Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Redo-transcatheter aortic valve implantation (TAVI) is the treatment of choice for failed transcatheter aortic valves. Currently, implantation of a SAPIEN 3 (S3) is indicated for redo-TAVI in degenerated CoreValve/Evolut (CV/EV) transcatheter aortic valves (TAVs) but is not well understood.
Aims: We aimed to evaluate S3 function following implantation in explanted calcified CV/EV TAVs and to assess the impact of CV/EV pathology on redo-TAVI outcomes.
Methods: Ex vivo hydrodynamic testing was performed per the International Organization for Standardization (ISO) 5840-3 standard on 4 S3 TAVs implanted at node 5 in calcified CV/EV explants. The mean gradient (MG), effective orifice area (EOA), peak velocity, regurgitant fraction (RF), geometric orifice area (GOA), leaflet overhang, leaflet pinwheeling, neoskirt height, and frame deformation were evaluated.
Results: CV/EV explants were calcified and stenotic. Following S3 implantation, the MG and peak velocity decreased. As per the ISO standard, all S3 implants showed adequate EOA, and 3 out of 4 had an RF within the accepted value (<20%). CV/EV leaflet overhang ranged from 25-37%. Calcified leaflets remained stationary throughout the cardiac cycle (difference <9%) and were not pinned in a manner that constrained S3 systolic flow or appeared to prevent selective frame cannulation. The downstream CV/EV GOA was larger than the upstream S3 GOA during systole. S3 frame underexpansion was seen, resulting in leaflet pinwheeling (range 13-30%). Above the neoskirt, calcium protrusion was observed in contact with the S3 leaflets.
Conclusions: S3 implantation at node 5 in calcified CV/EV valves resulted in satisfactory hydrodynamic performance in most configurations tested with stable leaflet overhang throughout the cardiac cycle. The long-term implications of S3 underexpansion, leaflet pinwheeling, and calcium protrusion require future studies.
Transcatheter aortic valve implantation (TAVI) has been shown to be an effective therapy for patients with severe symptomatic aortic stenosis (AS) irrespective of surgical risk12. As TAVI is progressively utilised in lower-risk patients with the potential for longevity, transcatheter aortic valve (TAV) durability and the need for reintervention have become increasingly relevant34567. A subset of younger, low-risk patients that are undergoing TAVI will eventually require repeat interventions due to structural valve deterioration (SVD), non-SVD, and/or other failure mechanisms.
Redo-TAVI has emerged as a viable therapeutic option for patients with a failed TAV. Redo-TAVI serves as a minimally invasive alternative to surgical TAV explant, which has been shown to be associated with high morbidity and mortality due to patient risk factors and technical challenges such as valve adherence, tissue ingrowth, and damage to surrounding structures891011. However, redo-TAVI has many complexities and potential pitfalls. This procedure may not be feasible in all patients due to the risk of coronary obstruction and impaired coronary access, which requires careful consideration in procedural planning with respect to device combinations12131415161718.
While clinical experience with redo-TAVI continues to grow, many critical knowledge gaps remain. Ex vivo bench testing can provide important insights when clinical experience is limited14192021. A recent study evaluated redo-TAVI for the self-expanding Evolut R (Medtronic) TAV following implantation of a balloon-expandable SAPIEN 3 (S3 [Edwards Lifesciences]) TAV19. The neoskirt height, leaflet overhang, Evolut R frame expansion, and S3 hydrodynamic performance were evaluated at different S3 implant depths. However, like all redo-TAVI bench testing to date, brand new index TAVs were used, thus, not accounting for the potential effect of index TAV leaflet degeneration and calcification on redo-TAVI. Given the features of TAV degeneration, such as leaflet thickening, stiffening and calcium nodule formation222324, consideration of these pathological features in bench models is key to further inform clinical practice regarding redo-TAVI. Therefore, this ex vivo benchtop study aimed to evaluate the functional performance of the S3 TAV following implantation within degenerated calcified CoreValve/Evolut (CV/EV) TAVs.
Methods
CoreValve/Evolut TAVI explants
A total of 4 CV/EV explants were used in this study as index TAVs: 23 mm Evolut R, 29 mm CoreValve, 29 mm Evolut PRO, and 34 mm Evolut R (all Medtronic devices). Two explants were obtained from the international, multicentre EXPLANT THV registry at St. Paul’s Hospital (Vancouver, BC, Canada), with their study approved by the Providence Health Care Research Ethics Board. The remaining 2 TAV explants were acquired from clinical institutions and approved by local institutional review boards. Surgical explantation occurred for clinical indications as determined by local Heart Teams. Clinical and patient characteristics were obtained from each clinical institution, when available. This bench study did not involve animal participants. Additional details can be found in Supplementary Appendix 1.
SAPIEN 3 in CoreValve/Evolut redo-TAVI
Redo-TAVI was performed on the bench using new S3 TAVs in the following combinations: 20 mm S3 within the 23 mm Evolut R, 26 mm S3 within the 29 mm CV, 26 mm S3 within the 29 mm Evolut PRO, and 29 mm S3 within the 34 mm Evolut R. The S3 TAVs were implanted with their outflow at CV/EV node 5 (Figure 1A). Implant depth was chosen on the basis of prior bench testing and computed tomography (CT)-based simulation studies that evaluated the feasibility of coronary access at implantation depths between nodes 4-6 of the index CV/EV12181925. The S3 TAVs were implanted under fluoroscopy (ARTIS icono biplane C-arm system [Siemens Healthineers]) into the CV/EV TAVs using the manufacturer’s standard delivery systems at a nominal volume. Balloon pre- and post-dilatation were not performed.
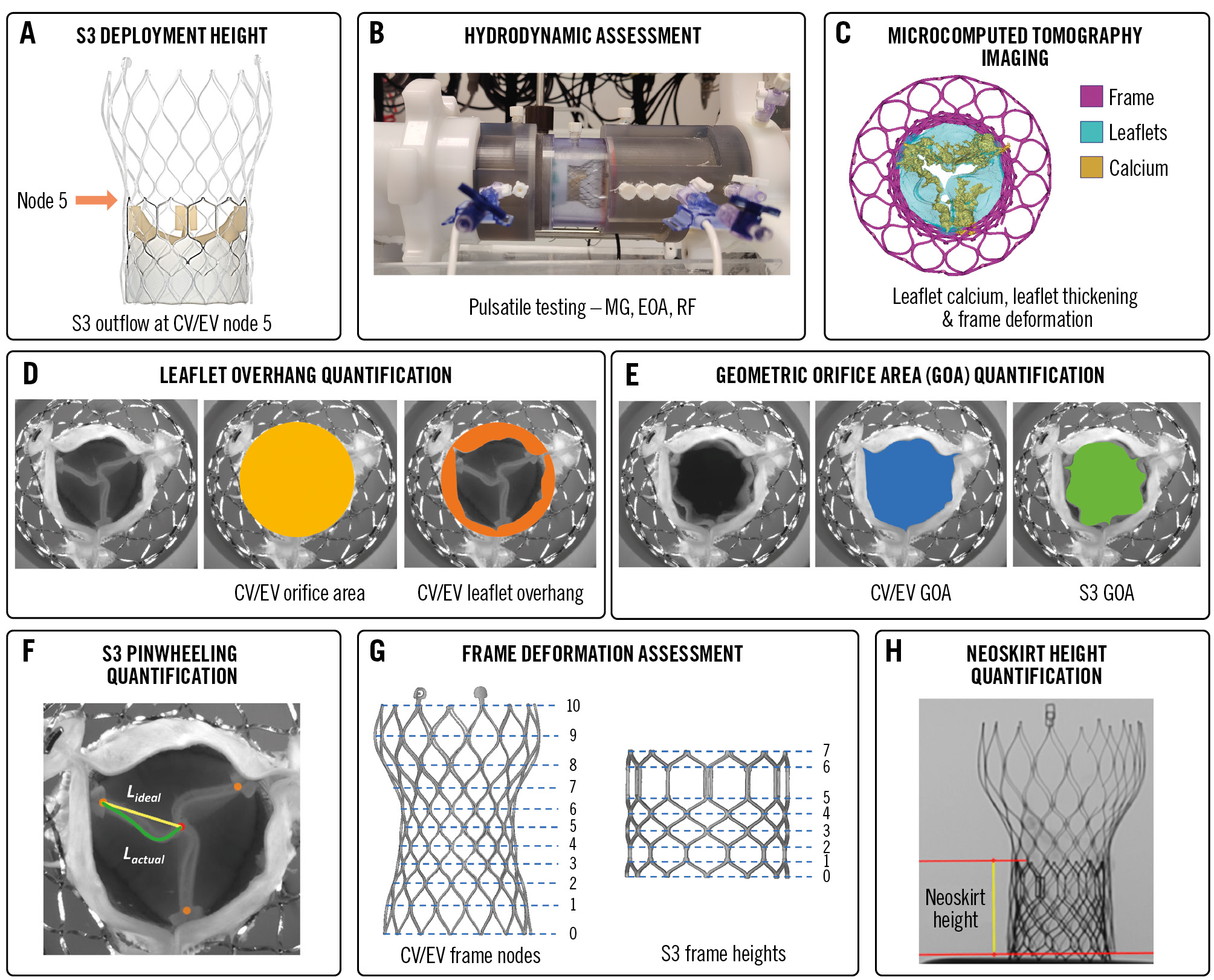
Figure 1. Study methodology. A) Redo-TAVI with S3 outflow at node 5 (orange arrow) of CV/EV; (B) pulse duplicator; (C) microcomputed tomography imaging; (D) degree of leaflet overhang (%) of the stenotic CV/EV leaflets following S3 implant; (E) GOA of S3 and CV/EV; (F) S3 leaflet pinwheeling; (G) CV/EV frame nodes and S3 frame heights; (H) neoskirt height. CV/EV: CoreValve/Evolut; EOA: effective orifice area; L: leaflet; MG: mean gradient; RF: regurgitant fraction; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation
Hydrodynamic bench testing
The HDTi-6000 heart valve pulse duplicator system (BDC Laboratories) was used to assess hydrodynamic performance before and after redo-TAVI in accordance with ISO 5840-3:2021 guidelines for in vitro pulsatile flow testing for heart valve substitutes implanted by transcatheter techniques (Figure 1B). The mean gradient (MG; mmHg), effective orifice area (EOA; cm2), peak velocity (m/s), and regurgitant fraction (RF; %) were quantified. An RF of >20% is considered significant in accordance with ISO guidelines. The EOA was derived from the continuity equation26. Additional details can be found in Supplementary Appendix 1.
Multimodality imaging analysis
Micro-CT imaging was used for calcium quantification of the index CV/EV leaflets, as previously described22. Briefly, calcification distribution on the CV/EV leaflets was assessed using volume measurement of the three-dimensionally (3D) reconstructed explanted TAVs. As shown in Figure 1C, segmentation and 3D reconstruction of the CV/EV frame (purple), leaflets (blue) and calcium burden (yellow) were performed in Mimics software, version 25.0 (Materialise). Calcium distribution analysis was performed in 3-matic software, version 17.0 (Materialise). Additional imaging details can be found in Supplementary Appendix 1.
Leaflet function analysis
CoreValve/Evolut neoskirt height
The neoskirt height was defined as the distance from the CV/EV frame inflow to the pinned leaflet height at the S3 frame outflow19 (Figure 1H). Neoskirt height was measured by fluoroscopy at the level of the S3 outflow following visual confirmation that the degenerated index leaflets were subjected to overhang and not pinned in a manner that extended the functional neoskirt above the S3 frame outflow. Neoskirt values were derived from averaging 4 height measurements per redo-TAVI combination using the DICOM viewer software, version 3.9.5 (MicroDicom).
CoreValve/Evolut leaflet overhang
As shown in Figure 1D, leaflet overhang was defined as the percentage of orifice obstruction at the level of the index CV/EV commissure pad region (leaflet outflow) due to inward flexing of the unpinned portion of the CV/EV leaflets19 (Supplementary Appendix 1).
CoreValve/Evolut and SAPIEN 3 geometric orifice area
As shown in Figure 1E, the CV/EV geometric orifice area (GOA; blue) and S3 GOA (green) following redo-TAVI were measured and averaged from 3 still-frame systolic images obtained from the high-speed videos using ImageJ software (ImageJ). The CV/EV GOA was compared to the S3 GOA at systole.
SAPIEN 3 pinwheeling
Pinwheeling, defined as twisting of the TAV leaflet's free edge as a consequence of redundant leaflet tissue was assessed for the S327. As shown in Figure 1F, pinwheeling − expressed as a percentage − was determined by tracing the contour of the length of the actual leaflet's free edge (Lactual) from the frame to the coaptation centre compared to the ideal configuration for the leaflet's free edge (straight line; Lideal) (Supplementary Appendix 1). Since pinwheeling might be difficult to measure in cases where major leaflet redundancy leads to a phenomenon of localised malcoaptation, regions where the leaflets' free edges failed to achieve full coaptation along their length were manually traced from images captured during diastole.
Frame deformation analysis
As shown in Figure 1G, micro-CT imaging performed before and after redo-TAVI was used for measuring the CV/EV perimeter-derived outer frame diameter in each of the 11 frame node levels from inflow to outflow (nodes 0-10). Similarly, the S3 area-derived outer frame diameter was measured at the 8 frame heights from inflow to outflow (heights 0-7) following redo-TAVI. Diameter measurements were originally taken mid-strut followed by the addition of the frame thickness to obtain the outer frame diameter values. The reference S3 frame diameter before redo-TAVI was the nominal frame diameter per the instructions for use, which was assumed to be constant across the frame height. The change in radius for the CV/EV TAVs following redo-TAVI was also quantified.
Statistical analysis
Continuous variables are presented as mean±standard deviation or median with interquartile range (IQR). Statistics were performed using Minitab software, version 20.1.3 (Minitab). Due to the small sample size, statistical comparisons between redo-TAVI configurations were not performed.
Results
CoreValve/Evolut explants characteristics
Clinical characteristics of the 4 CV/EV explants are provided in Supplementary Table 1. The median patient age at explant was 67 years (IQR 53-72 years), and 50% were female. The median time from index TAVI to explant was 4 years and 5 months (IQR 3 years and 1 month-4 years and 11 months). The degenerative mechanism responsible for explant in all cases was SVD, causing AS in 3 cases and mixed stenosis and concomitant regurgitation in 1 case.
Figure 2 and Supplementary Table 1 show the calcium burden, leaflet thickness, morphological appearance, and leaflet kinematics of the 4 CV/EV explants before redo-TAVI. Leaflet kinematics were abnormal in all explants (Moving image 1), and all explants were found to have leaflet calcification by micro-CT quantification (median 413 mm3 [IQR 120-597 mm3]). Table 1 shows the hydrodynamic performance of the CV/EV explants. Ex vivo MG, EOA and peak velocity median values were 48.9 mmHg (IQR 34.9-71.5 mmHg), 0.84 cm2 (IQR 0.70-1.04 cm2) and 4.8 m/s (IQR 4.0-5.9 m/s), respectively.
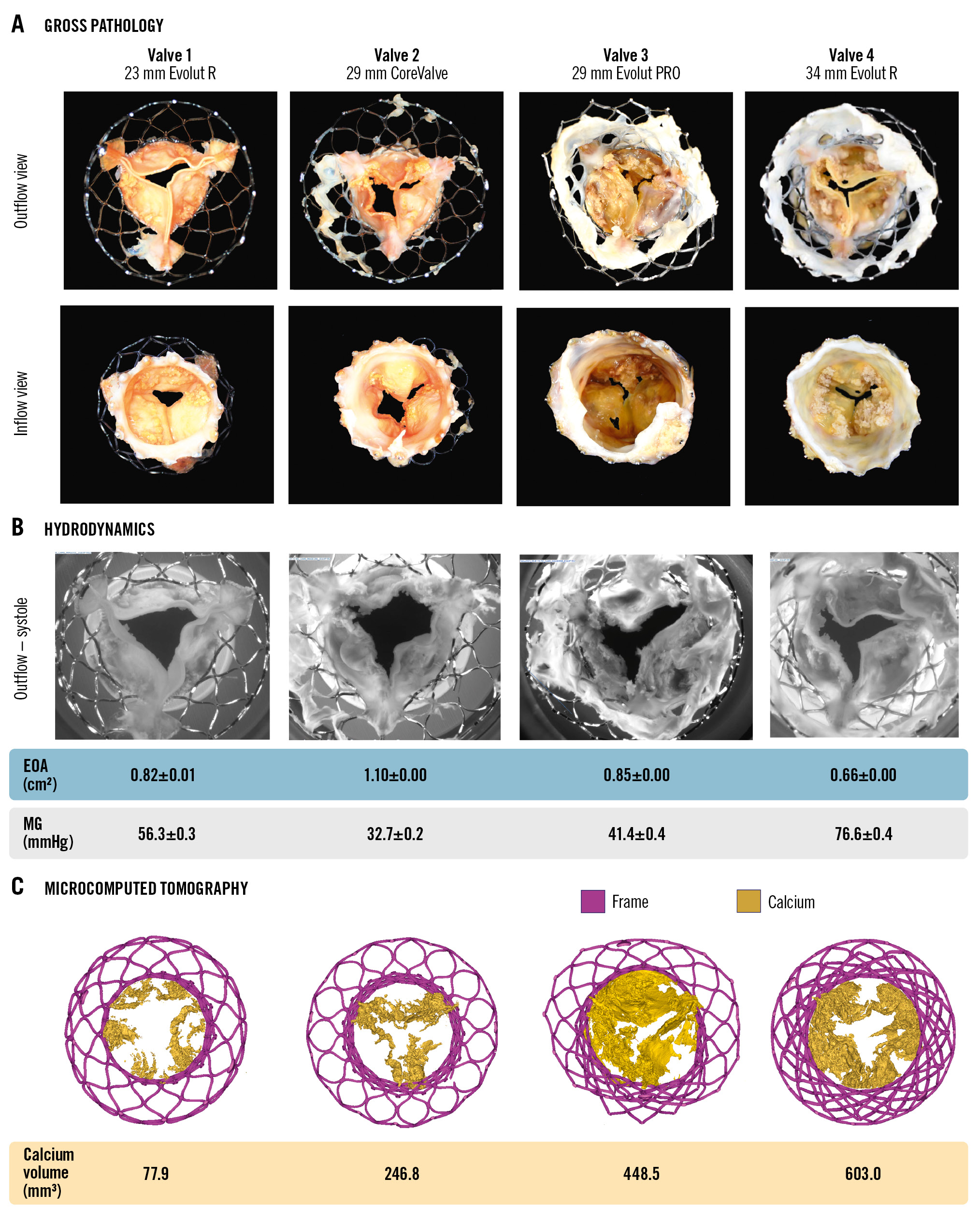
Figure 2. Appearance, hydrodynamics and calcium of degenerated CoreValve/Evolut. A) Gross pathology of the explanted calcified CV/EV used in the study. B) Images of explants during systolic opening prior to redo-TAVI with corresponding MG and EOA. C) Micro-CT of explants (calcification=yellow) with calcium volume per valve. CT: computed tomography; CV/EV: CoreValve/Evolut; EOA: effective orifice area; MG: mean gradient; TAVI: transcatheter aortic valve implantation
Table 1. Hydrodynamic performance before and after redo-TAVI.
| Mean gradient, mmHg | EOA, cm2 | Peak velocity, m/s | Regurgitant fraction, % | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Index CV/EV | Redo-TAVI | Index CV/EV | Redo-TAVI | ISO accepted | Index CV/EV | Redo-TAVI | Redo-TAVI | ISO accepted | |
| VALVE 120 mm S3 in23 mm Evolut R | 56.3±0.3 | 28.5±0.2 | 0.82±0.01 | 1.17±0.00 | 0.95 | 5.0±0.0 | 3.4±0.0 | 7.9±0.6 | 20 |
| VALVE 226 mm S3 in29 mm CoreValve | 32.7±0.2 | 9.5±0.1 | 1.10±0.00 | 2.16±0.02 | 1.60 | 3.8±0.0 | 1.9±0.0 | 18.9±0.4 | |
| VALVE 326 mm S3 in29 mm Evolut PRO | 41.4±0.4 | 10.2±0.1 | 0.85±0.00 | 2.08±0.01 | 1.60 | 4.6±0.0 | 1.9±0.0 | 12.3±0.4 | |
| VALVE 429 mm S3 in34 mm Evolut R | 76.6±0.4 | 7.0±0.1 | 0.66±0.00 | 2.54±0.02 | 2.10 | 6.2±0.1 | 1.6±0.0 | 25.8±0.3* | |
| Data presented as mean±standard deviation. *Subsequent hydrodynamic testing determined that central AR caused 21.7% of the total regurgitant fraction. AR: aortic regurgitation; CV/EV: CoreValve/Evolut; EOA: effective orifice area; ISO: International Organization for Standardization; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation | |||||||||
SAPIEN 3 in CoreValve/Evolut hydrodynamic performance
Table 1 and Figure 3 show the hydrodynamic performance and morphological appearance of the 4 redo-TAVI combinations. Following S3 implantation within the CV/EV at node 5 position, adequate S3 hydrodynamic function was achieved for 3 of the 4 redo-TAVI combinations per the ISO 5840-3 standard. The 29 mm S3 in 34 mm EV configuration showed an RF of 25.8%, above the 20% cutoff point defined by the standard. Further testing of this configuration was conducted by adding silicone sealant to the exterior surface of the 29 mm S3 to prevent inter-TAV leakage. Hydrodynamic retesting resulted in an RF of 21.7%, confirming the major contribution of central regurgitation to the high RF initially observed. The S3 in CV/EV redo-TAVI leaflet kinematics are presented in Moving image 2.
Hydrodynamic testing following redo-TAVI resulted in median MG, EOA and peak velocity measurements of 9.9 mmHg (IQR 7.6-23.9 mmHg), 2.1 cm2 (IQR 1.4-2.4 cm2) and 1.9 m/s (IQR 1.7-3.0 m/s), respectively (Figure 3B, Table 1). As expected, the highest MG/peak velocity and smallest EOA were noted for the 20 mm S3 in 23 mm EV redo-TAVI combination.
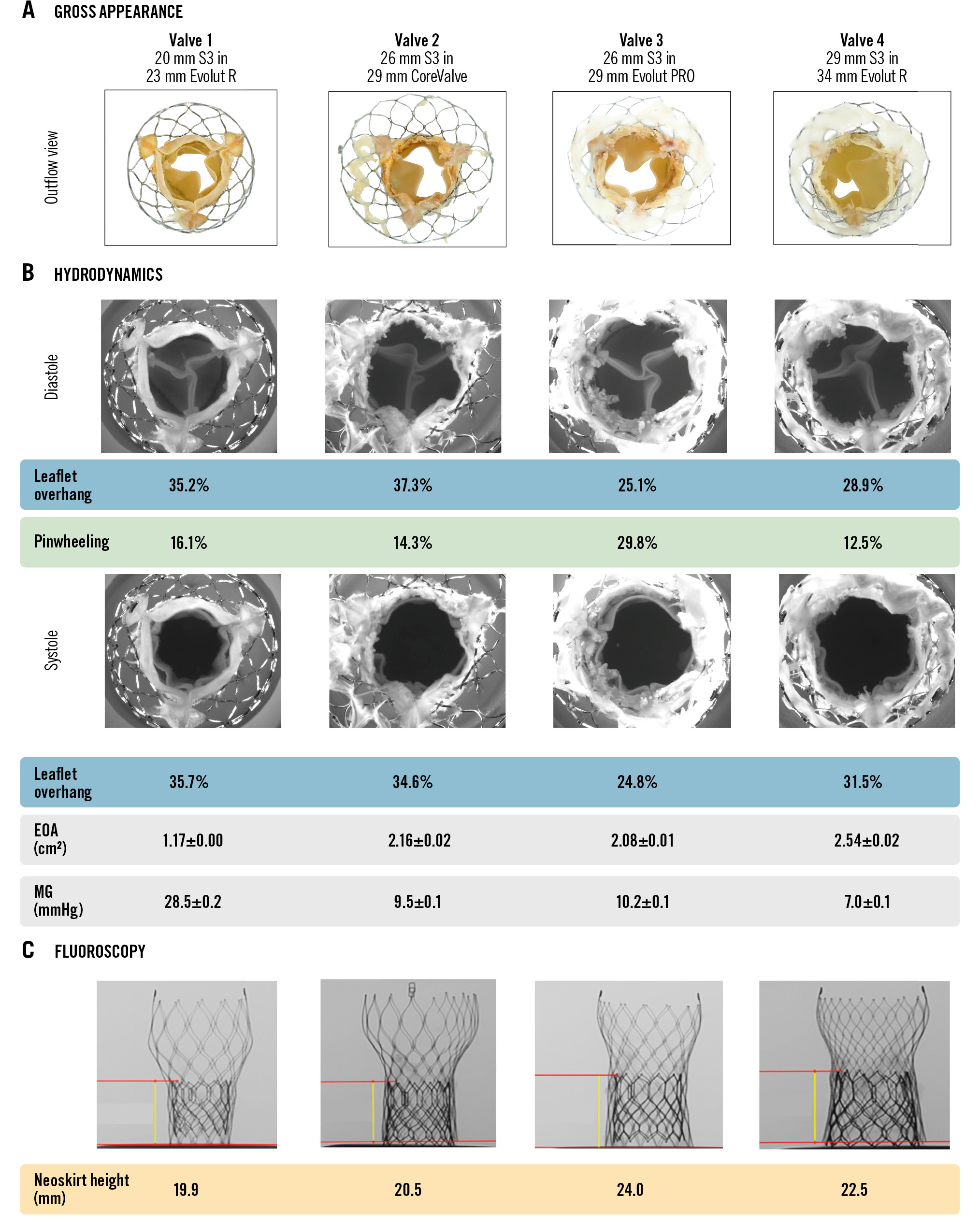
Figure 3. Redo-TAVI with SAPIEN 3 in CoreValve/Evolut at node 5. A) Appearance of redo-TAVI combinations following S3 implant from the outflow perspective. B) Following redo-TAVI, images of confirmed leaflet opening (systole) and closing (diastole) during hydrodynamic testing with resulting EOA, MG, leaflet overhang and S3 pinwheeling. C) Fluoroscopy of redo-TAVI configuration noting neoskirt heights. EOA: effective orifice area; MG: mean gradient; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation
Redo-TAVI leaflet measurements
As shown in Table 2 and Figure 3C, the S3 in CV/EV redo-TAVI at node 5 position resulted in neoskirt heights ranging between 19.9-24.0 mm. The lowest neoskirt value was measured for the 20 mm S3 in 23 mm EV combination (19.9 mm), and the highest neoskirt was noted for the 26 mm S3 in 29 mm EV combination (24.0 mm). Of note, despite leaflet rigidity due to calcification, the neoskirt was not found to extend above the S3 frame. Table 2 shows the degree of CV/EV leaflet overhang during diastole and systole. Leaflet overhang ranged from 24.8% to 37.3% across the 4 redo-TAVI combinations (Figure 3B). Overall, index CV/EV leaflets were pinned open and remained stationary throughout the cardiac cycle, with a difference <9% in leaflet overhang between diastole and systole.
Index CV/EV GOA and S3 GOA were quantified for the 4 redo-TAVI combinations (Table 2). The CV/EV GOA ranged from 3.2 cm2 to 5.3 cm2 across the 4 test combinations, while the S3 GOA ranged between 2.8 cm2 and 4.2 cm2. As shown in Figure 4A, during systole, the downstream opening orifice area (CV/EV GOA) was always larger than the upstream orifice area (S3 GOA), demonstrating that S3 systolic flow is not constrained by the overhanging leaflets. S3 EOA values are also presented in Figure 4A; however, directly comparing GOA to EOA measurements is inaccurate, because the former only considers the physical dimensions of the orifice, while the latter accounts for fluid flow characteristics.
As shown in Table 2, S3 implanted within CV/EV resulted in average S3 pinwheeling values ranging from 12.5-29.8%. The largest degree of pinwheeling was noted for the 26 mm S3 in 29 mm EV combination. Supplementary Table 2 shows the degree of pinwheeling for each pair of S3 leaflets. Pinwheeling also resulted in deficits in free-edge coaptation along the leaflet length. Supplementary Figure 1 shows these areas highlighted in red where the free edges of each pair of leaflets did not meet.
Table 2. Neoskirt height, leaflet overhang, geometric orifice area, and pinwheeling following redo-TAVI.
| Neoskirt height, mm | Index CV/EV leaflet overhang, % | Index CV/EVGOA, cm2 | S3 GOA,cm2 | S3pinwheeling, % | |||
|---|---|---|---|---|---|---|---|
| Diastole | Systole | Difference | |||||
| VALVE 120 mm S3 in23 mm Evolut R | 19.9±0.1 | 35.2±0.6 | 35.7±0.4 | 1.4 | 3.22±0.03 | 2.81±0.02 | 16.1±5.7 |
| VALVE 226 mm 3 in29 mm CoreValve | 20.5±0.4 | 37.3±0.8 | 34.6±0.2 | 7.5 | 4.08±0.02 | 3.67±0.05 | 14.3±10.5 |
| VALVE 326 mm S3 in29 mm Evolut PRO | 24.0±0.5 | 25.1±1.0 | 24.8±1.4 | 1.2 | 5.11±0.05 | 3.76±0.01 | 29.8±9.3 |
| VALVE 429 mm S3 in34 mm Evolut R | 22.5±0.3 | 28.9±0.3 | 31.5±0.6 | 8.6 | 5.26±0.02 | 4.19±0.04 | 12.5±3.6 |
| Data presented as mean±standard deviation. CV/EV: CoreValve/Evolut; GOA: geometric orifice area; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation | |||||||
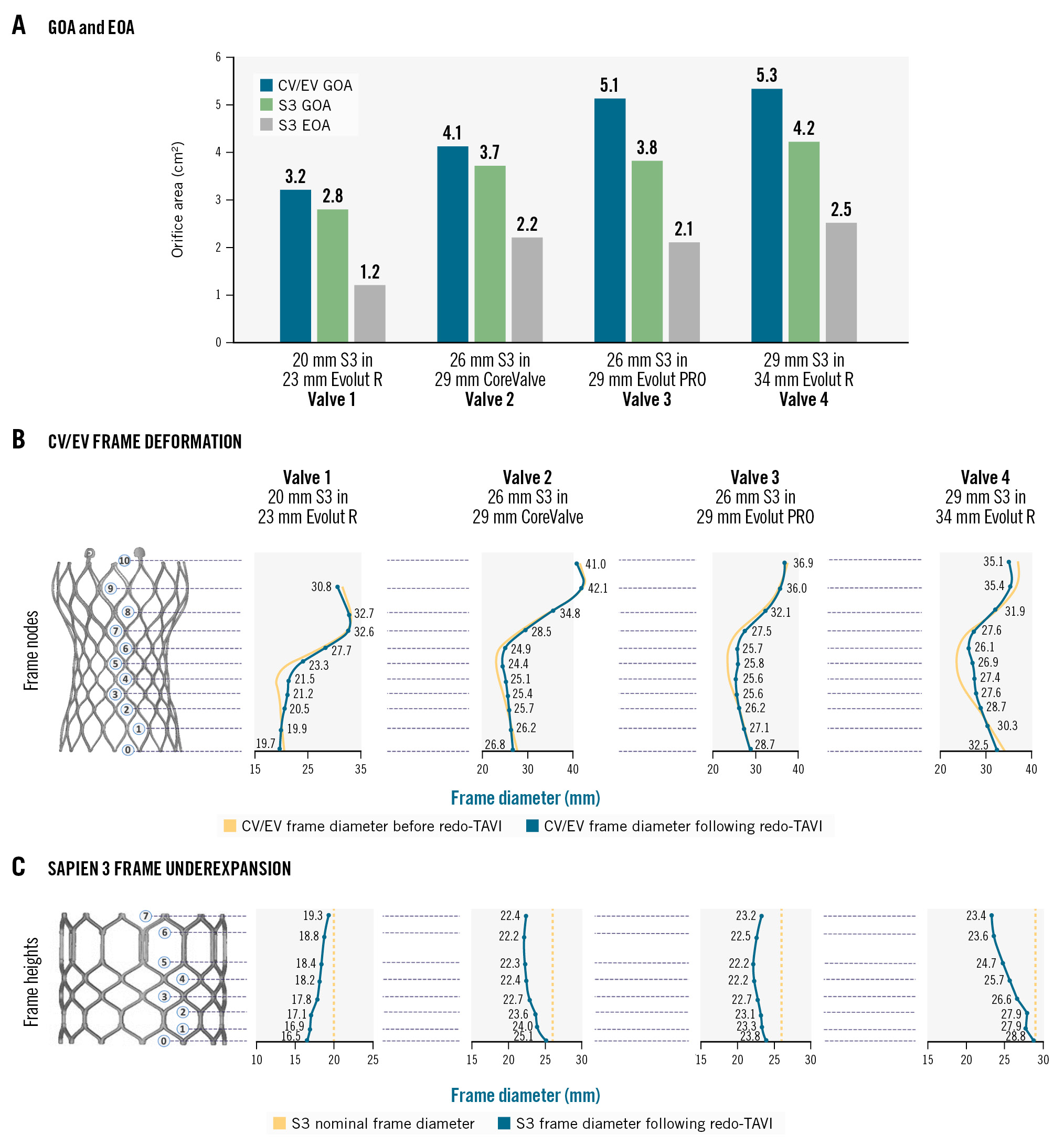
Figure 4. CoreValve/Evolut and SAPIEN 3 orifice areas and frame deformation. A) Comparison of CV/EV GOA, S3 GOA and S3 EOA. B) CV/EV frame deformation at nodes 0 to 10 comparing frame diameter before (yellow) and after (blue) redo-TAVI. C) S3 frame expansion comparing observed S3 frame diameter at node heights 0 to 7 at nominal state (yellow) and after redo-TAVI (blue). The data points shown correspond to frame diameter values following redo-TAVI. CV/EV: CoreValve/Evolut; EOA: effective orifice area; GOA: geometric orifice area; S3: SAPIEN 3; TAVI: transcatheter aortic valve implantation
CoreValve/Evolut frame deformation following redo-TAVI
Figure 4B presents the CV/EV outer frame diameter measurements at each node level before and after redo-TAVI. CV/EV frame expansion following redo-TAVI occurred more frequently closer to the waist and the functional leaflet region (nodes 4-7), and to a lesser extent at the inflow and outflow regions. CV/EV frame diameter measurements at each node level and change in radius following redo-TAVI are presented in Supplementary Table 3. The change in CV/EV radius was up to 1.7 mm across the tested combinations.
SAPIEN 3 frame expansion following redo-TAVI
Following redo-TAVI, S3 frame underexpansion was systematically observed to varying degrees along the frame height. Figure 4C shows the observed S3 frame diameter values compared to theoretical nominal expansion. In the case of the 20 mm S3 in 23 mm EV combination, the S3 showed a trumpet-like, “flared” frame geometry, with the highest frame underexpansion at the inflow and the lowest underexpansion at the outflow. In contrast, 2 of the remaining redo-TAVI combinations (valves 2 and 4) showed a funnel-like, “tapered” frame geometry, with the lowest frame underexpansion at the inflow and the highest underexpansion at the outflow, especially for the 29 mm S3 in 34 mm EV combination. The high S3 frame underexpansion observed for the 29 mm S3 in 34 mm EV combination appeared to have contributed to the high RF quantified for this configuration. The remaining redo-TAVI combination (valve 3) displayed a “dumbbell” geometry, with the midportion showing the highest degree of underexpansion. S3 frame underexpansion and degree of leaflet pinwheeling appears to be affected by redo-TAVI sizing and calcium location.
Calcification patterns and protrusion
Calcification was observable on both the outflow and inflow surfaces of the CV/EV TAVs and protruded from the inflow and outflow surfaces (Figure 5A). During hydrodynamic testing, CV/EV leaflet calcium protrusion from the inflow side through the S3 frame was observed for the 29 mm S3 in 34 mm EV combination, and to a lesser extent for the 26 mm S3 in 29 mm EV combination (Figure 5B). During systole, this calcification could be seen in contact with the S3 leaflets (Moving image 3). As shown in Figure 5A, this finding is in line with the substantial calcium burden and location identified by micro-CT at the inflow side of the 34 mm and 29 mm EV TAVs, which was deflected during redo-TAVI.
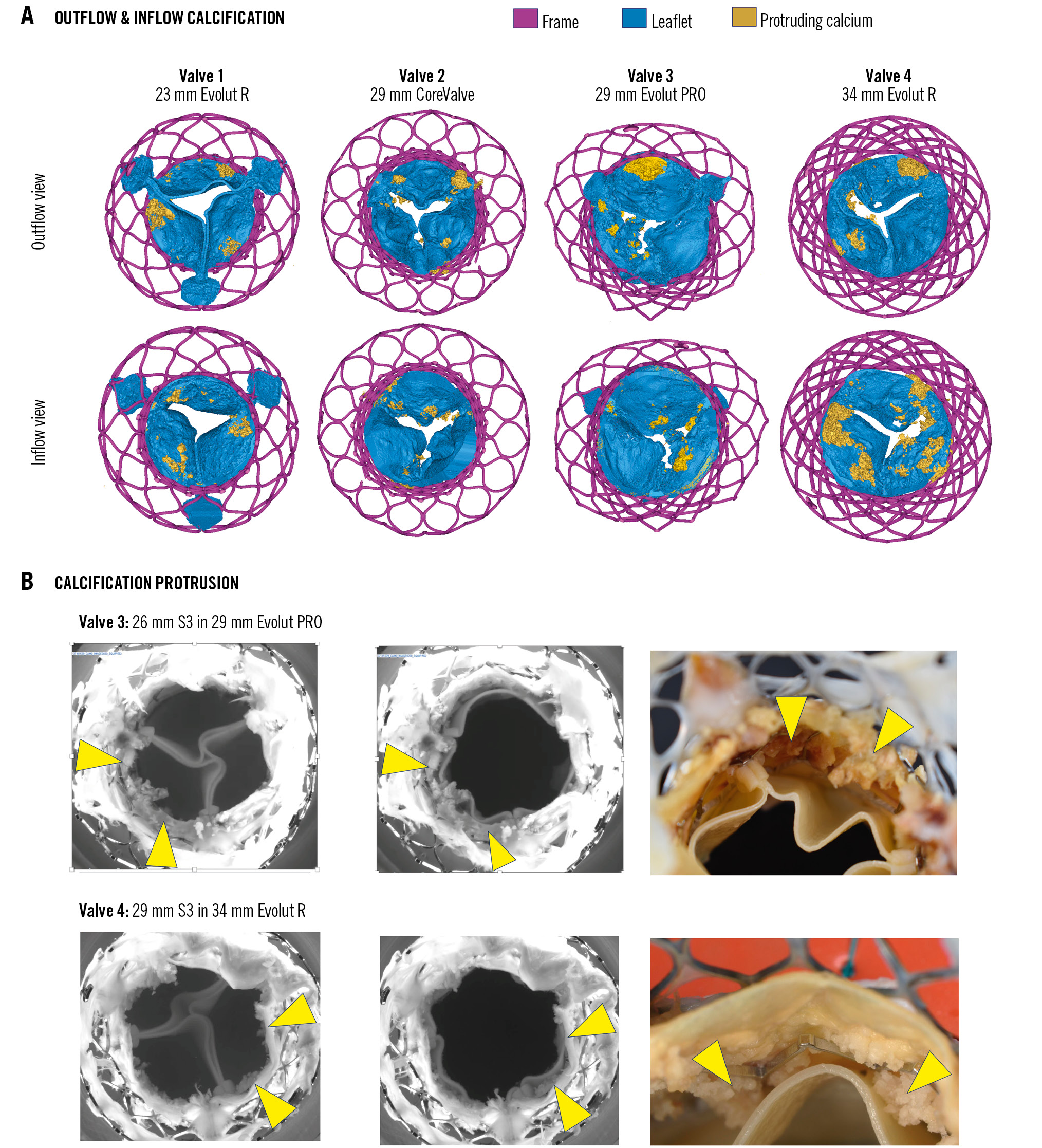
Figure 5. CoreValve/Evolut calcification patterns and protrusion following redo-TAVI. A) CV/EV micro-CT showing patterns of inflow and outflow calcification protrusions from the surface of the TAV leaflet. B) Images from hydrodynamic testing showing protruding calcification from degenerated CV/EV in contact with the implanted S3 and high-resolution gross pathology images of areas of protrusion. The yellow arrowheads denote protruding calcification. CT: computed tomography; CV/EV: CoreValve/Evolut; S3: SAPIEN 3; TAV: transcatheter aortic valve; TAVI: transcatheter aortic valve implantation
Frame cannulation needed for potential coronary access following redo-TAVI
Visually, ex vivo frame cannulation, which would be needed for coronary access, did not appear to be prevented by the positioning of the calcified CV/EV leaflets for all redo-TAVI combinations with the S3 outflow at node 5 (Supplementary Figure 2A). Supplementary Figure 2B shows modelling of catheter access for the 20 mm S3 in 23 mm EV and the 29 mm S3 in 34 mm EV redo-TAVI combinations.
Discussion
Redo-TAVI is an increasingly common procedure, and the assessment of its feasibility plays a central role in the lifetime management of patients with AS. However, clinical evidence remains limited, and bench testing can provide valuable insight to help fill this knowledge gap21282930. In the context of redo-TAVI with an S3 for a failed calcified CV/EV, this study provides the following key insights: first, node 5 positioning of the S3 outflow within a stenotic calcified CV/EV resulted in favourable hydrodynamics in 3 of the 4 configurations tested. Second, leaflet overhang – which was originally described in pristine valves – was also observed in degenerated calcified samples but remained stable throughout the cardiac cycle. In the current short-term hydrodynamic testing, this phenomenon did not seem to have a significant impact on S3 hydrodynamic performance, since all combinations exhibited an EOA above the ISO-defined cutoff points and the GOA of CV/EV TAVs with overhanging leaflets always exceeded the GOA of S3 valves. Further, the ability to cannulate the frame on the bench did not appear to be affected by leaflet overhang. Third, considerable S3 frame underexpansion and leaflet pinwheeling were observed and may be affected by TAV sizing and calcium location. Fourth, in highly calcified CV/EV, calcium nodules from the inflow side can protrude through the S3 open cells and make contact with the S3 leaflets during systole. Fifth, we observed different patterns of S3 frame underexpansion geometry across the considered valves assessed.
The present study addresses some of the limitations of previous bench work on redo-TAVI with S3 in CV/EV19. Indeed, in the prior analysis, brand new CV/EV TAVs were used, which did not reproduce the challenges and outcomes of calcified stenotic CV/EV TAVs. Therefore, the present analysis offers a thorough examination of redo-TAVI in calcified CV/EV TAVs, assessing hydrodynamics, leaflet displacement and kinematics, frame deformation and expansion, and the impact of leaflet displacement and calcium burden on redo-TAVI functional outcomes.
The index TAVs used in our study demonstrated overt SVD consistent with previously documented modes of TAV degeneration, resulting in stenosis or mixed stenosis and regurgitation. These valves, while a selected subset, provide insight into the challenges faced in the setting of redo-TAVI across the different ranges of size combinations. Two of the 4 redo-TAVI combinations tested demonstrated overall satisfactory hydrodynamic performance per the ISO 5840-3 standard and clinical guidelines. However, the 29 mm S3 in 34 mm EV combination exhibited suboptimal RF, and the 20 mm S3 in 23 mm EV configuration showed a residual MG >20 mmHg. Interestingly, the 20 mm S3 in 23 mm EV combination also demonstrated a residual MG >20 mmHg when pristine valves were used19.
These suboptimal results serve to highlight important clinical considerations for redo-TAVI. In the case of the high RF for the 29 mm S3 in 34 mm EV redo-TAVI, it was found that central leakage was the major contributor to the total RF initially observed. This was very likely secondary to S3 underexpansion, which was highest in this redo-TAVI combination, although it was observed in all redo-TAVI combinations. This observation is of utmost importance since the present study used a widely clinically accepted S3 sizing strategy of using an S3 that is one size smaller than the index CV/EV25 and provides a comparison to previous bench studies using new TAVs and the same sizing strategy19. However, our findings underline the importance of TAV sizing based on the in vivo CT-based internal diameter of the index TAV, which may result in a smaller S3 implant with improved expansion and a potential reduction in the RF. This is in line with emerging data13 and recent expert consensus from the freshly released Redo TAV app (KRUTSCH). Future redo-TAVI bench studies should consider the use of novel models that allow CT sizing. Our findings may also prove valuable for clinical studies on CT sizing. For example, current CT sizing does not incorporate aspects of TAV degeneration that may impact the internal diameter or the expansion of the index TAV. With respect to the high residual MG seen in the 20 mm S3 in 23 mm Evolut R configuration, this is in line with known complications of higher gradients in smaller TAVs, particularly when underexpanded, including the observation of gradients over 20 mmHg in the settings of index TAVI, valve-in-valve, and redo-TAVI with small S3 TAVs153132. Thus, these findings bring important focus on the importance of optimising TAV type and size selection at the time of the index TAVI procedure in order to maximise the chances of successful redo-TAVI. Indeed, when considering the lifetime management of young patients, it is critical to consider the potential need for a subsequent intervention at the time of the index TAVI procedure.
We previously showed that the presence of calcification on the surface of failed TAVs provides insights into the underlying mechanisms of valve degeneration22. The current analysis now unveils a potential functional consequence of this in the context of redo-TAVI: protrusion of calcium from the inflow and outflow surfaces can interact with the second TAV. This can impact redo-TAVI functional outcomes in two potential ways. First, outflow surface calcium can become pinned between the two TAVs during redo-TAVI and thus, as a component of leaflet thickening, impact S3 expansion, as observed. Second, inflow surface calcium can be displaced through the S3 frame, leading to contact with the S3 leaflets during systole, as was seen in 2 of the 4 redo-TAVI configurations tested. The significant calcium burden identified by micro-CT imaging on the inflow side of explanted TAVs suggests a potential link between calcium location and the observed protrusion phenomenon. Moreover, future studies will need to consider how to characterise valve degeneration, including leaflet thickening and calcification patterns, clinically in redo-TAVI. Indeed, even if the long-term consequences of repeated contact/friction with the calcium nodules are unknown, procedural planning to optimise sizing and minimise the risk of potential complications associated with calcium protrusion may be necessary.
This is also important when considering post-dilatation. While post-dilatation could improve S3 expansion, CV/EV deformation, and pinwheeling, there are potential concerns of damage to the S3 leaflets from protruding calcium, and further S3 expansion has implications for coronary flow obstruction and the extent and stability of leaflet overhang. In the current study, post-dilatation was not performed, and this may have resulted in the notable S3 underexpansion, associated pinwheeling, and high RF observed in the 29 mm S3 in 34 mm EV configuration. Notably, S3 pinwheeling in the setting of considerable central leakage can be lower than expected, given the lack of central coaptation, which impacts the assessment of the length of the actual leaflet's free edge (Lactual). This can also be impacted by differences in the extent of coaptation along the length of the free edges of leaflet pairs, as leaflet redundancy is taken up by flexion of the leaflet in an axis that pinwheeling measurements are not able to assess. We highlight this in Supplementary Figure 1. Our findings regarding pinwheeling also suggest that factors such as differences in calcification volume and pattern may impact the extent of pinwheeling, likely related to differences in expansion geometry and central coaptation location.
Importantly, leaflet overhang, originally described on the bench using new TAVs19, was still present in degenerated TAVs, although to a slightly lesser degree in some combinations. Furthermore, we showed that, in the presence of calcified leaflets, leaflet overhang is stable throughout the cardiac cycle with minimal difference between systole and diastole. While requiring more study, this may have implications for flow dynamics in the sinus, as an overhanging leaflet − whether stable or not − may contribute to turbulent flow, but how and the relative magnitude by which stable or unstable leaflet overhang impacts flow are unknown. The persistence of the leaflet overhang phenomenon in the presence of calcified leaflets has important clinical implications when considering redo-TAVI planning. Indeed, here we showed that leaflet overhang and neoskirt height findings are consistent: as the leaflets overhung and were not pinned straight or deflected outwards, the neoskirt was not found to extend above the S3 frame outflow. This is critical, since the neoskirt height is a key consideration for coronary access and assessment of risk of coronary obstruction. Thus, the fact that, on the bench, the neoskirt height can still be predicted by the level of the S3 outflow offers some reassuring elements in terms of the clinical predictability of procedural results. Current investigations have primarily focused on how leaflet overhang impacts the acute hydrodynamic function of redo-TAVI. However, future studies should explore other aspects of leaflet overhang and its effect on longer-term redo-TAVI outcomes, including evaluating how leaflet overhang and hydrodynamic function may change with implantation at higher or lower node positions. However, performing this in explants will always remain a significant challenge since implants in a degenerated CV/EV generally cause damage to the leaflets that preclude multiple S3 deployments.
Despite leaflet thickening and stiffness, and given the presence of leaflet overhang, we observed that these did not later prevent, at least on the bench, the ability to selectively cannulate the index TAV frame. This is of importance when considering the high prevalence of concomitant coronary artery disease in patients undergoing TAVI, especially when long-term survival is anticipated. However, given the patient-to-patient anatomical variation in aortic root dimensions, coronary height, and inconsistent rate of commissural alignment, selective coronary engagement might still remain extremely challenging in some cases, and further studies are required to better understand this issue.
Finally, we found that depending on the index TAV size and type, S3 frame expansion could follow several patterns. The smallest combination exhibited a “flared” frame geometry; 2 samples, particularly the largest configuration, displayed a “tapered” frame geometry, while one sample had a “dumbbell” geometry. This is of interest since S3 frame deformation has been shown to potentially impact outcomes such as risk for leaflet thrombosis33. While the current sample size does not allow for further characterisation of this phenomenon, future studies will need to look at the impact of parameters like degenerative changes of the index TAV or redo-TAVI sizing on patterns of expansion following redo-TAVI.
Limitations
There are limitations to be considered in this analysis. First, the ex vivo bench-testing setup may not reflect physiological conditions in clinical practice including the challenges of frame cannulation to achieve coronary access. In vivo expansion of the index and second TAVs may vary compared with a bench model, and the CV/EV holder used may not reflect patient anatomy but was in accordance with ISO 5840-3 guidance. Second, this is a limited redo-TAVI population which does not reflect all pathologies, patterns of calcification, size combinations, etc., but does reflect common challenges for failed CV/EV TAVs. Further, irreversible S3 frame deformation, risk of explanted CV/EV leaflet damage/tear during S3 removal, and CV/EV explant availability limited the assessment of additional redo-TAVI implant depth configurations. Third, sizing the S3 was based on recommendations of downsizing that did not consider CT sizing. Fourth, no balloon pre- or post-dilatation was performed, which may have impacted S3 frame expansion, CV/EV frame deformation, and S3 hydrodynamic performance. This gives insight into the standard nominal S3 deployment − a conservative approach that is used in clinical settings where the risk of coronary obstruction precludes the use of post-dilatation, which would further expand the CV/EV frame and increase the risk. However, this approach does not reflect the best-case scenario, where S3 nominal expansion can be achieved and where improved valve function could be expected. However, as mentioned earlier, it is also possible that aggressive post-dilatation could result in damage to the S3 leaflets from the calcium protrusion, and the issue of pre-/post-dilatation would warrant a full-scale future study considering method variations and outcomes of pre-/post-dilatation. Indeed, although 3 out of 4 redo-TAVI configurations had adequate hydrodynamic performance immediately after the procedure, S3 underexpansion, leaflet pinwheeling and calcium protrusion may have important longer-term clinical implications that were out of the scope of this study. While this study provides highly valuable insights, further validation using real-world clinical data on redo-TAVI is necessary.
Conclusions
Redo-TAVI with the S3 outflow at node 5 of a calcified stenotic CV/EV resulted in adequate systolic hydrodynamic performance (EOA, MG, velocity) per the ISO standard in all combinations tested, while 3 out of 4 combinations had an RF within the accepted ISO range (RF <20%). On the bench, leaflet overhang did not seem to have a significant impact on S3 short-term hydrodynamic performance. CV/EV leaflets remained open and stationary throughout the cardiac cycle and were not pinned in a manner that constrained S3 systolic flow or appeared to prevent selective frame cannulation on the bench. S3 frame underexpansion and leaflet pinwheeling, however, were observed. Overall, our findings contribute to the understanding of redo-TAVI in the context of failed CV/EV TAVs, offering valuable insights into patient selection, procedural planning with routine use of in vivo CT sizing, and optimisation of TAV performance. Further research and clinical studies are warranted to validate our findings and refine treatment strategies for patients requiring redo-TAVI, particularly in cases of valve degeneration and calcification.
Impact on daily practice
Implantation of a SAPIEN 3 is indicated for redo-transcatheter aortic valve implantation (TAVI) in the setting of degenerated transcatheter aortic valves (TAVs) but has never been modelled in failed calcified CoreValve/Evolut TAVs. Redo-TAVI with the SAPIEN 3 outflow at node 5 of the degenerated calcified CoreValve/Evolut led to favourable haemodynamics in 3 of the 4 configurations tested, stable leaflet overhang, acceptable neoskirt heights, SAPIEN 3 underexpansion and pinwheeling, and some instances of index TAV calcium touching the SAPIEN 3 leaflets.
Acknowledgements
The authors wish to acknowledge that Amanda Tenhoff, PhD, an employee of Medtronic, provided technical input for the study. Andres Caballero, PhD, an employee of Medtronic, assisted in the preparation of the manuscript including drafting the methods and results sections, creating tables and figures, ensuring the accuracy of the data presented, and providing editorial support under the direction of the lead and senior authors.
Funding
This work was supported by Medtronic, USA, and the Cardiovascular Translational Laboratory at St. Paul’s Hospital, Canada.
Conflict of interest statement
D. Meier has received an institutional grant from Edwards Lifesciences. A. Nigade, K. Dorman, and S. Javani are employees and shareholders of Medtronic. M. Akodad is a consultant to Edwards Lifesciences, Medtronic, and Abbott. D.A. Wood is a consultant and receives unrestricted grant support from Medtronic, Edwards Lifesciences, and Abbott. T. Rogers is a consultant to Edwards Lifesciences, Medtronic, Abbott, Anteris, and Boston Scientific; is an advisory board member to Medtronic and Boston Scientific; has equity in Transmural Systems; and is a co-inventor on patents, assigned to NIH, for transcatheter electrosurgery devices. R. Puri is a consultant, speaker and proctor for Medtronic and Abbott; consults for Centerline Biomedical, Philips, P+F Products + Features, Shockwave Medical, VDyne, VahatiCor, Advanced NanoTherapies, NuevoSono, TherOx, GE HealthCare, Anteris, T45 Labs, Pi-Cardia, Protembis, and Nyra Medical; and has equity interest in Centerline Biomedical, VahatiCor, and NuevoSono. K.B. Allen has received grant support, proctor and speaker bureau fees from Edwards Lifesciences, Medtronic, and Abbott, with no personal compensation. A.K. Chhatriwalla is a proctor for Edwards Lifesciences and Medtronic; is on the speakers bureau for Abbott, Edwards Lifesciences, and Medtronic; and has a research grant from Boston Scientific. M.J. Reardon has received fees to his institution from Medtronic for consulting and providing educational services. G.H.L. Tang has received speaker honoraria and served as a physician proctor, consultant, advisory board member, TAVI publications committee member, APOLLO trial screening committee member and IMPACT MR steering committee member for Medtronic; has received speaker honoraria and served as a physician proctor, consultant, advisory board member and TRILUMINATE trial anatomic eligibility and publications committee member for Abbott; has served as an advisory board member for Boston Scientific and JenaValve; a consultant and physician screening committee member for Shockwave Medical; a consultant for NeoChord, Shockwave Medical, Peija Medical, and Shenqi Medical Technology; and has received speaker honoraria from Siemens Healthineers. J.G. Webb is a consultant to and has received research funding from Edwards Lifesciences, Abbott, and ViVitro Labs. S. Fukuhara is a consultant for Medtronic, Terumo Aortic, and Artivion. S.L. Sellers is a consultant for Medtronic, Edwards Lifesciences, Excision Medical, and Anteris Technologies; has received research support from Medtronic, Edwards Lifesciences, Vivitro Labs, and HeartFlow; and has stock options in Excision Medical. V.N. Bapat is a consultant for Edwards Lifesciences, Medtronic, Abbott, Anteris Technologies, and Meril. The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Baseline CoreValve/Evolut explant function. Visualisation of valve kinematics during baseline hydrodynamic testing of explanted calcified CoreValve and Evolut TAVs.
Moving image 2. Hydrodynamic evaluation following redo-TAVI. Visualisation of valve kinematics, pinwheeling, and leaflet overhang following S3 in CV/EV redo-TAVI.
Moving image 3. Calcium protrusion. Visualisation of S3 leaflet contact with protruding CV/EV calcification following redo-TAVI.

