously determined based on the dimensions of the previously implanted valves, which were comparable between groups. This approach, which is uniquely suited for ViV TAVI, may minimise variables that could affect the outcomes of the study.
Postprocedural CT analysis revealed that underexpansion at both the waist and minimum area levels was significantly associated with HALT. While no significant differences in post-dilatation outcomes were noted between the groups, mid-segment dilatation of the newly implanted TAVI valve was likely constrained by the metal ring and structural components of the previous prosthetic valve. Fukui et al reported that the mid-segment dilatation of the SAPIEN valve was relatively underexpanded, with patients exhibiting HALT showing a higher incidence of underexpansion at the waist level, corroborating our findings25. Our results further revealed that patients with HALT exhibited a significantly higher rate of underexpansion at the minimum area level, establishing it as a critical independent factor for HALT. Although the rates of predilatation and post-dilatation in this study were low, and neither emerged as significant predictors of HALT, their appropriate use could potentially improve underexpansion at the waist and minimum area levels. However, it is also plausible that in ViV TAVI procedures, the pre-existing valve may act as a constraint, limiting the effectiveness of balloon dilatation. This warrants further investigation with a larger sample size to better understand the impact of these interventions in ViV TAVI. Conversely, no significant differences were observed in the eccentricity of the prosthetic valve on follow-up CT scans between the groups. Additionally, the eccentricity of the THV was not identified as a significant predictor of HALT in the multivariable analysis. This finding may seem contrary to previous reports suggesting that the eccentricity of the THV is a risk factor for leaflet thrombosis after TAVI2526. However, it is conceivable that eccentricity is a less prevalent concern in ViV TAVI procedures because of fewer contributing factors, such as calcific protrusion and bicuspid-induced distortions, which are more prevalent in native valve procedures. These findings underscore the importance of precise monitoring of THV expansion at the waist and minimum area levels using CT to predict and potentially prevent thrombus formation following ViV TAVI.
Our multivariable analysis did not conclusively identify commissural irregularity as a HALT predictor, although its prevalence was significantly higher in patients with HALT. Previous research indicated that commissure misalignment may contribute to HALT because of mechanical stress and altered blood flow dynamics27. Therefore, additional studies with larger sample sizes should validate whether these findings are similar in patients undergoing ViV TAVI.
Anticoagulation therapy was determined to be an independent predictor of HALT, along with underexpansion at the minimal area level. Studies have demonstrated that anticoagulation therapy is more effective than antiplatelet therapy in reducing the risk of leaflet thrombosis following TAVI1. Although ViV TAVI increases the risk of leaflet thrombosis, the potential benefits of long-term anticoagulation must be carefully balanced against the increased risk of bleeding.
Although previous studies have found no definitive correlation between HALT and stroke or mortality41428, the association between post-TAVI leaflet thrombosis and clinical outcomes remains controversial. In our study, no significant differences in all-cause mortality or stroke were observed among the ViV TAVI patients at the 3-year follow-up. The notably low incidence of stroke in both groups could potentially be linked to the proactive use of cerebral protection systems in nearly half of the cohort. Echocardiographic findings at 2 years did not show significant differences between the two groups in terms of aortic valve area or left ventricular ejection fraction. However, there was a trend towards higher mean pressure gradients in patients with HALT, which, although not statistically significant, aligns with the findings of Rashid et al that HALT was associated with increased transvalvular gradients29. We found a significant elevation in the composite outcome in patients with HALT compared to those without HALT, suggesting that HALT may contribute to structural valve deterioration and negatively impact the prognosis of patients undergoing ViV TAVI. Considering these implications, it is essential to extend our research to larger cohorts with longer follow-up periods to thoroughly verify these associations and refine our understanding of the effect of HALT on long-term outcomes after TAVI.
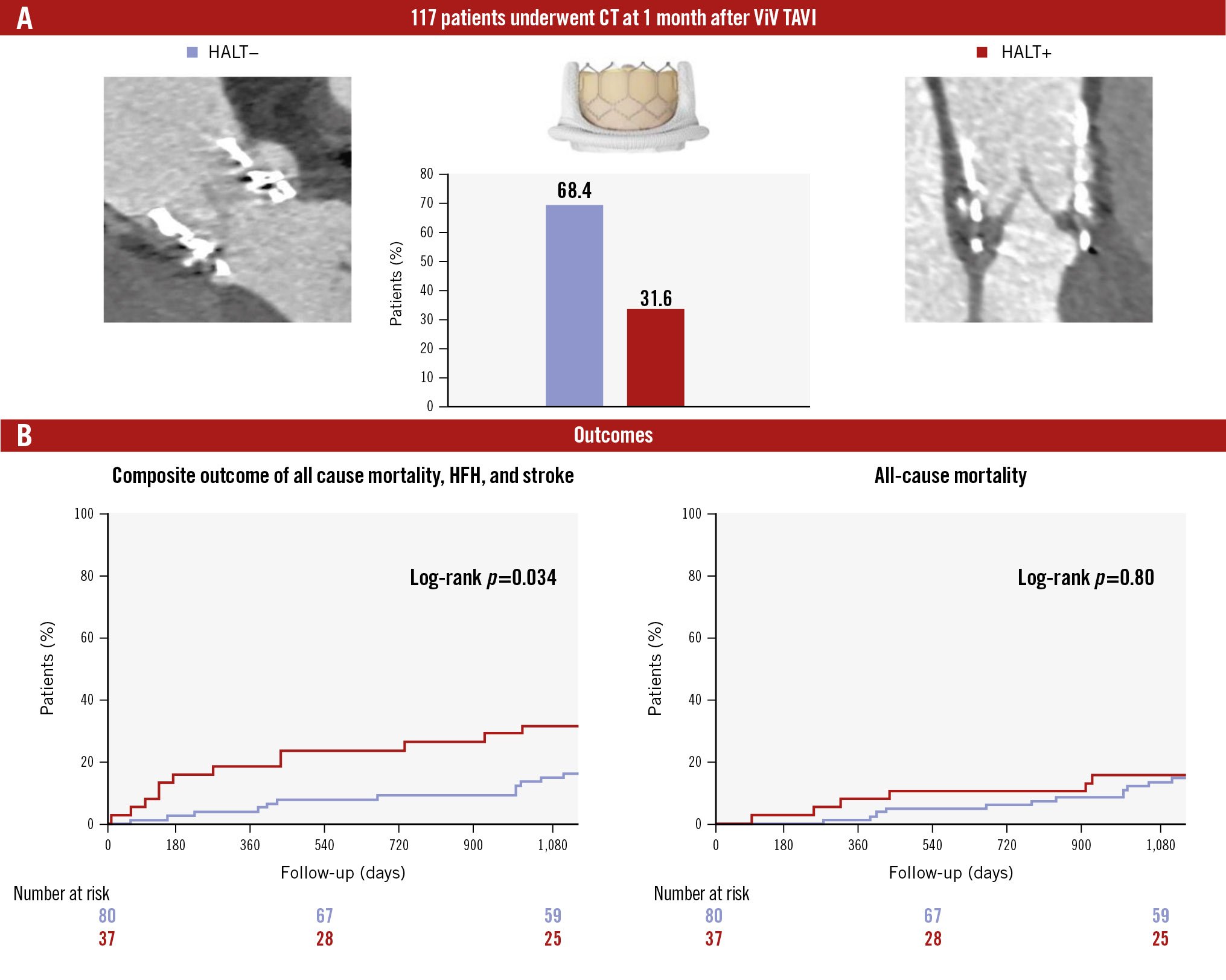
Central illustration. HALT detected by CT after ViV TAVI. A) Prevalence of HALT at 1 month after ViV TAVI. B) Composite and all-cause mortality outcomes in patients with and without HALT. CT: computed tomography; HALT: hypoattenuated leaflet thickening; HFH: heart failure hospitalisation; TAVI: transcatheter aortic valve implantation; ViV: valve-in-valve
Limitations
Our study has several limitations. Conducted as a retrospective cohort study at a single centre, the potential for selection bias cannot be disregarded, particularly concerning ViV TAVI procedures and antithrombotic management. The post-detection anticoagulation strategy for HALT was left to the discretion of the attending clinicians, which could have influenced the outcomes. Our analysis was limited to balloon-expandable valves for ViV TAVI, leaving the effects of self-expanding valves unexplored. Additionally, the evaluation of HALT based on CT was presented without sufficient corroborative validation studies to substantiate the extent of its association with thrombosis. Moreover, our analysis was confined to early postprocedural CT assessments of leaflet thrombosis, conducted 30 days after ViV TAVI, without subsequent CT imaging, to monitor the long-term progression or resolution of HALT.
Conclusions
In this study, HALT was frequently observed in patients who underwent ViV TAVI. We identified a significant correlation between HALT detected 30 days post-procedure and underexpansion of the THV at both the prosthetic waist and minimum area levels. Moreover, HALT was associated with an increased incidence of composite outcomes, including all-cause mortality, hospitalisation for HF, and stroke. To advance our understanding of the clinical implications and thrombotic predictors after ViV TAVI, it is crucial to extend the follow-up period and expand the cohort size.
Impact on daily practice
This study emphasises the importance of peri- and postprocedural computed tomography analysis in detecting hypoattenuated leaflet thickening (HALT), a subclinical valve thrombosis that may impair valve function after ViV TAVI. The identification of suboptimal valve expansion as a risk factor for HALT highlights the need for optimised procedural techniques. The association of HALT with adverse outcomes, including mortality, heart failure hospitalisation, and stroke at 3 years, underscores the importance of refining interventional strategies to improve long-term outcomes.
Acknowledgements
The study was supported in part by the California Chapter of the American College of Cardiology through the Save a Heart Foundation.
Conflict of interest statement
R.R. Makkar has received grant support from Edwards Lifesciences; he is a consultant for Abbott, Cordis, and Medtronic; and holds equity in Entourage Medical. T. Chakravarty is a consultant, proctor, and speaker for Edwards Lifesciences and Medtronic; he is a consultant for Abbott; and a consultant and speaker for Boston Scientific. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

