Abstract
Background: Redo-transcatheter aortic valve implantation (TAVI) may be unfeasible because of the risk of compromising coronary flow or coronary access by the pinned back leaflets of the index transcatheter aortic valve.
Aims: We aimed to evaluate the feasibility of redo-TAVI using the balloon-expandable SAPIEN 3 (S3) implanted within the self-expanding ACURATE neo2 (ACn2) valve and to identify predictors associated with a high risk of compromising coronary flow.
Methods: A total of 153 post-ACn2 TAVI cardiac computed tomography scans were analysed. Redo-TAVI using an S3 was simulated in two positions: S3 outflow to the ACn2 upper crown (low implant) and S3 outflow to the base of the ACn2 commissural posts (high implant). The risk for coronary flow compromise and inaccessibility was determined by the height of the neoskirt created by the pinned back leaflets and the valve-to-aorta distances.
Results: At a low S3 implant position, risk of coronary flow compromise was predicted in only 8% of patients and this increased to 60% with a high S3 position. In accordance, coronary access was predicted to be unrestricted in 52% versus 13% of patients with a low versus high S3 implantation. Female sex, a small aortic annular dimension and a sinotubular junction-to-aortic annulus mean diameter ratio <1.15 were independent predictors associated with a high risk for coronary flow compromise.
Conclusions: The feasibility of redo-TAVI with an S3 in an ACn2 depends on the implant depth of the S3 and the geometry of the surrounding aorta. A low S3 implant may reduce the risk of coronary flow compromise and inaccessibility.
Globally, the median age of patients undergoing transcatheter aortic valve implantation (TAVI) is decreasing, and the long-term durability of transcatheter aortic valves (TAVs) remains unknown1. Therefore, an increasing proportion of younger patients are expected to outlive their index TAV2. For these patients, a redo-TAVI procedure compares favourably against surgical explantation3456.
Redo-TAVI is predicted to be unfeasible for a significant proportion of TAVI patients because of an increased risk of coronary obstruction or coronary inaccessibility789. Few data exist concerning the feasibility of redo-TAVI after failure of the self-expanding ACURATE neo2 (ACn2) TAV (Boston Scientific). In vitro studies1011 and isolated case reports1213 have confirmed the technical feasibility and favourable haemodynamic outcomes following implantation of the balloon-expandable SAPIEN 3 (S3) TAV (Edwards Lifesciences) to treat a degenerated ACn2; however, the subsequent impact on coronary flow and coronary access remains unknown.
In this study, we evaluated the feasibility of S3-in-ACn2 redo-TAVI, based on post-ACn2 TAVI computed tomography (CT) imaging and determined predictors associated with a high risk of compromising coronary flow and coronary access.
Methods
Study population
Amongst 1,024 patients who underwent TAVI with the ACn2 TAV in two centres in Germany and Denmark, 153 patients had high-quality post-implant cardiac CT scans, which were analysed for this study. Patients treated with an ACn2 TAV for a degenerated surgical bioprosthesis were excluded. All cardiac CT scans were electrocardiographically gated, contrast enhanced and had <1 mm slice thickness. CT analysis was performed using 3Mensio software (Pie Medical Imaging), and all measurements were performed and verified independently by two experienced physicians (G. Bieliauskas, Y. Kobari). Ethical approval for the study was granted by the local ethics committees, and written informed consent was obtained from all included patients.
Second TAV sizing and positioning
The post-TAVI CT scans were used to implant the virtual S3 inside the ACn2 valves. Selection of the size of the S3 TAV for the simulation was based on the size and expansion of the index ACn2 as well as the native aortic annular dimensions derived from the pre-TAVI CT. The expected expansion of the redo-TAV complex was taken into consideration based on findings from previous bench-testing data and real-world case examples1113. Accordingly, a 21 mm virtual S3 was implanted into a small ACn2 (ACn2 S), a 23 or 24 mm virtual S3 implanted into a medium ACn2 (ACn2 M), and a 25 mm virtual S3 into a large ACn2 (ACn2 L). The decision to use a 23 mm or 24 mm for the ACn2 M was based on the expansion of the index TAV and native aortic annular dimension (Figure 1).
Two different implant positions of the S3 were evaluated: in case of a low S3 implant, the outflow of the S3 was positioned at the level of the upper crown of the ACn2. In case of a high S3 implant, the S3 outflow was positioned at the bottom of the commissural posts of the ACn2 (Figure 1) – these positions were based on prior bench test work.
The resulting height of the pinned-up leaflets, termed neoskirt, was defined as the distance from the ACn2 inflow to the outflow of the virtual S3, and this position was defined as the neoskirt plane (NSP). The residual leaflet length of the ACn2 above the outflow of the S3 or NSP was defined as leaflet overhang, and the extent of leaflet overhang was predicted based on data from bench testing1011.
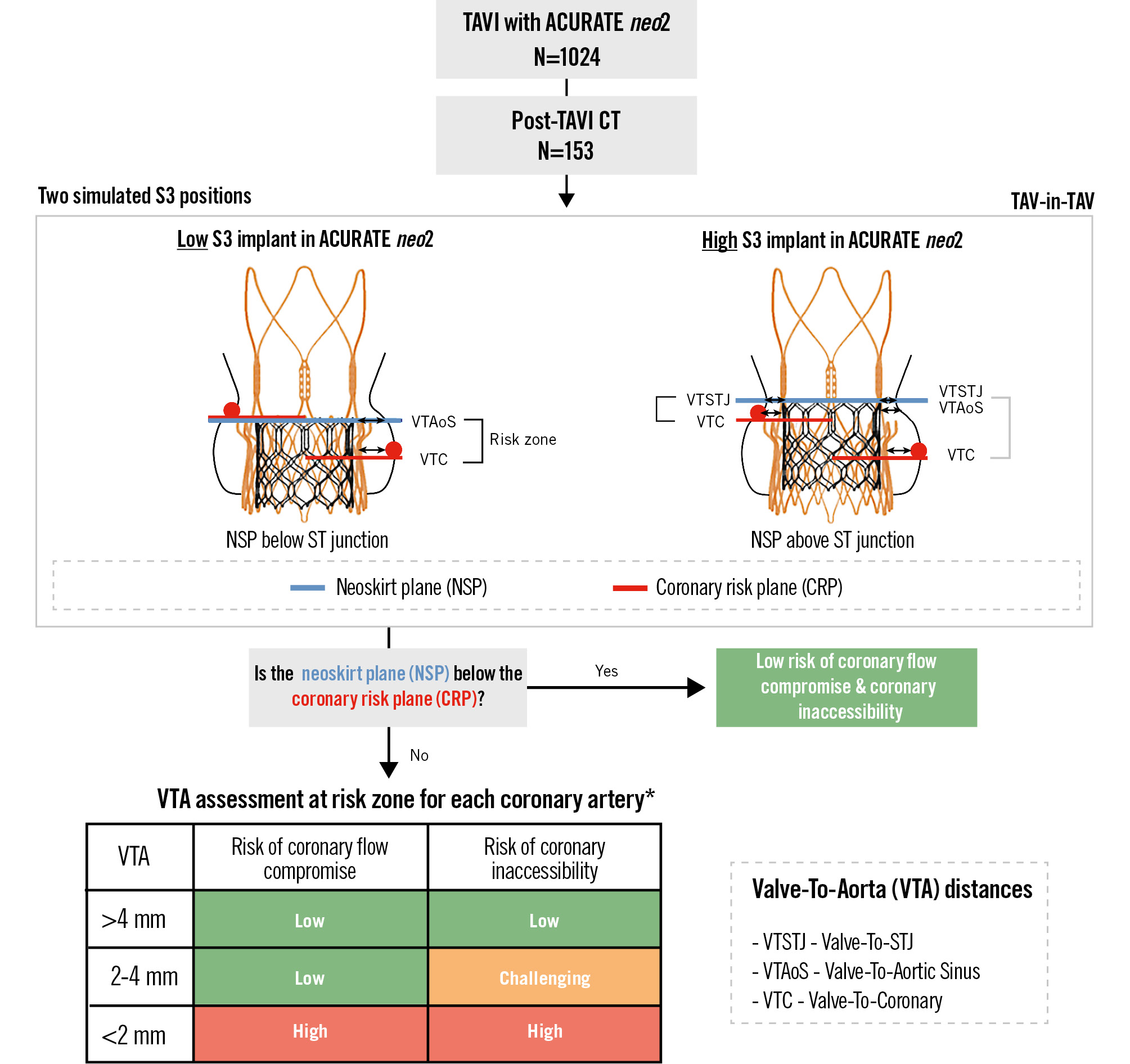
Figure 1. Study concept and methodology. Methodology used to evaluate the risk of coronary flow compromise and coronary inaccessibility following virtual SAPIEN 3 (S3) implantation in an ACURATE neo2 (ACn2) index TAV using a post-index TAVI CT scan. Sizing of the virtual S3 was based on prior bench-testing data to account for predicted S3-in-ACn2 valve expansion. Definitions of the neoskirt plane and coronary risk plane can be found in the Methods section and on the redo TAV App (KRUTSCH). *Risk based on narrowest VTA measurement and simulated further (ACn2 expansion by S3 implantation − based on bench test data: 23 mm S3 in ACn2 S [21 mm]; 23 mm S3 in ACn2 M [23 mm]; 26 mm S3 in ACn2 M [24 mm]; 26 mm S3 in ACn2 L [25 mm]). CT: computed tomography; L: large; M: medium; S: small; S3: SAPIEN 3; ST: sinotubular; TAV: transcatheter aortic valve; TAVI: transcatheter aortic valve implantation
Determining the risk of compromising coronary flow or coronary access
In order to determine the risk of compromising coronary flow or access, two planes were identified: the NSP and the coronary risk plane (CRP). The neoskirt is the portion of the redo-TAV combination covered with the inner skirt and the pinned-open prosthetic leaflets of the index TAV – the NSP is defined as the plane at the top of the neoskirt1415. The CRP is defined as the plane parallel with the aortic annulus at the base of the coronary ostium and was obtained for both the left and right coronary ostia. The relationship between the NSP and the CRP for each coronary ostium was noted.
In a short-axis view, the distance between the simulated redo-TAV complex (expanded ACn2 with a virtual S3) and the surrounding aortic wall was measured and termed the valve-to-aorta (VTA) distance. Considering the thickness of the stent frame and the blooming artefact on CT, the VTA distances were measured from the middle of the stent frame.
Depending on the patients’ anatomy, the VTA distance can be evaluated at three different levels: (1) valve-to-coronary (VTC), (2) valve-to-aortic sinus (VTAoS), and (3) valve-to-sinotubular junction (VTSTJ). Each of these VTA measurements was performed for the left (LCA) and right coronary artery (RCA). Based on these measurements, the risk of compromising coronary flow or access was determined (Figure 1).
Redo-TAV with an S3-in-ACn2 was deemed to be low risk for both coronary flow compromise and coronary inaccessibility if the NSP was below the CRP. If the NSP was above the CRP, then the narrowest of the three VTA measurements was used to further define the risk. The risk of coronary flow compromise was deemed to be low if the VTA was >2 mm; it was deemed high risk for compromise if the VTA was <2 mm. For coronary access, the risk of inaccessibility was deemed to be low if the VTA was >4 mm, challenging if the VTA was 2-4 mm, and high if the VTA was <2 mm. These evaluations were conducted for both the LCA and RCA. The higher risk for the two coronary arteries was determined as the overall risk level for each patient.
Statistical analysis
Categorical variables are expressed as numbers (percentages) and continuous variables as medians (interquartile range). A stepwise uni- and multivariate logistic regression analysis was utilised in order to identify associated factors and independent predictors of coronary flow compromise in case of a high S3 implantation into an index ACn2 TAV. Clinical, cardiac CT and TAVI-specific variables were included in this analysis. Variables which were associated with a higher risk for coronary flow compromise in the univariate model (defined as p<0.1) were included in the multivariate model in order to identify independent predictors of coronary flow compromise. In case of similar variables (e.g., aortic annulus perimeter or area), only the variable with the highest statistical power was tested in the multivariate model in order to avoid multicollinearity issues. In case of a “ratio” variable with a p-value<0.1 in the univariate analysis, it was prespecified that the optimal cutoff value would be determined and tested in the multivariate analysis. A 2-sided p-value<0.05 was considered statistically significant. All statistical analyses were performed using SPSS, version 27.0 (IBM).
Results
For the purpose of this study, 153 patients with a post-TAVI cardiac CT following ACn2 TAV implantation were included. Baseline clinical, CT and procedural details are summarised in Table 1. The median age of the study cohort was 81 (77-84) years, 61% were female, and the median Society of Thoracic Surgeons risk score was 2.9% (2.2-4.6%). A small, medium, or large ACn2 was implanted in 30 (19.6%), 57 (37.3%), and 66 (43.1%) of patients, respectively. Redo-TAVI was simulated using a virtual 21 mm S3 for all ACn2 S and a 25 mm S3 for all ACn2 L valves. For ACn2 M, a virtual 23 mm and 24 mm S3 were simulated in 44/57 and 13/57 of cases, respectively.
Table 1. Baseline characteristics.
| N=153 | |
|---|---|
| Clinical variables | |
| Age, years | 81 (77-84) |
| Female | 94 (61.4) |
| Arterial hypertension | 125 (81.7) |
| Diabetes mellitus | 44 (28.8) |
| Coronary artery disease | 75 (49.0) |
| Prior PCI | 53 (34.6) |
| Prior CABG | 14 (9.2) |
| Atrial fibrillation | 47 (30.7) |
| Permanent pacemaker | 13 (8.5) |
| Prior stroke | 13 (8.5) |
| Peripheral arterial disease | 16 (10.5) |
| Reduced renal function§ | 59 (38.6) |
| STS risk score, % | 2.9 (2.2-4.6) |
| CT variables | |
| Aortic annulus perimeter, mm | 74.7 (71.0-78.6) |
| Aortic annulus area, mm2 | 432 (383-477) |
| Sinus of Valsalva mean diameter, mm | 30.9 (28.8-33.5) |
| STJ mean diameter, mm | 27.2 (25.6-29.4) |
| STJ height, mm | 25.0 (22.7-28.0) |
| Left coronary artery height, mm | 13.5 (12.0-15.8) |
| Right coronary artery height, mm | 16.8 (14.5-18.6) |
| TAVI procedure | |
| ACURATE neo2 | |
| Small, 23 mm | 30 (19.6) |
| Medium, 25 mm | 57 (37.3) |
| Large, 27 mm | 66 (43.1) |
| Predilatation | 148 (96.7) |
| Implant depth, mm | 5.0 (4.0-5.6) |
| Post-dilatation | 50 (32.7) |
| In-hospital outcomes | |
| Myocardial infarction | 0 |
| Stroke | 4 (2.6) |
| Permanent pacemaker implantation | 14 (9.2) |
| Predischarge echocardiographic outcomes | |
| Transprosthetic mean gradient, mmHg | 8.0 (5.0-10.8) |
| Paravalvular regurgitation | |
| None-trace | 71 (46.4) |
| Mild | 77 (50.3) |
| Moderate or greater | 5 (3.3) |
| Data are given as median (IQR) or n (%). §Reduced renal function is defined as estimated glomerular filtration rate <30 mL/min/1.73 m2. CABG: coronary artery bypass graft; CT: computed tomography; IQR: interquartile range; PCI: percutaneous coronary intervention; STJ: sinotubular junction; STS: Society of Thoracic Surgeons; TAVI: transcatheter aortic valve implantation | |
Predicted risk of coronary flow compromise
The predicted risk of coronary flow compromise was dependent on the implantation depth of the S3, with a greater risk seen with a higher implant of the S3 (Figure 2). In case of a high S3 implant, 60% of patients were deemed to be at a high risk and 40% at a low risk of coronary flow compromise, whilst for a low S3 implant, only 8% were at a high risk and the remaining 92% at a low risk. Irrespective of the implant depth of the S3, the RCA was associated with a greater risk of coronary flow compromise compared to the LCA (Figure 2).
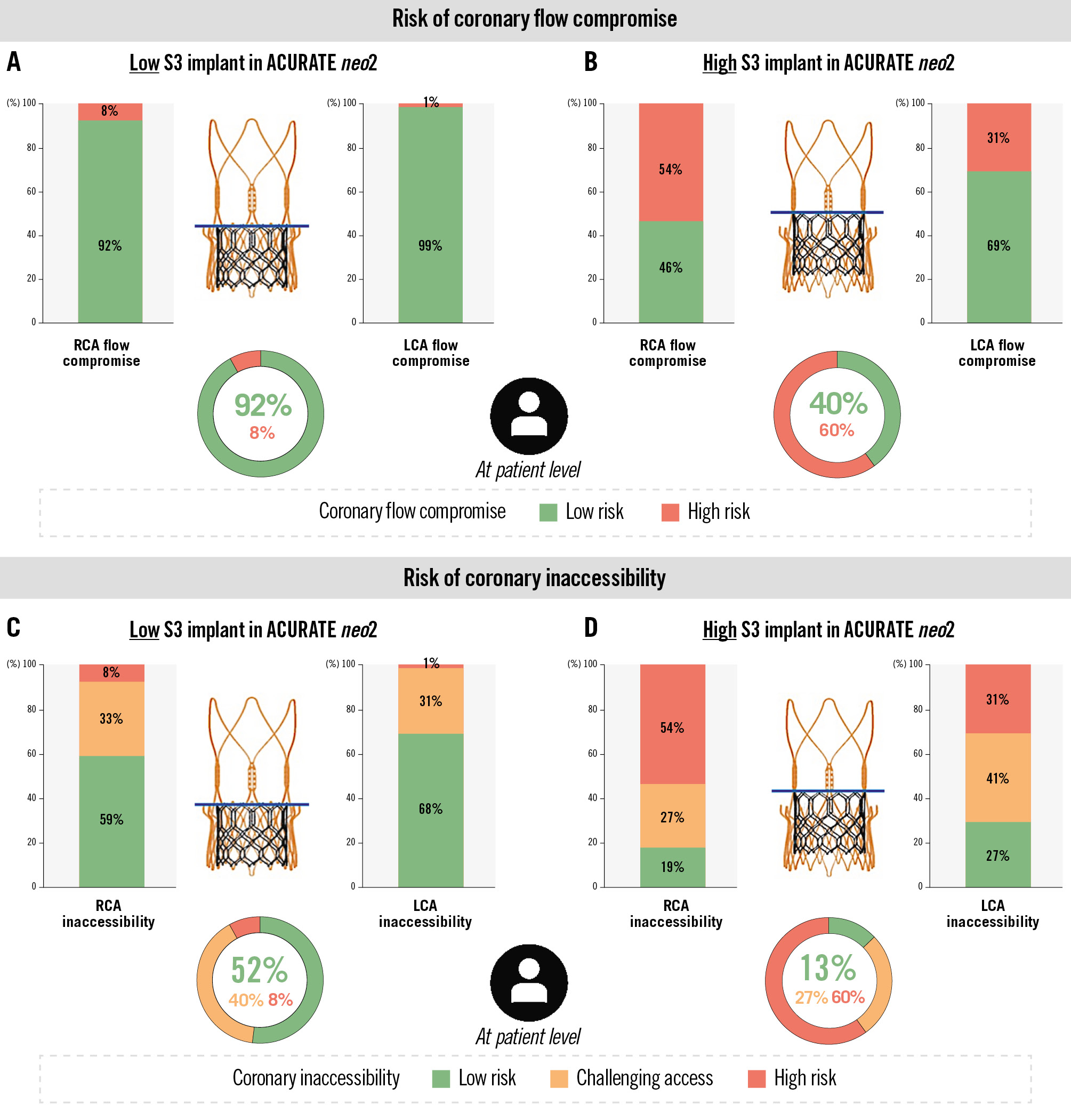
Figure 2. CT-predicted risk of coronary flow compromise and coronary inaccessibility following redo-TAVI with S3-in-ACn2 TAV. A) The risk for coronary flow compromise was low (8%) with a low S3 implant position, aligning with the upper crown of the ACn2 and (B) relatively high (60%) with a high S3 implant position, aligning with the base of the ACn2 commissural posts. Similarly, the risk of a challenging coronary access or coronary inaccessibility was (C) low with a low S3 implant position and (D) higher with a high S3 position. ACn2: ACURATE neo2; CT: computed tomography; LCA: left coronary artery; RCA: right coronary artery; S3: SAPIEN 3; TAV: transcatheter aortic valve; TAVI: transcatheter aortic valve implantation
Predicted risk of coronary inaccessibility
The risk of coronary inaccessibility was also greater with a higher implantation of the S3 TAV (Figure 2). A total of 60% of patients would be at high risk for coronary inaccessibility to one or both coronary ostia with a high S3 implant, with a further 27% having challenging coronary access. Implanting the S3 lower would lead to only 8% of patients being at high risk for coronary inaccessibility, but 40% could still have challenging coronary access. A straightforward coronary access was predicted for 13% of patients with a high S3 implant; this increased to 52% with a low S3 implant. Again, the RCA was at an increased risk for coronary inaccessibility compared to the LCA.
Predictors of coronary flow compromise
The risk of coronary flow compromise is highest in case of a high S3 implant position. A list of clinical, CT and procedural variables was screened in order to identify variables associated with this increased risk. Besides female sex, coronary artery disease and atrial fibrillation, numerous CT variables reflecting a small aortic annulus, small aortic root and low coronary ostia were identified (p<0.1). None of the procedural variables were associated with a high risk of coronary flow compromise (Table 2).
In the multivariate analysis, aortic annulus perimeter (odds ratio [OR] 0.91, 95% confidence interval [CI]: 0.83-0.99; p=0.035), female sex (OR 2.79, 95% CI: 1.29-6.10; p=0.01) and a sinotubular junction/aortic annulus mean diameter (STJ/AAØ) ratio <1.15 (OR 3.91, 95% CI: 1.55-9.88; p<0.01) were the only independent predictors of a high risk of coronary flow compromise following a high S3 implant (Table 3); the latter variable being the strongest predictor.
The analysis to identify predictors of coronary inaccessibility (high risk) was, by definition, the same as reported in Table 2 and Table 3. A separate analysis to identify variables associated with a high-intermediate risk (challenging access) of coronary inaccessibility resulted in similar findings and can be found in Supplementary Table 1.
Table 2. Characteristics and univariate analysis of a high risk of coronary flow compromise with a high S3 implant.
| Coronary flow compromise | Univariate model | |||
|---|---|---|---|---|
| High risk N=92 | Low risk N=61 | Odds ratio (95% CI) | p-value | |
| Clinical variables | ||||
| Age, years | 81 (77-84) | 81 (78-86) | 0.96 (0.91-1.02) | 0.151 |
| Female | 68 (73.9) | 26 (42.6) | 3.81 (1.92-7.59) | <0.001* |
| Arterial hypertension | 76 (82.6) | 49 (80.3) | 1.16 (0.51-2.67) | 0.721 |
| Diabetes mellitus | 76 (82.6) | 49 (80.3) | 1.16 (0.51-2.67) | 0.721 |
| Coronary artery disease | 39 (42.4) | 36 (59.0) | 0.51 (0.27-0.98) | 0.045* |
| Prior PCI | 29 (31.5) | 24 (39.3) | 0.71 (0.36-1.40) | 0.320 |
| Prior CABG | 7 (7.6) | 7 (11.5) | 0.64 (0.21-1.91) | 0.420 |
| Atrial fibrillation | 22 (23.9) | 25 (41.0) | 0.45 (0.23-0.91) | 0.026* |
| Prior stroke | 6 (6.5) | 7 (11.5) | 0.54 (0.17-1.69) | 0.288 |
| Peripheral arterial disease | 8 (8.7) | 8 (13.1) | 0.63 (0.22-1.78) | 0.385 |
| CT variables | ||||
| Aortic annulus perimeter, mm | 73.8 (70.4-77.0) | 76.1 (72.1-79.4) | 0.94 (0.88-1.00) | 0.040* |
| Aortic annulus area, mm2 | 417 (373-454) | 445 (396-486) | 0.99 (0.99-1.00) | 0.041* |
| SoV-LCC, mm | 30.3 (28.5-32.9) | 33.4 (31.0-35.7) | 0.75 (0.66-0.85) | <0.001* |
| SoV-RCC, mm | 29.0 (27.3-31.0) | 32.0 (30.3-33.9) | 0.73 (0.65-0.83) | <0.001* |
| STJ mean diameter, mm | 26.2 (24.6-27.8) | 29.3 (27.7-31.3) | 0.61 (0.51-0.73) | <0.001* |
| STJ/aortic annulus mean diameter ratio | 1.12 (1.07-1.17) | 1.22 (1.14-1.31) | 0.39 (0.27-0.57) | <0.001* |
| STJ height, mm | 24.0 (22.2-27.0) | 26.8 (23.9-30.0) | 0.89 (0.82-0.97) | 0.005* |
| Left coronary ostium height, mm | 13.0 (11.7-15.0) | 14.0 (12.9-16.8) | 0.84 (0.74-0.94) | 0.003* |
| Right coronary ostium height, mm | 16.0 (14.0-18.0) | 17.5 (15.2-19.4) | 0.83 (0.74-0.93) | 0.002* |
| TAVI procedure | ||||
| ACURATE neo2 | ||||
| Small, 23 mm | 19 (20.7) | 11 (18.0) | Reference | |
| Medium, 25 mm | 38 (41.3) | 19 (31.1) | 1.16 (0.46-2.92) | 0.756 |
| Large, 27 mm | 35 (38.0) | 31 (50.8) | 0.65 (0.27-1.59) | 0.347 |
| Predilatation | 90 (97.8) | 58 (95.1) | 2.33 (0.38-14.6) | 0.363 |
| Post-dilatation | 31 (33.7) | 19 (31.1) | 1.12 (0.56-2.25) | 0.742 |
| Commissural alignment | 67 (72.8) | 47 (77.0) | 0.80 (0.38-1.70) | 0.558 |
| Data are given as median (IQR) or n (%). *P-value<0.10 for variable with the highest statistical power in case of multicollinearity. CABG: coronary artery bypass graft; CI: confidence interval; CT: computed tomography; IQR: interquartile range; LCC: left coronary cusp; PCI: percutaneous coronary intervention; RCC: right coronary cusp; S3: SAPIEN 3; SoV: sinus of Valsalva; STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation | ||||
Table 3. Independent predictors of a high risk of coronary flow compromise for S3 implanted in a high position.
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Clinical variables | ||||
| Female | 3.81 (1.92-7.59) | <0.001 | 2.79 (1.29-6.10) | 0.010* |
| Coronary artery disease | 0.51 (0.27-0.98) | 0.045 | 0.62 (0.28-1.38) | 0.242 |
| Atrial fibrillation | 0.45 (0.23-0.91) | 0.026 | 0.51 (0.30-1.03) | 0.114 |
| CT variables | ||||
| Aortic annulus perimeter, mm | 0.94 (0.88-1.00) | 0.041 | 0.91 (0.83-0.99) | 0.035* |
| SoV-LCC, mm | 0.75 (0.66-0.85) | <0.001 | 0.99 (0.78-1.26) | 0.959 |
| SoV-RCC, mm | 0.73 (0.65-0.83) | <0.001 | 0.85 (0.65-1.11) | 0.220 |
| STJ/aortic annulus mean diameter ratio <1.15 | 3.56 (2.05-6.20) | <0.001 | 3.91 (1.55-9.88) | 0.004* |
| STJ height, mm | 0.89 (0.82-0.97) | 0.005 | 1.04 (0.93-1.16) | 0.492 |
| Left coronary ostium height, mm | 0.84 (0.74-0.94) | 0.003 | 0.95 (0.82-1.09) | 0.439 |
| Right coronary ostium height, mm | 0.83 (0.74-0.93) | 0.002 | 0.87 (0.74-1.02) | 0.081 |
| *P-value<0.05. CI: confidence interval; CT: computed tomography; LCC: left coronary cusp; OR: odds ratio; RCC: right coronary cusp; S3: SAPIEN 3; SoV: sinus of Valsalva; STJ: sinotubular junction | ||||
Discussion
We used post-TAVI CT scans to determine the real-world feasibility of redo-TAVI for a degenerated ACn2 using an S3 valve at two different implant depths. The key conclusions are as follows: (1) redo-TAVI with an S3-in-ACn2 valve was potentially feasible for up to 92% of patients, (2) a low S3 implant position, in which the S3 outflow is aligned with the upper crown of the ACn2 valve, predicted a lower risk for coronary flow compromise and coronary inaccessibility, and (3) an STJ/AAØ ratio <1.15 was a strong independent predictor for high risk of coronary flow compromise in case of a high S3 implant position (Central illustration).
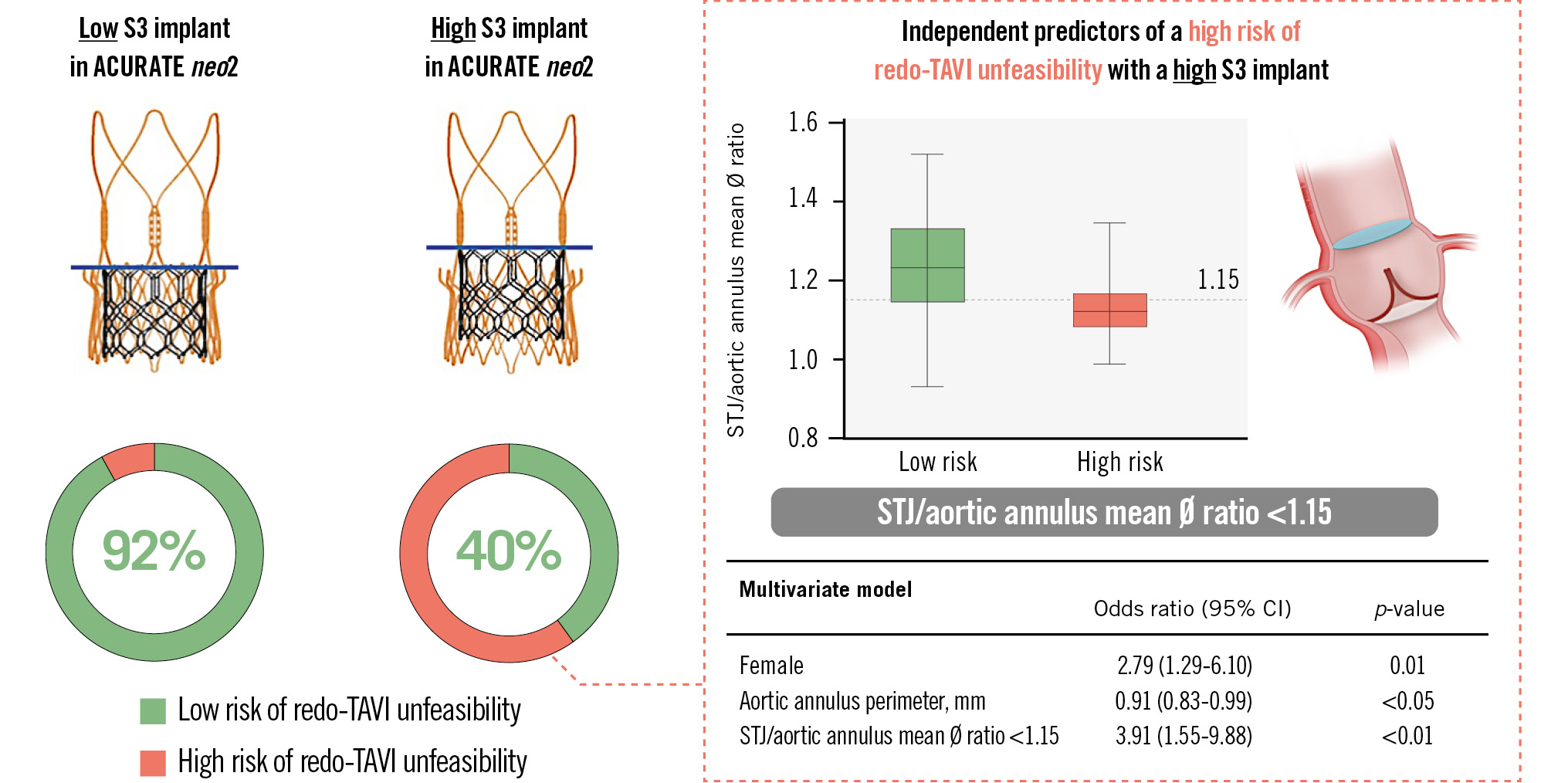
Central illustration. Feasibility of redo-TAVI in ACURATE neo2 valves: a CT analysis. The risk of redo-TAVI unfeasibility is low (8%) in case of a low SAPIEN 3 (S3) implantation in an index ACURATE neo2; this risk increases to 60% in case of a high S3 implant. Independent predictors of a high risk of redo-TAVI unfeasibility in case of a high S3 implant are female sex, a small aortic annulus and an STJ/aortic annulus mean diameter ratio <1.15; the latter variable is the strongest predictor. CI: confidence interval; CT: computed tomography; STJ: sinotubular junction; TAVI: transcatheter aortic valve implantation
Optimal implant depth of S3
Implanting an S3 inside the supra-annular ACn2 valve pins back the leaflets of the index ACn211. The outflow of the S3 determines the maximum height of the pinned back leaflets and is termed the NSP. One of the advantages of using a balloon-expandable TAV to revalve an ACn2 is that the operator can adjust the position of the S3, thereby impacting the NSP and subsequent risk for coronary issues14. However, the final position of a balloon-expandable TAV can be influenced by several procedural factors. In our study, 60% of patients were deemed high risk for coronary flow compromise or inaccessibility following a high implantation of the S3. In contrast, lowering the S3 implantation to match the S3 outflow at the level of the upper crown resulted in only 8% of patients having a high risk for coronary flow compromise or inaccessibility. The benefits of a low S3 implantation must be balanced against the potential impact of leaving residual leaflet tissue overhanging the S3. In vitro studies of S3-in-ACn2 demonstrated favourable haemodynamic outcomes associated with both tested S3 implant depths irrespective of the extent of leaflet overhang11. Still, it is not certain whether a low S3 position is sufficient to treat a degenerated ACn2 with stiff calcified leaflets, and the longer-term consequences remain unknown. As a result, we strongly advocate the study of real TAV-in-TAV cases and emphasise the need for future research to validate our findings and hypotheses.
Results from prior CT-based simulation studies evaluating S3 implantation in supra-annular TAVs are aligned with our findings. Analyses from pre- and post-TAVI CT scans from the Evolut Low Risk trial demonstrated that the risk of compromising coronary flow and access was lowest (20%) with the S3 implanted in the lower position (node 4). In case of a higher S3-in-Evolut (Medtronic) implant position (node 6), there was a high risk of coronary flow compromise reported in 75% of patients916, which is a less favourable result as compared to the 60% of patients with a high risk of coronary flow compromise in case of a high S3-in-ACn2 implant. Taken together, these studies demonstrate that, although the absolute difference between a low and high S3 implant may only be 2-3 mm, the resulting consequences on coronary flow and access may be significant. This highlights the importance of adopting an individualised, systematic approach to preprocedural planning using cardiac CT to ensure optimal outcomes following redo-TAVI131517.
Predicting the feasibility of redo-TAVI
Multiple anatomical and device-related factors can impact the feasibility of redo-TAVI1718. In our study, following adjustment of clinical, CT-based and procedural variables, an STJ/AAØ ratio <1.15 proved to be the strongest independent predictor for a high risk of coronary flow compromise following a high S3 implant in an ACn2 index TAV.
Although aortic annulus and STJ dimensions are known to be positively correlated, variations in their relative dimensions can occur, with some individuals having a proportionally larger STJ relative to their aortic annulus dimensions, and vice versa1920. The aortic annulus diameter dictates the size of the index ACn2 and the subsequent size of the implanted S3. The STJ/AAØ ratio encapsulates the geometric relationship between the S3-in-ACn2 redo-TAV complex with the surrounding aortic wall. Thus, for a given aortic annulus size, a proportionally smaller STJ may result in the redo-TAV complex being in closer proximity to the aortic wall, decreasing the VTA distances and increasing the risk of compromising coronary flow. A relatively high STJ above the aortic annulus plane may be beneficial and reduce the risk for coronary flow and/or access issues. Still, the STJ diameter will always dictate the overall VTA gap (VTSTJ and lower VTAoS) available for coronary flow or access after redo-TAVI, even when the STJ is above the projected functional neoskirt plane.
The STJ/AAØ ratio may have clinical utility when planning an index TAVI as well as a redo-TAVI procedure for S3-in-ACn2. If a ratio of <1.15 is noted, a lower S3 implant position could be pursued, if feasible, to reduce the risk of coronary flow compromise. Further clinical studies are required to validate the accuracy and reproducibility of this parameter as well as to determine its applicability to different combinations and configurations of redo-TAVI.
Finally, it is worth noting that in this study involving ACn2 valves, the risk of coronary flow compromise or inaccessibility following redo-TAVI was higher for the RCA than for the LCA, even though the RCA ostium is often a few millimetres higher than the LCA ostium. A clear explanation for this observation is lacking, although it can be hypothesised that the ACn2 implantation technique and final position – leaning more towards the outer aortic curvature – might be an explanation for this observation. Interestingly, this may indirectly also reduce the risk of coronary issues with the left main.
Additional strategies to improve redo-TAVI feasibility
Despite a low S3 implantation, 8% of patients were still deemed to be at a high risk of coronary flow compromise and would not be suitable for redo-TAVI. For these patients, an alternative treatment strategy involving surgical explantation would carry a high risk of morbidity and mortality56. The potential benefit of adjunctive techniques such as leaflet modification merits evaluation. Techniques such as Bioprosthetic Aortic Scallop Intentional Laceration to prevent Iatrogenic Coronary Artery obstruction (BASILICA) may help to increase coronary flow by creating a splay in the pinned ACn2 leaflets2122. Similarly, despite a low S3 implant, 40% of patients were still deemed to have a challenging coronary access. This highlights the importance of ensuring commissural alignment during index ACn2 implantation and the use of dedicated valve-specific cannulation techniques to facilitate coronary access232425, as well as not setting too high a threshold to consider leaflet modification in case of redo-TAVI.
Limitations
There are several limitations to note in this study. The patients did not undergo an actual redo-TAVI procedure; therefore the CT-based predictions may not fully reflect the physiological conditions in real-world practice. Our simulations may not fully capture the in vivo expansion of the ACn2 or S3 valves that may occur during redo-TAVI. Although we tried to account for the predicted S3 expansion, this was based on in vitro work using non-calcified, non-degenerated TAVs11. Previous studies did not consider this further expansion of the redo-TAV complex at all. Another limitation of this CT study is that it is impossible to predict what the impact will be of “overhanging” ACn2 leaflets in case of redo-TAVI with a low S3 implantation – both on coronary access and flow as well as on the haemodynamic valvular performance. Finally, a patient selection bias cannot be excluded, as only 153 of 1,024 patients treated with an ACURATE neo2 valve had a post-implant cardiac CT scan; these were most often performed in the context of studies investigating TAV leaflet thickening. Moreover, none of the patients in this study had a native bicuspid aortic valve anatomy – hence, extrapolation of the study results to this particular patient group should be avoided. Finally, the impact of leaflet modification on coronary flow and accessibility was not evaluated.
Conclusions
Post-TAVI CT analysis of the ACURATE neo2 valve confirmed that redo-TAVI using the short-frame balloon-expandable SAPIEN 3 valve is feasible. The lowest risk for coronary flow compromise and inaccessibility is observed when the S3 is implanted low. Clinical and anatomical factors may predict when a high S3 implant may be unfeasible to treat a degenerated ACURATE neo2, with an STJ-to-aortic annulus mean diameter ratio <1.15 being a strong predictor of redo-TAVI unfeasibility.
Impact on daily practice
This study investigated the feasibility of redo-transcatheter aortic valve implantation (TAVI) using a SAPIEN 3 (S3) to treat an index ACURATE neo2 valve. The risk of coronary flow compromise and coronary inaccessibility is highly dependent on the implant position of the S3, with a low position being the most favourable. A sinotubular junction-to-aortic annulus mean diameter ratio <1.15 is a strong predictor of redo-TAVI unfeasibility due to coronary issues. Careful preprocedural planning requires a detailed analysis of pre- and post-implant TAVI computed tomography scans to determine the optimal implant size and depth of the S3 to preserve coronary flow and future coronary access.
Funding
An unrestricted grant was provided by Boston Scientific, USA.
Conflict of interest statement
G. Bieliauskas received institutional research grants and consulting fees from Boston Scientific. Y. Kobari has received financial support from the Japan Heart Foundation and Boston Scientific for his fellowship. A.A. Khokhar received speaker fees from Boston Scientific. M. Abdel-Wahab received consulting fees from Medtronic and Boston Scientific; and honoraria for lectures or advisory boards from Medtronic, Boston Scientific, and Edwards Lifesciences (paid to his institution). D. Dudek has received research funding from Boston Scientific. J. Cavalcante received consulting fees from 4C Medical, Abbott, Alleviant Medical, Anteris, Boston Scientific, Edwards Lifesciences, JenaValve, JC Medical, Medtronic, and Novo Nordisk; and has received research grant support from Abbott, Allina Health Foundation, JenaValve, and NIH/NHLBI. K. Hayashida is a clinical proctor for Edwards Lifesciences, Medtronic, and Abbott. G.H.L. Tang has received speaker honoraria and served as a physician proctor, consultant, advisory board member, TAVR publications committee member, APOLLO trial screening committee member and IMPACT MR steering committee member for Medtronic; has received speaker honoraria and served as a physician proctor, consultant, advisory board member and TRILUMINATE trial anatomic eligibility and publications committee member for Abbott; has served as an advisory board member for Boston Scientific and JenaValve; a consultant and physician screening committee member for Shockwave Medical; a consultant for NeoChord, Peija Medical, and Shenqi Medical Technology; and has received speaker honoraria from Siemens Healthineers. D. Mylotte has been a consultant for Boston Scientific, Medtronic, and MicroPort. V.N. Bapat has received consulting fees from Abbott, Medtronic, Boston Scientific, and Edwards Lifesciences. O. De Backer received institutional research grants and consulting fees from Boston Scientific. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

