Cory:
Unlock Your AI Assistant Now!
Abstract
Background: The latest-generation SAPIEN 3 Ultra RESILIA (S3UR) transcatheter heart valve (THV) incorporates several changes in leaflet design, including an improved anticalcification coating and modified commissural attachment. There are no established data on hypoattenuating leaflet thickening (HALT) following transcatheter aortic valve implantation (TAVI) using the S3UR.
Aims: Our study aimed to elucidate the clinical features of HALT following S3UR implantation.
Methods: As a subset of the OCEAN (Optimized CathEter vAlvular INtervention)-TAVI registry, we prospectively assessed patients who underwent cardiac computed tomography (CT) 30 days after S3UR implantation. HALT and potentially relevant THV geometry were analysed using four-dimensional CT data by an independent core laboratory.
Results: Of the 445 patients studied, HALT was detected in 95 patients (21.3%) 30 days after TAVI. The modification of the commissural attachment specific to the 20 mm and 23 mm S3UR THVs did not affect the incidence of HALT (22.1% for ≤23 mm; 20.2% for ≥26 mm; p=0.636). The hourglass-shaped THV frame (p<0.001) and asymmetricity of THV leaflets (p=0.002) were independently associated with HALT development. A trend toward higher mean aortic gradients at 30 days with greater degrees of HALT (HALT >25% vs HALT ≤25%: 10.3 [interquartile range [IQR] 7.0-13.0] mmHg vs 8.6 [IQR 6.3-11.6] mmHg; p=0.007; HALT >50% vs HALT ≤50%: 11.5 [IQR 7.0-14.3] mmHg vs 8.9 [IQR 6.3-11.9] mmHg; p=0.002) was noted.
Conclusions: The incidence of HALT for the S3UR was comparable with the already reported incidences for the previous-generation SAPIEN 3 THV. Given the haemodynamic impact of HALT severity and multiplicity, strategic planning to avoid deformation of the implanted THV might be required. (Clinical trial registration: UMIN000020423)
In the past decades, recommendations for transcatheter aortic valve implantation (TAVI), which was established as a therapeutic alternative to surgical aortic valve replacement for inoperable or high-risk patients with severe aortic stenosis (AS), have expanded to include low-risk patients. Despite significant advances in transcatheter heart valve (THV) technologies and increased operator experience, there have been concerns about hypoattenuating leaflet thickening (HALT) with or without reduced leaflet motion (RLM), which potentially increases the risk of systemic embolisation and affects prosthesis function or durability123. In patients who received the previous-generation balloon-expandable SAPIEN 3 (S3) THV (Edwards Lifesciences), the incidence of HALT was reported to be 13-21%, and non-uniform expansion of the THV, including frame deformation and asymmetric leaflet, has been identified as a strong predictor of HALT4567. The latest-generation balloon-expandable SAPIEN 3 Ultra RESILIA (S3UR) THV (Edwards Lifesciences), with new features such as dry tissue storage in combination with the anticalcification technology of RESILIA tissue and the alteration in the leaflet attachment method at the commissural posts specific to smaller 20 mm and 23 mm THV sizes, has been in clinical use for several years. Although recent papers have reported the significantly better valve performance of the S3UR89, no data on its HALT have been established. Therefore, this study aimed to investigate the incidence, predictors, and clinical impact of HALT following TAVI with the S3UR using Japanese multicentre prospective registry data.
Methods
Study population and design
As a subset of the ongoing, prospective, Japanese multicentre Optimized CathEter vAlvular INtervention (OCEAN)-TAVI registry, beginning on April 2023, we prospectively performed an electrocardiogram (ECG)-gated, contrast-enhanced cardiac computed tomography (CT) scan 30 days following TAVI to detect HALT and RLM and assess the feasibility of future redo-TAVI, unless medical or social contraindication precluded contrast administration. This study included all patients who underwent such CT examinations following S3UR implantation at 12 institutions in Japan between April 2023 and March 2024. Patients with transfemoral and non-transfemoral approaches were included. To evaluate the clinical characteristics of HALT and RLM for the S3UR, patients with previous aortic bioprosthetic valves, a second THV implantation for bailout, or inadequate CT image quality for leaflet assessment were excluded from the analysis. The decision to proceed with TAVI and the selection of the THV type and size were made with the consensus of a dedicated Heart Team at each site. During TAVI, an activated clotting time of ≥250 s was achieved by intravenous administration of unfractionated heparin. After the procedure, the recommended antithrombotic regimen was dual antiplatelet therapy (DAPT) consisting of aspirin and thienopyridine for 6 months, followed by lifelong aspirin therapy, as per the Japanese guidelines10; however, single antiplatelet therapy (SAPT) or no antiplatelet therapy (NAPT) was selected for patients deemed to be at high bleeding risk. A single oral anticoagulant without antithrombotic agents was selected when long-term anticoagulation was indicated because of pre-existing atrial fibrillation or deep vein thrombosis. The study protocol of the OCEAN-TAVI registry was approved by the local institutional review board of each participating centre and registered with the University Hospital Medical Information Network (UMIN000020423). All the patients provided written informed consent before undergoing TAVI. Data collection was conducted as shown in Supplementary Appendix 1.
CT image acquisition and interpretation
For both the baseline and 30-day assessments, an ECG-gated, contrast-enhanced cardiac CT scan was performed based on a pre-established protocol (Supplementary Appendix 2). Centralised core laboratory assessment of the CT data using 3mensio Valves software, version 8.0 (Pie Medical Imaging) was performed at Toyohashi, Nagoya, and Gifu Heart Centers by experienced imaging specialists who were not aware of all clinical results. Baseline variables were assessed using pre-TAVI CT with standard definitions11. Post-TAVI CT data were analysed using multiplanar reformats aligned with the short- and long-axis dimensions of the THV. HALT and RLM were meticulously evaluated using the entire cardiac cycle data, whereas the other variables, such as prosthesis expansion, implantation depth, and commissural alignment, were assessed in one phase of mid-diastole (70-80% of the R-R interval).
Assessment of the implanted THV using post-TAVI CT
Prosthetic leaflet abnormalities
HALT and RLM were assessed per leaflet according to the Valve Academic Research Consortium (VARC)-3 criteria (Supplementary Figure 1)12. HALT was defined as a visually identified increased leaflet thickness with a typically meniscal appearance on long-axis views. The extent of leaflet thickening was semiquantitatively graded on long-axis views carefully aligned with the leaflet centre, focusing on involvement along the curvilinear leaflet beginning at the base, using a 5-tier grading scale: none, ≤25%, >25% to ≤50%, >50% to ≤75%, and >75%. If HALT was identified, the presence of RLM throughout the entire systolic phase was also evaluated using a 4-tier grading scale along the curvilinear leaflet beginning at the base: none, <50%, ≥50% to <100%, and immobile (100%). The location of HALT was also evaluated with regard to which of the 3 leaflets of a THV was involved. Among the 3 leaflets of a THV, the one facing more than half of the native non-coronary cusp (NCC) on the THV short-axis CT image was defined as the NCC-side leaflet. Subsequently, based on the location of the NCC-side leaflet, the left coronary cusp (LCC)-side leaflet and right coronary cusp (RCC)-side leaflet were determined. The Valsalva thrombus was defined as a low-density space without contrast enhancement between the native sinus of Valsalva (SOV) and the implanted THV stent frame.
THV stent frame deformation
The geometry of the THV stent frame was assessed for orthogonal major and minor diameters and expansion area at 3 levels of the prosthesis: leaflet inflow (i.e., the nadir of the prosthesis leaflets), prosthesis waist, and leaflet outflow (i.e., the top of the 3 commissural tabs) levels (Supplementary Figure 2)5. To minimise measurement errors due to blooming artefacts, these stent frame measurements were obtained by tracing or connecting the middle of the stent struts at a window width of 3,000 and a window level of 2,000. The THV implantation depth was defined as the mean of the distances from the inflow of the THV to the SOV floor, measured at each coronary cusp, as previously described1314. The degree of canting was calculated as the difference between the maximum and minimum THV implantation depths. The postprocedural native annulus level was defined as the plane with a mean THV implantation depth above the THV frame inflow level. Post-implant THV oversizing (%) was calculated as follows: (measured THV area at the native annulus level/native annulus area − 1) × 100. THV expansion (%) was calculated as follows: (measured THV area/nominal THV area) × 100. The nominal THV areas of the S3UR valves used in the above calculations were 300 mm2, 390 mm2, 503 mm2, and 621 mm2 for 20 mm, 23 mm, 26 mm, and 29 mm THVs, respectively, consistent with the previous paper where the nominal CT-derived area was measured by tracing the centre of the stent frame15. The prosthesis deformation index was calculated as follows: (THV area at the leaflet inflow level + THV area at the leaflet outflow level) / (2 × THV area at the prosthesis waist level)5. THV eccentricity (%) was calculated as follows: √1 − (minor diameter)2/(major diameter)2}× 100.
Asymmetric leaflet expansion
The expansion of each prosthetic valve leaflet was evaluated by measuring the angle (°) formed by the border stent struts corresponding to each prosthetic valve leaflet and the THV centre point at the coaptation level (Supplementary Figure 2). Assuming that the full expansion of each leaflet would be 120°, the asymmetric leaflet expansion was calculated as the sum of the difference between 120° and each measured leaflet angle.
Area ratio of the THV to the SOV
To assess the extent to which the THV occupied the SOV, the SOV and THV areas were measured at the level where the SOV area was visually the largest (Supplementary Figure 3). The area ratio (%) of the THV to the SOV was calculated16.
Overlap between the THV commissures and coronary ostia
The angle between the THV commissure and each coronary ostium was measured, as previously reported13. Briefly, the positions of the THV commissures were identified and marked at the cross-sectional level of the THV leaflet coaptation, and the ostium of each coronary artery was identified in a cross-section perpendicular to the axis of the aorta. Subsequently, the two angles through the centre of the aorta were measured: one from the left coronary artery ostium to the nearest THV commissure, and the other from the right coronary artery ostium to the nearest of the THV commissures. The severity of the overlap between the THV commissures and coronary ostia was defined as follows: severe (0-20°), moderate (21-35°), and mild/no (36-60°) overlap17.
Statistical analysis
Categorical variables are described as numbers and percentages and were compared using the chi-square or Fisher’s exact test, as appropriate. Continuous variables, whose normality of distribution was assessed using the Shapiro-Wilk test, are described as means±standard deviations or medians (interquartile ranges [IQRs]) and were compared using the unpaired Student’s t-test or Wilcoxon rank-sum test based on their distributions. To determine the risk factors for HALT 30 days after S3UR implantation, logistic regression analyses were conducted. Variables deemed to be clinically relevant to HALT were included in the multivariable model. Odds ratios (ORs) were reported with corresponding 95% confidence intervals (CIs). The variance inflation factor was used to check for multicollinearity for each variable, and the obtained variance inflation factor value was between 1 and 2. Receiver operating characteristic curves were utilised to illustrate and assess the predictive performance of the variables for HALT. Furthermore, the c-statistics between the potential predictors were compared using the method of DeLong et al18. Linear mixed models were used to identify independent predictors of the prosthesis deformation index and asymmetric leaflet expansion. Variables with p-values<0.10 in the univariate analysis were considered eligible for inclusion in the multivariable model. Regarding the post-TAVI CT analysis, intraobserver (2-week interval) and interobserver agreements were evaluated using the intraclass correlation coefficient (ICC) in 20 patients who were randomly selected. All statistical analyses were performed using JMP, version 14.2.0 (SAS Institute) and R, version 4.0.2 (R Foundation for Statistical Computing). A 2-tailed p-value of 0.05 was used for significance testing.
Results
Study population and incidence of HALT and RLM
A total of 481 patients undergoing TAVI using the S3UR at 12 participating institutions between April 2023 and March 2024 were enrolled in this post-TAVI CT study. After exclusion of 36 patients due to a previous aortic bioprosthetic valve (n=3), second THV requirement for bailout (n=1), or inadequate CT image quality for leaflet assessment (n=32), 445 patients were included in the final analysis. The median age of the patients was 84 years, and 40% of them were male. Of these patients, 95 (21.3%) with HALT were identified at the 30-day post-TAVI CT (Central illustration). The incidence of HALT was statistically comparable between the four S3UR sizes, albeit with a relatively lower incidence for the 20 mm valve (Figure 1). Although the unique modification of the S3UR in the sawing manoeuvres for commissures is applied only in the 20 mm and 23 mm valves, this modification did not affect the incidence of HALT (22.1% for 20 mm and 23 mm valves, 20.2% for 26 mm and 29 mm valves; p=0.636) (Central illustration). Overall, 45 patients (10.1%) showed HALT involving multiple leaflets. A leaflet-level analysis revealed that HALT was detected in 13.5%, 10.5%, and 11.9% of the leaflets on the NCC, LCC, and RCC sides, respectively (p=0.406). All leaflets with >25% HALT exhibited signs of RLM, whereas no RLM was identified in 56 (83.6%) of 67 leaflets with ≤25% HALT (Supplementary Table 1). Baseline and procedural characteristics were comparable between patients with and without HALT, except for the prevalence of atrial fibrillation (10.5% vs 21.4%; p=0.011) and usage of anticoagulant therapy at discharge (13.7% vs 23.4%; p=0.033) (Table 1). In a subanalysis focusing on 350 patients without anticoagulation therapy at discharge, DAPT was associated with a significantly decreased risk of HALT occurrence as compared with SAPT and NAPT (8.7% vs 25.3% vs 26.7%; p=0.021). Meanwhile, in 95 patients with anticoagulant therapy at discharge, the incidence of HALT was comparable between those taking oral vitamin K antagonists and those taking direct oral anticoagulants (17.7% vs 12.8%; p=0.697) (Supplementary Figure 4). 
Central illustration. HALT following S3UR implantation. A) Thirty-day incidence of HALT for the S3UR. B) The two independent predictors of HALT and the risk factors for these variables. C) Box plots of postprocedural mean aortic gradient according to the degree and multiplicity of HALT. The white horizontal lines within the boxes denote the median, and the bottom and top edges of the boxes represent the 25th and 75th percentiles, respectively. The whiskers extend from the edges of the boxes to the lowest and highest values within 1.5 times the interquartile range. CT: computed tomography; HALT: hypoattenuating leaflet thickening; NS: non-significant; S3UR: SAPIEN 3 Ultra RESILIA; THV: transcatheter heart valve
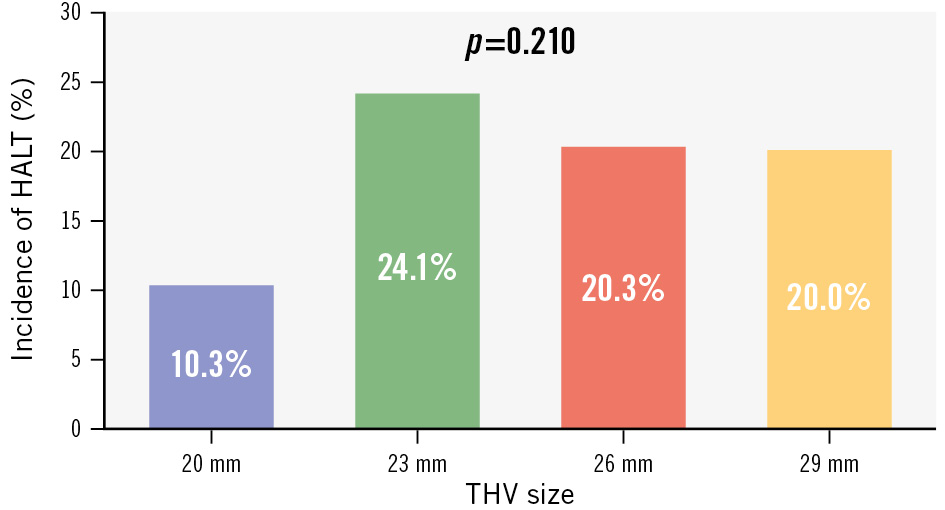
Figure 1. Incidence of HALT for each THV size. Bar graph showing the incidence of HALT for each size of the SAPIEN 3 Ultra RESILIA prosthesis. HALT: hypoattenuating leaflet thickening; THV: transcatheter heart valve
Table 1. Baseline clinical and procedural characteristics.
| Overall (N=445) |
HALT | p-value | ||
|---|---|---|---|---|
| Yes (N=95) | No (N=350) | |||
| Demographics | ||||
| Age, years | 84 (80-87) | 84 (80-86) | 84 (80-88) | 0.753 |
| Male | 178 (40.0) | 36 (37.9) | 142 (40.6) | 0.636 |
| BSA, m2 | 1.48 (1.35-1.61) | 1.47 (1.34-1.64) | 1.48 (1.35-1.60) | 0.843 |
| Clinical Frailty Scale ≥4 | 189 (41.5) | 39 (41.1) | 150 (42.9) | 0.773 |
| NYHA Functional Class III/IV | 80 (18.0) | 21 (22.1) | 59 (16.9) | 0.247 |
| STS-PROM score, % | 6.0 (3.8-9.5) | 5.1 (3.5-7.6) | 6.2 (4.0-9.8) | 0.062 |
| Comorbidities | ||||
| Hypertension | 325 (73.0) | 70 (73.7) | 255 (72.9) | 0.872 |
| Dyslipidaemia | 222 (50.0) | 45 (47.4) | 177 (50.6) | 0.580 |
| Diabetes | 147 (33.0) | 29 (30.5) | 118 (33.7) | 0.556 |
| Atrial fibrillation | 85 (19.1) | 10 (10.5) | 75 (21.4) | 0.011 |
| CAD | 161 (36.2) | 29 (30.5) | 132 (37.7) | 0.192 |
| Previous CABG | 19 (4.3) | 5 (5.3) | 14 (4.0) | 0.598 |
| PAD | 53 (11.9) | 11 (11.6) | 42 (12.0) | 0.910 |
| CKD: eGFR <60 mL/min/1.73 m2 | 316 (71.0) | 67 (70.5) | 249 (71.1) | 0.907 |
| Haemodialysis | 78 (17.5) | 15 (15.8) | 63 (18.0) | 0.612 |
| COPD | 28 (6.3) | 4 (4.2) | 24 (6.9) | 0.325 |
| Previous stroke/TIA | 41 (9.2) | 6 (6.3) | 35 (10.0) | 0.252 |
| Previous pacemaker | 23 (5.2) | 4 (4.2) | 19 (5.4) | 0.627 |
| Echocardiographic data | ||||
| AVA, cm2 | 0.71 (0.59-0.82) | 0.73 (0.62-0.84) | 0.70 (0.58-0.81) | 0.492 |
| Mean aortic gradient, mmHg | 41.0 (35.0-48.8) | 40.0 (33.3-47.2) | 41.0 (35.1-49.3) | 0.245 |
| LVEF, % | 63.5 (57.6-68.4) | 63.3 (58.4-69.6) | 63.7 (56.3-68.0) | 0.662 |
| Preprocedural CT data | ||||
| Bicuspid aortic valve | 15 (3.4) | 4 (4.2) | 11 (3.1) | 0.618 |
| Annulus area, mm2 | 413.0 (365.0-472.0) | 402.0 (357.0-457.0) | 409.9 (359.6-470.2) | 0.464 |
| LVOT calcification | 40 (10.8) | 12 (12.6) | 36 (10.3) | 0.520 |
| Procedural variables | ||||
| Transfemoral access | 425 (95.7) | 91 (95.8) | 334 (95.7) | 0.970 |
| Predilatation | 34 (7.7) | 12 (12.6) | 22 (6.3) | 0.052 |
| Post-dilatation | 59 (13.3) | 14 (14.7) | 45 (12.9) | 0.643 |
| THV size | 0.210 | |||
| 20 mm | 39 (8.8) | 4 (4.2) | 35 (10.0) | |
| 23 mm | 228 (51.2) | 55 (57.9) | 173 (49.4) | |
| 26 mm | 148 (33.3) | 30 (31.6) | 118 (33.7) | |
| 29 mm | 30 (6.7) | 6 (6.3) | 24 (6.9) | |
| Area oversizing for THV, % | 9.8 (2.1-15.7) | 11.7 (4.4-17.3) | 9.4 (1.4-15.2) | 0.121 |
| Medications at discharge | ||||
| Antiplatelet therapy | 287 (64.5) | 62 (65.3) | 225 (64.3) | 0.860 |
| Anticoagulant therapy | 95 (21.4) | 13 (13.7) | 82 (23.4) | 0.033 |
| Values are n (%) or median (interquartile range). AVA: aortic valve area; BSA: body surface area; CABG: coronary artery bypass grafting; CAD: coronary artery disease; CKD: chronic kidney disease; COPD: chronic obstructive pulmonary disease; CT: computed tomography; eGFR: estimated glomerular filtration rate; HALT: hypoattenuating leaflet thickening; LVEF: left ventricular ejection fraction; LVOT: left ventricular outflow tract; NYHA: New York Heart Association; PAD: peripheral artery disease; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; THV: transcatheter heart valve; TIA: transient ischaemic attack | ||||
Postprocedural echocardiographic data
Compared with patients without HALT, those with HALT had a numerically higher mean aortic gradient; however, the difference was not significant. The degree of paravalvular leakage was also not associated with HALT development (Table 2). A greater proportion of patients with HALT fulfilled the VARC-3 criteria for prosthesis-patient mismatch (PPM) compared to those without HALT (15.8% vs 8.6%; p=0.049). An additional sensitivity analysis according to the degree of HALT showed significant differences in the mean aortic gradient on setting 25% or 50% as the cutoff for HALT severity (HALT >25% vs HALT ≤25%: 10.3 [IQR 7.0-13.0] mmHg vs 8.6 [IQR 6.3-11.6] mmHg; p=0.007; HALT >50% vs HALT ≤50%: 11.5 [IQR 7.0-14.3] mmHg vs 8.9 [IQR 6.3-11.9] mmHg; p=0.002). The presence of multiple HALT involving two or three leaflets was also associated with a higher mean aortic gradient (multiple HALT>0% or not: 11.0 [IQR 7.5-14.9] mmHg vs 8.6 [IQR 6.3-11.6] mmHg; p=0.001; multiple HALT >25% or not: 14.0 [IQR 8.9-17.5] mmHg vs 9.0 [IQR 6.3-11.9] mmHg; p=0.025) (Central illustration).
Table 2. Postprocedural echocardiographic data.
| Overall (N=445) |
HALT | p-value | ||
|---|---|---|---|---|
| Yes (N=95) | No (N=350) | |||
| EOA, cm2 | 1.77 (1.47-2.11) | 1.72 (1.32-2.10) | 1.79 (1.48-2.11) | 0.207 |
| Indexed EOA, cm2/m2 | 1.18 (1.01-1.44) | 1.14 (0.94-1.44) | 1.19 (1.03-1.43) | 0.123 |
| Mean aortic gradient, mmHg | 9.0 (6.4-12.0) | 9.1 (6.5-13.0) | 8.7 (6.3-11.6) | 0.080 |
| PVL | 0.433 | |||
| None-to-trivial PVL | 381 (85.6) | 78 (82.1) | 303 (86.6) | |
| Mild PVL | 62 (13.9) | 16 (16.8) | 46 (13.1) | |
| Moderate-to-severe PVL | 2 (0.5) | 1 (1.1) | 1 (0.3) | |
| MR ≥moderate | 30 (6.7) | 4 (4.2) | 26 (7.4) | 0.358 |
| TR ≥moderate | 47 (10.6) | 10 (10.5) | 37 (10.6) | 0.990 |
| SPAP, mmHg | 29.0 (24.0-35.0) | 27.0 (22.5-32.5) | 29.2 (24.4-36.0) | 0.038 |
| Values are n (%) or median (interquartile range). EOA: effective orifice area; HALT: hypoattenuating leaflet thickening; MR: mitral regurgitation; PVL: paravalvular leakage; SPAP: systolic pulmonary artery pressure; TR: tricuspid regurgitation | ||||
Implanted THV assessment using post-TAVI CT
Compared with the nominal THV areas offered by the manufacturer, underexpansion of the implanted THV was observed at all levels of the S3UR frame in post-TAVI CT (Table 3). Regardless of the presence or absence of HALT, the expansion area percentage of the THV was the smallest at the prosthesis waist level with a median prosthesis deformation index of 1.06, indicating an hourglass-shaped implantation of the THV. However, patients with HALT had a larger prosthesis deformation index than those without HALT (1.11 [IQR 1.09-1.13] vs 1.06 [IQR 1.04-1.07]; p<0.001), and this difference was observed for all THV sizes (Figure 2A). THV eccentricity, ranging from 0.29 to 0.35 at each level of the THV, was comparable between the two groups at all levels, whereas asymmetric leaflet expansion was associated with HALT development (16° [IQR 9-22°] vs 8° [IQR 6-12°]; p=0.001). Larger asymmetric leaflet expansion in patients with HALT was also identified for all THV sizes (Figure 2B). The intra- and interobserver agreement was acceptable for the prosthesis deformation index (intraobserver ICC=0.94; interobserver ICC=0.91) and the asymmetric leaflet expansion (intraobserver ICC=0.95; interobserver ICC=0.91).
Table 3. Post-TAVI CT data.
| Overall (N=445) |
HALT | p-value | ||
|---|---|---|---|---|
| Yes (N=95) | No (N=350) | |||
| Measured oversizing, % | –7.5 (–12.4 to –2.3) | –7.6 (–12.7 to –1.9) | –7.5 (–12.4 to –2.5) | 0.740 |
| Expansion area of THV, % | ||||
| Leaflet inflow | 85.6 (82.2-89.5) | 87.0 (82.2-89.7) | 85.4 (82.2-89.4) | 0.279 |
| Prosthesis waist | 82.8 (78.7-86.7) | 80.6 (76.2-83.4) | 83.8 (79.8-87.1) | <0.001 |
| Leaflet outflow | 91.1 (86.9-94.4) | 91.2 (88.6-95.0) | 91.0 (86.2-94.3) | 0.179 |
| THV eccentricity | ||||
| Leaflet inflow | 0.32 (0.26-0.37) | 0.31 (0.24-0.36) | 0.32 (0.27-0.37) | 0.104 |
| Prosthesis waist | 0.31 (0.26-0.36) | 0.30 (0.23-0.35) | 0.32 (0.27-0.36) | 0.143 |
| Leaflet outflow | 0.29 (0.24-0.34) | 0.28 (0.22-0.33) | 0.29 (0.25-0.35) | 0.154 |
| Prosthesis deformation index | 1.06 (1.05-1.08) | 1.11 (1.09-1.13) | 1.06 (1.04-1.07) | <0.001 |
| Asymmetric leaflet expansion, degrees | 10 (6-12) | 16 (9-22) | 8 (6-12) | <0.001 |
| Implantation depth, mm | 2.3 (1.7-3.5) | 2.4 (1.7-3.7) | 2.3 (1.6-3.4) | 0.785 |
| Canting, mm | 2.0 (1.3-3.0) | 2.3 (1.5-3.1) | 2.0 (1.2-2.9) | 0.115 |
| Area ratio of THV to SOV, % | 47.6 (43.5-51.3) | 47.6 (42.6-51.3) | 47.6 (43.6-51.3) | 0.646 |
| Overlap between THV commissure and LCA | 0.452 | |||
| Severe | 144 (32.4) | 32 (33.7) | 112 (32.0) | |
| Moderate | 123 (27.6) | 30 (31.6) | 93 (26.6) | |
| Mild/none | 178 (40.0) | 33 (34.7) | 145 (41.4) | |
| Overlap between THV commissure and RCA | 0.638 | |||
| Severe | 141 (31.7) | 28 (29.5) | 113 (32.3) | |
| Moderate | 110 (24.7) | 27 (28.4) | 83 (23.7) | |
| Mild/none | 194 (43.6) | 40 (42.1) | 154 (44.0) | |
| Moderate or severe overlap between THV commissure and either coronary artery | 341 (76.6) | 80 (84.2) | 15 (14.4) | 0.042 |
| SOV thrombus | 102 (22.9) | 34 (35.8) | 68 (19.4) | 0.001 |
| Values are n (%) or median (interquartile range). CT: computed tomography; HALT: hypoattenuating leaflet thickening; LCA: left coronary artery; RCA: right coronary artery; SOV: sinus of Valsalva; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve | ||||
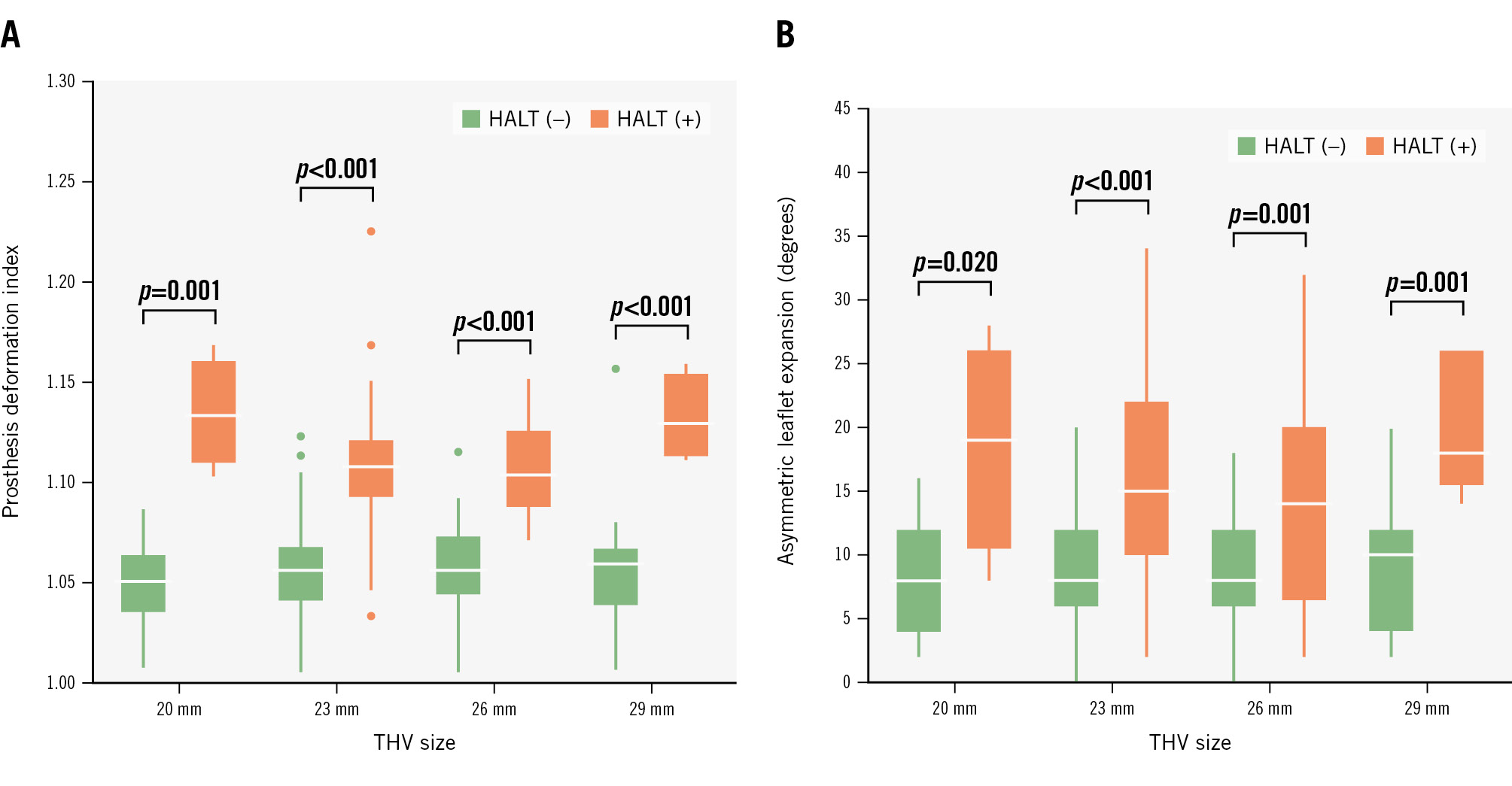
Figure 2. Implanted THV deformation and HALT by THV size. Box plots of prosthesis deformation index (A) and asymmetric leaflet expansion (B) according to THV size. The white horizontal lines within the boxes denote the median, and the bottom and top edges of the boxes represent the 25th and 75th percentiles, respectively. The whiskers extend from the edges of boxes to the lowest and highest values within 1.5 times the interquartile range. The dots outside the whiskers represent the outliers. HALT: hypoattenuating leaflet thickening; THV: transcatheter heart valve
Predictors of HALT following S3UR implantation
Logistic regression analysis was utilised to determine the predictors of HALT in patients who received S3UR implantation (Supplementary Table 2, Table 4). The multivariable model showed that the prosthesis deformation index (OR 2.87 per 0.01 increase, 95% CI: 2.23-3.70; p<0.001) and asymmetric leaflet expansion (OR 1.10 per 1° increase, 95% CI: 1.03-1.18; p=0.004) were independently associated with HALT development. Anticoagulant therapy at discharge and moderate or severe overlap between the THV commissure and either coronary artery exhibited significance in the univariate analysis, but not in the multivariable analysis. A comparative analysis assessing the discrimination values of these factors is shown in Figure 3. The prosthesis deformation index was more predictive of HALT than asymmetric leaflet expansion, although asymmetric leaflet expansion also had a relatively high discriminatory value (c-statistic: 0.88 vs 0.77; p=0.001). The best discriminatory cutoffs of the prosthesis deformation index and asymmetric leaflet expansion were 1.08 and 14°.
Table 4. Multivariable logistic regression analysis for predictors of HALT.
| Variables | Adjusted OR | 95% CI | p-value |
|---|---|---|---|
| Age (per 1-year increase) | 0.95 | 0.89-1.02 | 0.194 |
| Male sex | 1.55 | 0.58-4.15 | 0.379 |
| Anticoagulant therapy at discharge | 0.39 | 0.13-1.20 | 0.105 |
| THV size ≤23 mm | 1.25 | 0.49-3.23 | 0.640 |
| Prosthesis deformation index (per 0.01 increase) | 2.87 | 2.23-3.70 | <0.001 |
| Asymmetric leaflet expansion (per 1° increase) | 1.10 | 1.03-1.18 | 0.004 |
| Implantation depth (per 1 mm increase) | 0.97 | 0.75-1.26 | 0.839 |
| Canting (per 1 mm increase) | 0.96 | 0.71-1.32 | 0.816 |
| Area ratio of THV to SOV (per 5% increase) | 1.03 | 0.79-1.35 | 0.790 |
| Moderate or severe overlap between THV commissure and either coronary artery | 1.49 | 0.49-4.58 | 0.478 |
| CI: confidence interval; HALT: hypoattenuating leaflet thickening; OR: odds ratio; SOV: sinus of Valsalva; THV: transcatheter heart valve | |||

Figure 3. Predictive ability of implanted THV geometry for HALT. Receiver operating characteristic curves with comparative analyses of the predictability of prosthesis deformation index (A), asymmetric leaflet expansion (B), implantation depth (C), canting (D), and area ratio of THV to SOV (E) for HALT. CI: confidence interval; HALT: hypoattenuating leaflet thickening; NPV: negative predictive value; PPV: positive predictive value; SOV: sinus of Valsalva; THV: transcatheter heart valve
Factors associated with THV deformation
Linear regression analyses were applied to identify the factors associated with THV deformation (Supplementary Table 3). In the multivariable models, a greater mean aortic gradient at baseline, underfilling implantation of the THV, and moderate or severe overlap between THV commissures were independently related to a larger prosthesis deformation index, whereas only the canting degree of the THV was an independent predictor of a larger asymmetric leaflet expansion.
Discussion
To the best of our knowledge, this is the first multicentre study evaluating the clinical characteristics of HALT for the latest-generation balloon-expandable S3UR THV. The main findings of this study are summarised as follows: (i) HALT was detected in 21.3% of the patients in this cohort; (ii) the revised commissural leaflet suspension method specific to the 20 mm and 23 mm THVs to maximise the leaflet opening was not associated with an increased risk of HALT; (iii) the degree and multiplicity of HALT were associated with a higher postprocedural mean aortic gradient; and (iv) the prosthesis deformation index and asymmetric leaflet expansion were independently associated with an increased risk of HALT development. With the expansion of TAVI indications for lower risk profiles of severe AS, the occurrence of HALT, which may be related to impaired prosthesis function and durability23, has been recognised as a serious concern. Regarding the previous-generation balloon-expandable S3 THV, two prospective CT studies have investigated the clinical characteristics of HALT in detail. The first is an imaging substudy (n=221) of the Placement of Aortic Transcatheter Valves (PARTNER) 3 trial, showing that the incidence of HALT for the S3 increased from 13.3% at 30 days to 27.5% at 1 year4. The second is a single-centre but large study that analysed the prospectively acquired CT data of 352 patients treated with the S3, elucidating that the 30-day incidence of HALT was 20.7%5. In the present study evaluating 445 patients treated with the latest-generation balloon-expandable S3UR THV, the incidence of HALT at 30 days following TAVI was 21.3%, suggesting that the introduction of this newer device was not associated with an increased risk of HALT development. Fundamentally, RESILIA technology is designed to prevent calcification rather than thrombosis. However, the lack of a significant difference in the incidence of HALT between RESILIA and non-RESILIA leaflets is noteworthy, particularly given prior histological evidence suggesting a progression from thrombosis to fibrosis and then to calcification19. The susceptibility of a leaflet thrombus to organisation should be assessed in future studies with longer-term follow-up and repeated CT examinations. Furthermore, although we had been concerned about the potential effect of the revised commissural leaflet suspension method specific to the 20 mm and 23 mm S3UR THVs on HALT occurrence, the present study found a comparable incidence of HALT between THV sizes ≤23 mm and ≥26 mm (22.1% vs 20.2%). HALT development includes a multifactorial process involving foreign body materials and patient-specific blood chemistry. However, previous studies with in vitro models have indicated local blood flow stagnation on the THV leaflets, particularly at the basal neosinus, which is consistent with the region where HALT has been detected clinically20. In this context, an intra-annular THV may be somewhat disadvantaged because its neosinus is surrounded by both its inner skirt and native leaflets and is structurally larger than that of a supra-annular THV. In fact, some studies have suggested a greater incidence of HALT in balloon-expandable THVs compared with self-expanding THVs121. Therefore, detecting modifiable risk factors for HALT in patients who are treated with balloon-expandable THVs is quite important. Although some previous studies have identified that the non-uniform expansion of the previous-generation S3 THV is strongly related to the development of HALT, no data on HALT for the latest-generation S3UR THV have been established. Our study successfully validated the robust association between HALT development and THV deformation, including an hourglass-shaped frame and leaflet eccentricity, in a larger, independent cohort that exclusively included patients treated with the S3UR. As in vitro simulation studies showed that such THV distortion can alter the flow characteristics and increase the shear stress of leaflets22, the highly predictive performance of those factors for HALT development is mechanistically understandable. Linear regression analyses were also performed for implanted THV deformation, identifying baseline mean aortic gradient, underfilling implantation, coronary malalignment, and THV canting as risk factors. These insights shed light on critical aspects that could inform clinical decision-making and preprocedural planning, such as THV type, size, and position. From a practical aspect, post-dilatation would be useful to mitigate the risk of THV deformation; however, this strategy entails a trade-off with the risk of procedural complications, including aortic root injury, conduction disturbances, and THV leaflet damage. In other words, in cases where the underfilling implantation of the S3UR is likely to be required because of severe calcification, other THVs with supra-annular leaflets may be a reasonable choice. The impact of HALT on THV haemodynamic status is not well established and varies across studies, which can be attributed to the heterogeneity in the severity of HALT included in those studies. Theoretically, the haemodynamic impact of the THV is likely to differ depending on the severity of HALT, but prior studies with a relatively small number of patients may not have possessed the statistical power to perform severity-specific analyses. Our study, taking advantage of its larger cohort, successfully showed that greater degrees (>25%) and/or multiplicity (≥2 leaflets) of HALT were associated with a higher mean aortic gradient (Central illustration), which may be clinically relevant. Although further study is needed to determine whether these small increases (3-4 mmHg) noted at 30 days will be amplified over time and cause structural valve deterioration, an elevated aortic gradient detected during clinical follow-up may indicate the occurrence of extensive HALT, which should probably be confirmed by cardiac CT. We also showed a higher incidence of fulfilling the criteria for PPM in patients with HALT; however, the incidence of PPM in relation to HALT should be carefully interpreted because PPM is essentially a subtype of non-structural valve dysfunction and should be evaluated after excluding HALT. Although numerous studies have investigated the impact of PPM following TAVI2324, the majority of them have not evaluated HALT for patients with PPM, potentially influencing the true incidence and prognostic effect of PPM. The possible sequelae of subclinical leaflet thrombosis include central and systemic thromboembolism, progressive valve stenosis, and a negative impact on long-term valve durability. However, leaflet thrombosis involves a dynamic process that may develop and resolve spontaneously with or without anticoagulant use and may pose challenges in diagnosis and treatment425. Thus, the routine use of preventive anticoagulants or follow-up cardiac CT screenings following TAVI remains debatable. On the other hand, the growing evidence regarding the negative impact of a major burden of HALT (>50%) on long-term THV outcomes is of great interest26. Considering this finding in conjunction with a recent histological study suggesting that the organisation of leaflet thrombus to pannus begins within a year, earlier initiation of anticoagulation could be more effective19. Our study only evaluated the 30-day clinical characteristics of HALT for the S3UR, and a longer-term follow-up is warranted to assess the full effect of HALT on THV performance and structural valve dysfunction.
Limitations
This study has several limitations. First, this study has inherent limitations as it is based on a retrospective analysis of prospectively registered dataset. However, the participation of several institutions in the study may have attenuated the potential selection and ascertainment biases. Second, this study only examined the 30-day clinical features of HALT for the S3UR despite the transient and dynamic nature of HALT. Future studies with longer-term follow-up and detailed antithrombotic regimens are needed to assess the full clinical impact of HALT. In addition, the single-arm design that enrolled only patients who received an S3UR precluded direct comparison with those who received a previous-generation S3. Third, CT analyses were performed by an independent core laboratory blinded to clinical outcomes, but the presence or absence of HALT as a primary outcome measure would be apparent on CT images to the reviewers, resulting in some inevitable measurement biases. Fourth, among a total of 481 patients, 32 patients (6.7%) were excluded from our analysis because of poor image quality caused by beam artefacts. Although the CT acquisition protocol of this study adopted a tube voltage of 100-120 kV based on previous studies, the aggressive use of a higher tube voltage (~140 kV) could have mitigated such artefacts27. Finally, the information on baseline oral anticoagulant, such as dose, regimens, and targeted international normalised ratios for warfarin users, was unavailable, which could have affected the outcomes.
Conclusions
On 30-day CT, HALT was detected in 21.3% of the patients who were treated with the latest-generation balloon-expandable S3UR THV. The modified commissural leaflet suspension method specific to the 20 mm and 23 mm S3UR THVs was not associated with an increased risk of HALT, whereas the prosthesis deformation index and asymmetric leaflet expansion were independently associated with an increased risk of HALT development regardless of the THV size. These findings may shed light on important aspects that could inform clinical decision-making, preprocedural planning, and amelioration of THV designs.
Impact on daily practice
The 30-day incidence of hypoattenuating leaflet thickening (HALT) in patients who were treated with the SAPIEN 3 Ultra RESILIA (S3UR) transcatheter heart valve (THV) was 21.3%, which was comparable with recently reported incidences for the previous-generation SAPIEN 3 THV. The revised commissural leaflet suspension method specific to the 20 mm and 23 mm S3UR THVs was not associated with an increased risk of HALT, whereas the prosthesis deformation index and asymmetric leaflet expansion were independently associated with an increased risk of HALT development regardless of the S3UR THV size. Both greater degrees (>25%) and multiplicity (≥2 leaflets) of HALT were associated with a higher mean aortic gradient after transcatheter aortic valve implantation.
Acknowledgements
The authors thank the investigators and institutions that have participated in the OCEAN-TAVI registry.
Funding
The OCEAN-TAVI registry is supported by Edwards Lifesciences, Medtronic, Boston Scientific, Abbott, and Daiichi Sankyo.
Conflict of interest statement
F. Yashima and Y. Ohno are clinical proctors for Medtronic. M. Asami is a clinical proctor for Abbott and Medtronic. Y. Fuku is a clinical proctor for Edwards Lifesciences and Medtronic. G. Nakazawa is a clinical proctor for Edwards Lifesciences and Abbott. M. Yamamoto, S. Shirai, D. Hachinohe, and K. Hayashida are clinical proctors for Edwards Lifesciences, Abbott, and Medtronic. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.

