Left ventricular assist devices (LVADs) have revolutionised the treatment of patients with advanced heart failure (HF). Ongoing engineering advances in LVAD design have markedly improved survival and quality of life. However, potentially life-threatening long-term complications have been observed, even with the HeartMate 3 (HM3 [Abbott]) LVAD. One of these is outflow graft obstruction (OGO), which is known to have different aetiologies and clinical presentations [1].
In this single-centre experience from a large tertiary care centre, we report detection principles and interventional treatment of OGO within the portion covered by the bend relief in patients on HM3. Between January 2015 and April 2023, 433 patients underwent LVAD implantation at our institution. In all cases, the outflow graft (OG) was connected to the ascending aorta and placed alongside the right ventricle; it was not wrapped in protective material. All patients were followed up until October 2023. Written informed consent for data collection was given by all patients.
We established a standardised diagnostic algorithm to confirm OGO (Figure 1). OGO was defined as external compression of the OG lumen due to the accumulation of a gelatinous substance between the OG and the bend relief. The decision on management strategy was made by a multidisciplinary HF team. Treatment was indicated if the patient showed clinical impairment by a low-flow alarm or by critical stenosis (>70% OG narrowing).
The intervention was performed in the catheter lab (Figure 1). Intravascular ultrasound was performed with the use of an IVUS catheter (Eagle Eye Platinum [Philips]) to interrogate the OG regarding the severity of the stenosis and to confirm the absence of thrombus (Moving image 1). In some cases, percutaneous transluminal angioplasty (PTA) was performed prior to stent implantation to prepare the optimal landing zone within the OG. A VIABAHN VBX stent (GORE VIABAHN VBX 11x79 mm [W. L. Gore & Associates]) was positioned with its distal end overlapping the metal pump housing. LVAD flow was stopped briefly for final stent positioning and deployment of the primary stent, and any subsequent stents were placed to prevent restenosis due to debris migration, as well as for PTA using a 14x20 mm Atlas Gold balloon (BD) for complete stent adaptation. Norepinephrine bolus was administered when needed during LVAD cessation.
Adverse event rates are presented as a percentage of patients and as events per patient-year (EPPY). All statistical analyses were done with SPSS 27.0 (IBM).
Relevant OGO was observed only in patients supported with the HM3 LVAD, with an incidence of 2.8% (10/357). The EPPY rate was 0.014. A total of 80% of the patients with OGO had undergone LVAD implantation via full sternotomy. The mean LVAD support time until OGO diagnosis was 1,375.3±586.2 days. The mean age of the patients at the time of percutaneous intervention was 61.8 years (range 36-77). Most patients were male (90%), and half of the patients had ischaemic cardiomyopathy as the primary cause of advanced HF. Six patients received an LVAD as destination therapy. In all patients with OGO, N-terminal pro-brain natriuretic peptide values were significantly elevated at the time of admission. Other biomarkers, such as lactate dehydrogenase, showed no significant difference in comparison to patients without OGO. Transthoracic echocardiography (TTE) was highly suggestive of OGO in 6 patients. Increased peak velocity values between 1.7 m/s and 3.3 m/s were observed over the OG. The procedure was successful in all patients. The mean number of implanted stents was 3.5 (range 2-4). There was no in-hospital mortality, and no intervention-related major adverse cardiac or cerebrovascular events occurred, nor any minor or major access site-related complications. After a median follow-up of 336 days, no restenosis or stent migration were observed. One patient died 152 days after the intervention due to COVID 19-related acute respiratory distress syndrome.
OGO caused by extrinsic compression is a late complication after HM3 implantation. The incidence of OGO in our study is comparable to previously reported values [2]. A step-by-step diagnostic approach is required for proper diagnosis (Figure 1). TTE should be considered as the primary imaging method, with special attention to a possible increase in peak velocity in the OG, the increase in left ventricular diameter and the opening of the aortic valve on every heartbeat. The role of graftoplasty in addition to computed tomography angiography and intravascular ultrasound [3] is discussed in the literature [4]. This combined approach makes it possible to differentiate between biodebris and twist, particularly in the case of stenosis located in the area of the kink. In all cases, we used balloon-expandable stents, which result in excellent adherence to the outflow graft wall. It is usually necessary to use more than one stent due to the discrepancy between the stent size and the inner diameter of the LVAD outflow graft, resulting in significant longitudinal stent shortening after PTA.
Our clinical series is limited to a single-centre experience with a small sample size. Nonetheless, in the absence of larger trials, our results highlight the value of stent graft implantation as a safe and feasible treatment option for these high-risk patients, making it a viable alternative to redo surgery, which is recommended in the current mechanical circulatory support guidelines [5].
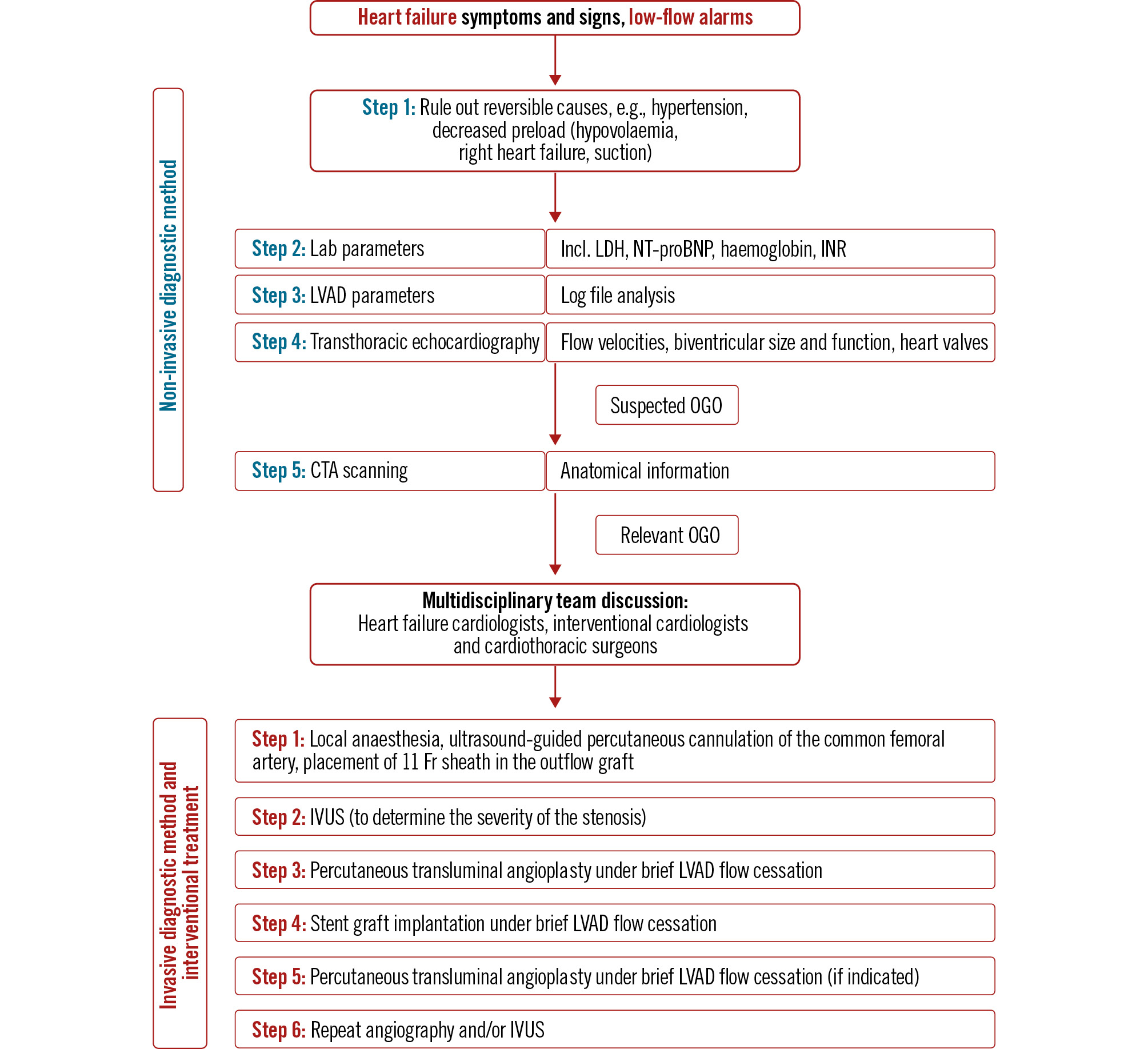
Figure 1. Diagnostic and therapeutic approach to outflow graft obstruction after left ventricular assist device implantation. CTA: computed tomography angiography; Fr: French; INR: international normalised ratio; IVUS: intravascular ultrasound; LDH: lactate dehydrogenase; LVAD: left ventricular assist device; NT-proBNP: N-terminal pro-brain natriuretic peptide; OGO: outflow graft obstruction; TTE: transthoracic echocardiography
Conflict of interest statement
C. Krieghoff reports lecturer fees from Edwards Lifesciences and FUJIFILM Irvine Scientific (payments to Heart Center Leipzig at University Leipzig, Germany). The other authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. Intravascular ultrasound assessment to determine the severity of the outflow graft stenosis.

