Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Transcatheter aortic valve implantation (TAVI) in pure aortic regurgitation (AR) remains challenging because of inadequate anchoring forces. Traditional approaches, which rely solely on virtual annulus oversizing, have demonstrated limited success. We propose a novel anatomical classification system and dual-anchoring theory to optimise the TAVI strategy in patients with pure AR.
Aims: We aimed to evaluate the efficacy and safety of TAVI in pure AR using a novel anatomical classification system and dual-anchoring theory.
Methods: The AURORA trial is a prospective, multicentre, single-arm study conducted across 16 centres in China. Patients with severe pure AR underwent comprehensive anatomical assessment using multidetector computed tomography (CT). Based on the ability to provide adequate anchoring forces (≥10% of oversizing) in three zones (left ventricular outflow tract, anatomical annulus, and ascending aorta), patients were classified into 4 types. Those with anatomical types 1-3 were enrolled and underwent TAVI using the VitaFlow valve system. The primary efficacy endpoint was device success, and the primary safety endpoints included 30-day mortality and major complications.
Results: Among 187 screened patients, 100 patients with suitable anatomy (types 1-3) were enrolled. The mean age was 72.7±7.2 years, and the mean Society of Thoracic Surgeons Predicted Risk of Mortality score was 9.10±5.81%. Device success was achieved in 91% of cases, with no procedural mortality. The new permanent pacemaker implantation rate was 9%. Postprocedural CT analysis in 43 patients revealed that the maximum contact forces were primarily localised between the virtual annulus and the sinotubular junction (83.7% of cases). No device failure occurred in later cases.
Conclusions: The AURORA classification system shows that comprehensive anatomical assessment can lead to favourable outcomes in pure AR using conventional TAVI devices. The low pacemaker implantation rate and the absence of device failure in later cases suggest that optimal anatomical matching may be superior to aggressive oversizing strategies.
Severe aortic regurgitation (AR) represents a substantial therapeutic challenge, particularly in patients at high surgical risk. Traditional surgical aortic valve replacement (SAVR) is often contraindicated for these patients due to the presence of prohibitive comorbidities and surgical risks. Transcatheter aortic valve implantation (TAVI) has emerged as a promising, less invasive alternative, with proven efficacy in treating aortic stenosis, largely due to the presence of calcified annular structures that facilitate secure valve anchoring123. However, the application of TAVI in AR remains limited by the absence of such anchoring structures, resulting in a higher incidence of device malposition and paravalvular leak, which can adversely impact procedural success and long-term outcomes456.
Despite these challenges, recent advances in valve technology and procedural techniques have shown potential to improve TAVI outcomes in AR, warranting further investigation into optimised strategies for patient selection and device implantation.
This study aimed to evaluate the safety and efficacy of transfemoral TAVI in high-risk patients with severe AR utilising a novel anatomical classification system and dual-anchoring theory. The hypothesis underlying this approach is that optimal device stability can be achieved by identifying multiple anchoring sites along the aortic root, as delineated through multidetector computed tomography (MDCT).
This method seeks to overcome the challenges associated with the absence of calcified structures, which are typically critical for secure valve anchoring in conventional TAVI procedures.
Methods
Study design
The AURORA trial is a prospective, multicentre, single-arm cohort study conducted across 16 high-volume centres in China with expertise in TAVI. All patients with severe pure AR were initially screened by local Heart Teams, evaluating surgical risk based on available clinical data and obtaining written informed consent for further investigation. Transthoracic or transoesophageal echocardiographic data were subsequently uploaded to the echocardiographic core laboratory for confirmation of echocardiographic eligibility criteria in accordance with guidelines from the American Society of Echocardiography7. Patients deemed to meet the criteria for aortic valve replacement per established guidelines13 and who were at high surgical risk underwent further assessment via MDCT evaluation. All computed tomography (CT) imaging analyses were performed at the Beijing Anzhen Hospital Core Laboratory. Only those patients with anatomical suitability for TAVI, as defined by types 1, 2, and 3 of the novel AURORA classification, were enrolled in the trial, as previously described8. All complications and TAVI-specific endpoints were defined as per the Valve Academic Research Consortium-3 definitions9. All adverse events were assessed and adjudicated by an independent clinical events committee. The study was registered with the Chinese Clinical Trial Registry (ChiCTR2200055415)8.
Multidetector computed tomography and AURORA classification
All patients underwent preprocedural MDCT following a standardised TAVI protocol. The acquired images were analysed using 3mensio software (Pie Medical Imaging). Standardised techniques were employed to measure the annulus, left ventricular outflow tract (LVOT), and ascending aorta (40 mm above the annulus), as previously described81011. Following identification of the virtual annular plane, contours were traced at 2 mm intervals (2, 4, 6, 8, and 10 mm) above the annular plane using a perfect circle defined by 3 points at the valve commissures, allowing for an estimation of the anatomical aortic annulus (Figure 1).
The LVOT zone was defined as the region extending from 6 mm below the annulus to the annular plane, while the anatomical aortic annulus zone extended from the virtual annular plane to 10 mm above it. To assess the adequacy of anchoring forces, we established the following criteria using a 10% oversizing rate as the cutoff:
• For the LVOT zone, the bottom diameter of the transcatheter heart valve (THV) was used as a reference. The LVOT was considered to provide adequate anchoring force if at least 4 mm of the 6 mm LVOT zone allowed for a THV oversizing rate ≥10%.
• Similarly, the anatomical aortic annulus zone was considered capable of providing adequate anchoring force if at least 4 mm met the 10% oversizing criterion.
• For the ascending aorta, the circumference was measured 40 mm above the annulus and compared to the diameter at the top of the THV. The ascending aorta was deemed adequate to provide anchoring force if the THV’s top portion exceeded a 10% oversizing rate at this location.
Based on these criteria, anatomical classification was performed as follows (Figure 1):
• AURORA type 1: all three zones (LVOT, annulus, and ascending aorta) are capable of providing adequate anchoring force;
• AURORA type 2: only the annulus and the ascending aorta are capable of providing adequate anchoring force;
• AURORA type 3: only the LVOT and the annulus are capable of providing adequate anchoring force;
• AURORA type 4: only one zone, or no zones, can provide adequate anchoring force.
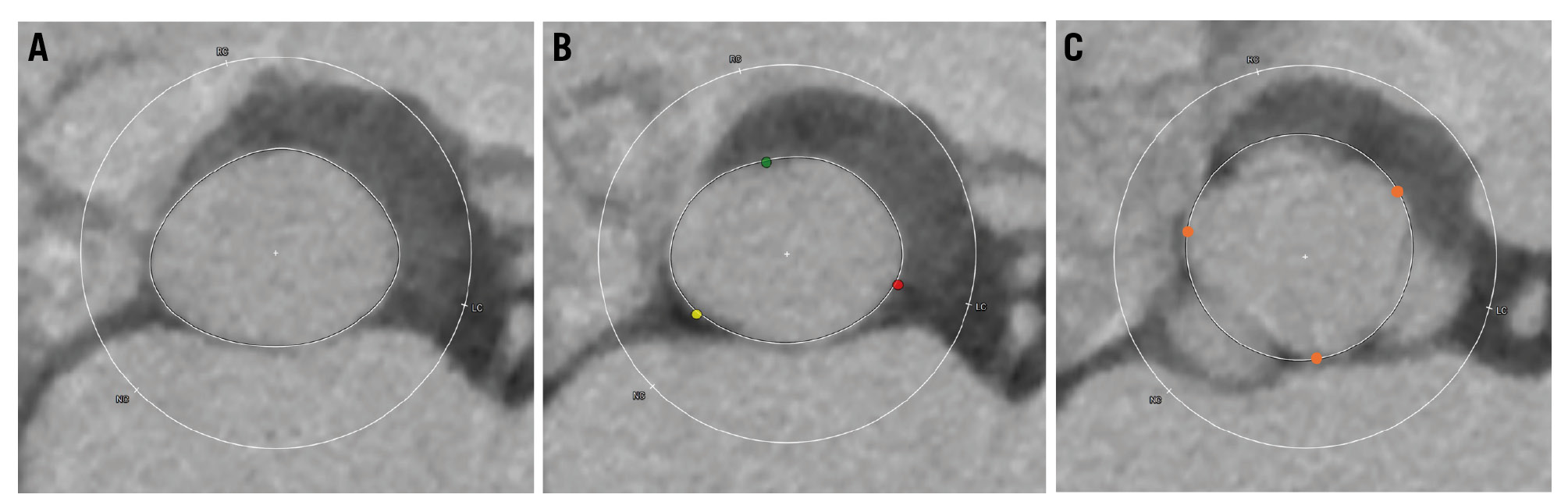
Figure 1. Multiplanar measurement in preprocedural CT. A) Left ventricular outflow tract and subannular plane measurements using standard techniques. B) Virtual annular plane measurement using the standard technique, defined by the plane connecting the three nadirs of the aortic sinuses (red dot: the nadir of the left coronary sinus; green dot: the nadir of the right coronary sinus; yellow dot: the nadir of the non-coronary sinus). C) Supra-annular plane measurement using a perfect circle defined by the three commissural points (orange dots). CT: computed tomography
TAVI strategy and procedure
In this study, all procedures were performed using the VitaFlow Valve (MicroPort), a domestic self-expanding TAVI device12. A multidisciplinary Heart Team conducted the interventions in a hybrid catheterisation laboratory under fluoroscopic guidance. Patients were administered either local anaesthesia or general anaesthesia with intubation, depending on the clinical indication. Transfemoral procedures followed standard protocols1314, with valve sizing and deployment strategies recommended by the Anzhen Hospital Core Laboratory.
The technical protocol8 employed during the procedure included rapid pacing at 180 beats per minute to reduce regurgitation volume and systolic blood pressure. Deployment strategies were adapted based on the anatomical classification: for type 2 anatomy, rapid pacing was maintained throughout both stages of deployment to improve THV stability. For type 1 and type 3 anatomies, rapid pacing continued until two-thirds of the THV frame was deployed. Deployment was completed only after confirmation of the correct positioning at the angiography (Figure 2). In cases of significant paravalvular regurgitation following initial THV deployment, a second THV could be implanted to address paravalvular leak, valve malposition, or to prevent embolisation of the first valve into the left ventricle.
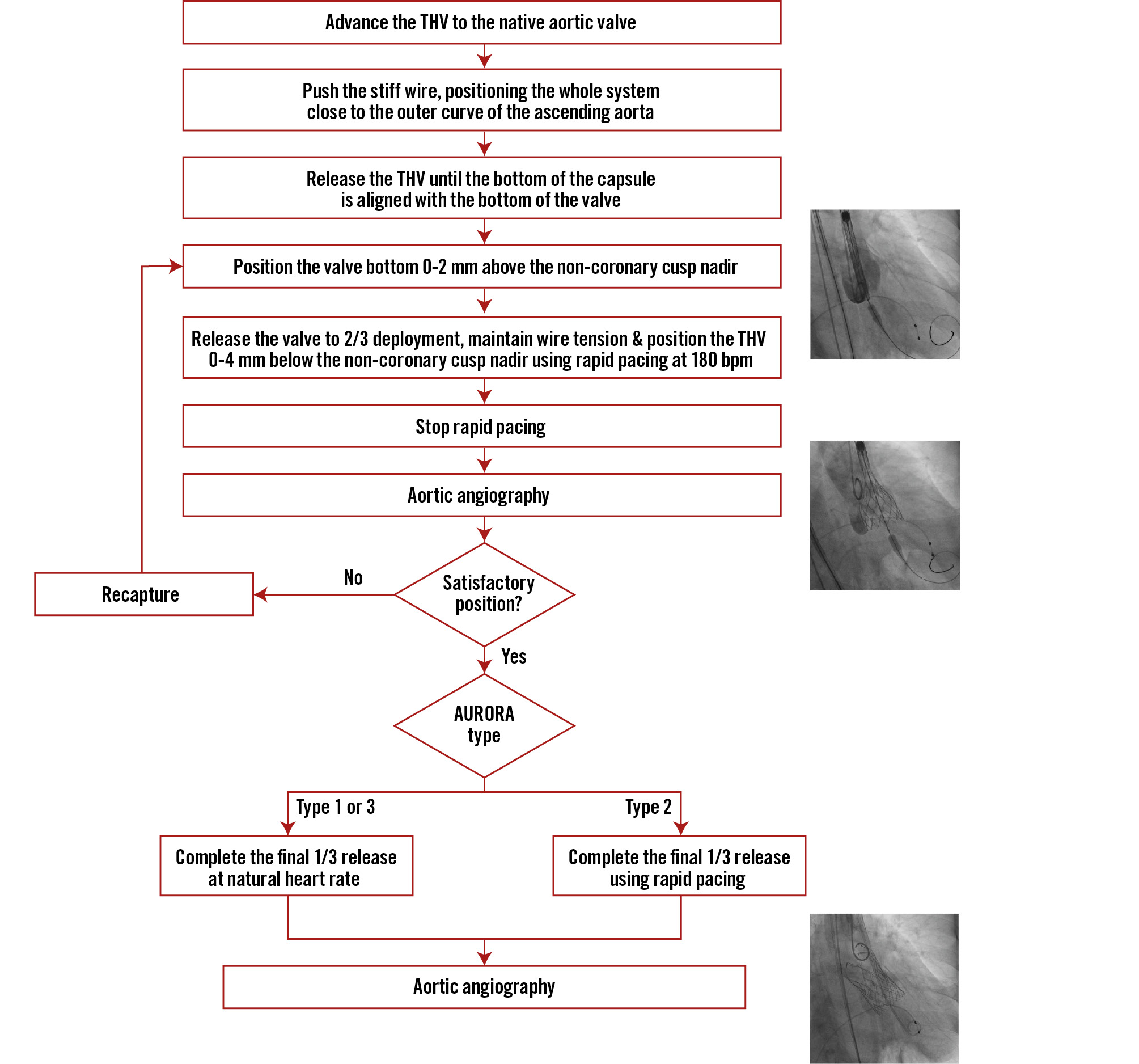
Figure 2. Step-by-step TAVI procedure using the AURORA protocol. bpm: beats per minute; TAVI: transcatheter aortic valve implantation; THV: transcatheter heart valve
Biomechanical analysis
Patient-specific computer simulations were conducted using finite element analysis (FEA) to predict device-host interactions during deployment. This platform integrates both the geometric and mechanical properties of the device and the patient’s anatomy. The device model was reconstructed based on manufacturer-provided data (MicroPort). Patient-specific anatomy was segmented from preoperative MDCT images, with assigned mechanical elastic properties for each anatomical structure: native aortic wall (E=0.6 MPa, ν=0.3), native leaflet tissue (E=2 MPa, ν=0.45) and calcium nodules (E=4 MPa, ν=0.3, yield stress=0.6 MPa). These details have been previously described1516.
The outward force exerted on the frame was calculated for each patient, and the total outward force was evaluated across 3 regions: the LVOT region, the anatomical annulus region, and the ascending aorta region (Figure 3).
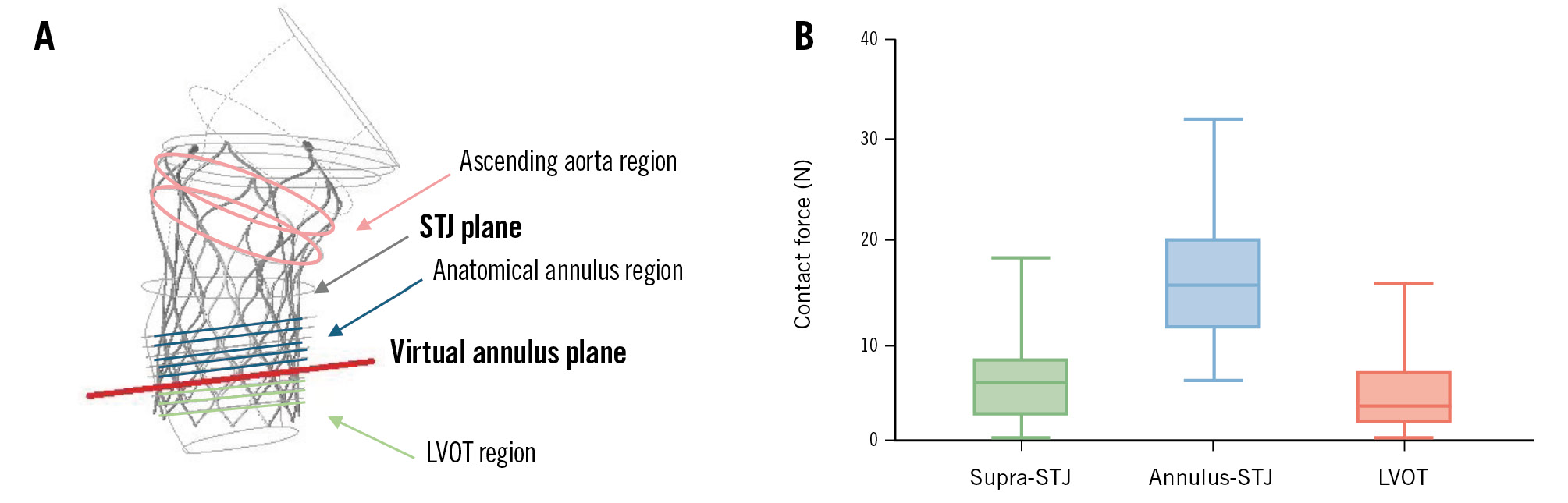
Figure 3. Biomechanical analysis. A) Patient-specific computer simulation showing the 3 anatomical regions. B) Contact force distribution calculated using the finite element analysis, demonstrating significantly higher forces in the anatomical annulus region compared to other regions. LVOT: left ventricular outflow tract; STJ: sinotubular junction
Statistical analysis
Statistical analysis was performed using SPSS, version 21.0 (IBM). Continuous variables are expressed as mean±standard deviation and were compared using either the unpaired Student’s t-test or the Mann-Whitney U test, depending on the data distribution. Categorical variables are presented as frequencies with corresponding percentages and were compared using the χ2 test or Fisher’s exact test, as appropriate.
Results
Baseline clinical characteristics
From February 2020 to March 2022, a total of 187 patients were screened for eligibility. Following anatomical assessment of the 187 screened patients using the AURORA classification system, 87 (46.5%) were classified as AURORA type 4 and were excluded from the study, resulting in 100 patients with anatomical types 1-3 being enrolled from 16 centres across China. The excluded patients were characterised by insufficient anchoring zones according to our prespecified criteria. The majority of these patients had either inadequate anchoring in multiple zones or anatomical dimensions that exceeded the available THV sizing ranges. Common anatomical features leading to exclusion included excessive LVOT dimensions, significant dilation of the ascending aorta, or a combination of unfavourable measurements across multiple zones that would prevent stable valve anchoring. The study included both tricuspid and bicuspid aortic valve anatomies. Among the 100 enrolled patients, 98 had tricuspid anatomy, while only 2 had bicuspid anatomy. Given the small number of bicuspid cases, a subgroup analysis based on valve morphology was not performed. Regardless of valve morphology, all patients were assessed using the same AURORA classification criteria for anatomical suitability.
The study cohort (n=100) had a mean age of 72.7±7.2 years, with a male predominance (63%). The mean Society of Thoracic Surgeons Predicted Risk of Mortality score was 9.10±5.81%, indicating a high surgical risk. Hypertension was present in 64% of patients, and 28% had atrial fibrillation. Preprocedural echocardiography revealed a mean left ventricular ejection fraction of 53.02±10.65% and a mean left ventricular end-systolic diameter (LVESD) of 43.52±10.45 mm. Most patients (64%) were in New York Heart Association (NYHA) Functional Class III or IV at baseline. The distribution of AURORA classifications was as follows: 40 patients (40%) were type 1, 8 patients (8%) were type 2, and 52 patients (52%) were type 3. Anatomical characteristics differed significantly between types, with type 3 patients showing larger ascending aortic diameters (41.29±3.47 mm) compared to type 1 (34.84±2.40 mm) and type 2 (35.40±1.85 mm; p<0.001) patients. Device success rates were comparable across all types, with 93% in type 1, 100% in type 2, and 88% in type 3 (p=0.663) (Central illustration).
Baseline clinical characteristics are summarised in Table 1.
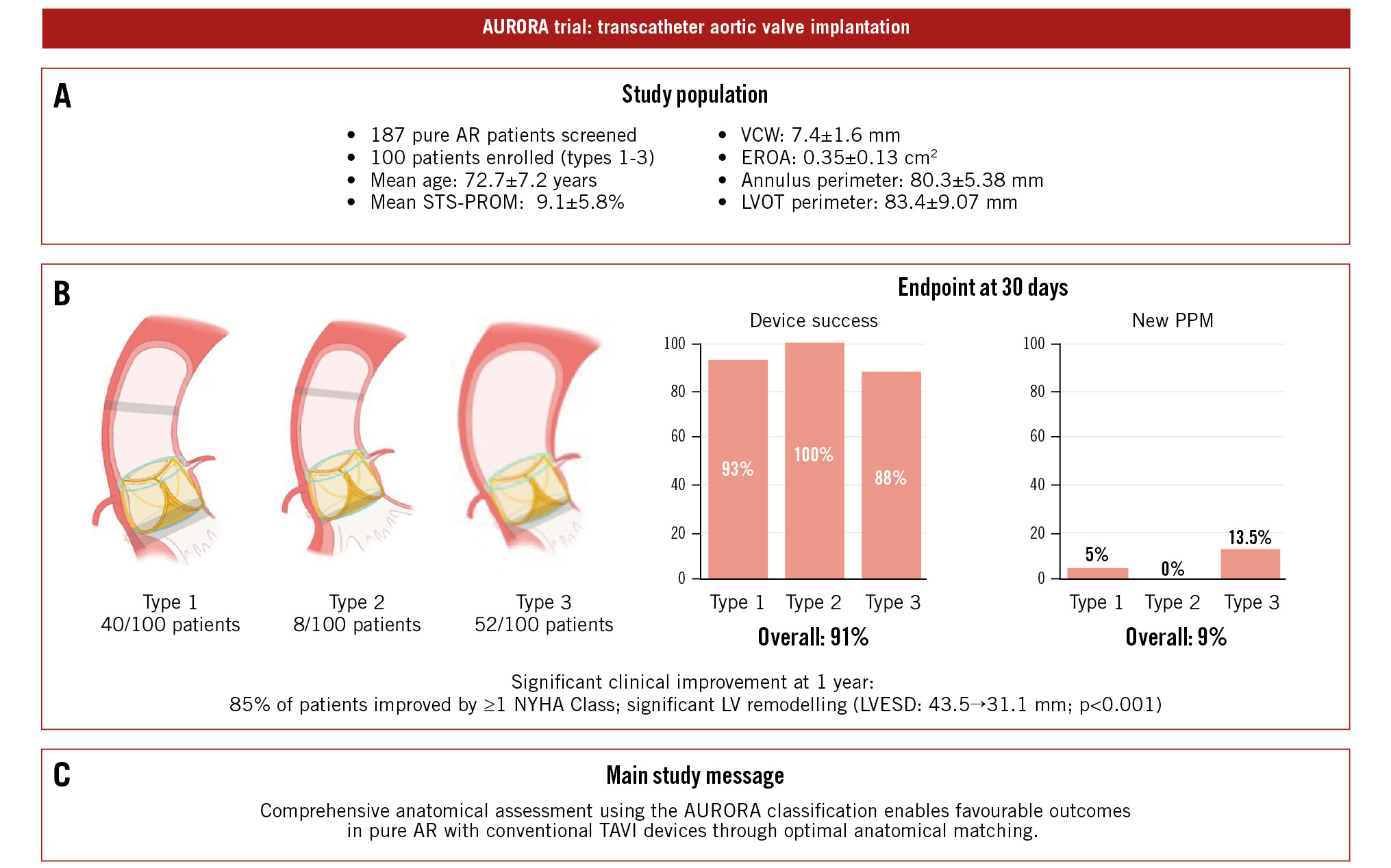
Central illustration. AURORA trial: transcatheter aortic valve implantation in pure aortic regurgitation. A) Study population. B) Key results stratified by the AURORA anatomical classification. C) Take-home message. AR: aortic regurgitation; AURORA: Anatomical classification and dUal anchoRing theory to Optimize the tavR strategy for pure severe Aortic regurgitation; EROA: effective regurgitant orifice area; LV: left ventricular; LVESD: left ventricular end-systolic diameter; LVOT: left ventricular outflow tract; NYHA: New York Heart Association; PPM: permanent pacemaker; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TAVI: transcatheter aortic valve implantation; TAVR: transcatheter aortic valve replacement; VCW: vena contracta width
Table 1. Baseline clinical characteristics.
| Characteristics | Patients (n=100) |
Type 1 n=40 |
Type 2 n=8 |
Type 3 n=52 |
p-value |
|---|---|---|---|---|---|
| Age, years | 72.74±7.16 | 72.30±7.56 | 69.25±7.30 | 73.69±6.87 | 0.230 |
| Female sex | 37 (37) | 12 (30) | 5 (63) | 20 (39) | 0.222 |
| BMI, kg/m2 | 22.94±3.45 | 22.41±3.21 | 23.04±2.51 | 23.33±3.75 | 0.460 |
| BSA, m2 | 1.64±0.17 | 1.65±0.17 | 1.61±0.14 | 1.64±0.17 | 0.835 |
| AF | 28 (28) | 7 (17.5) | 2 (25.0) | 19 (36.54) | 0.170 |
| COPD | 9 (9) | 2 (5.0) | 1 (12.5) | 6 (11.5) | 0.464 |
| Prior permanent pacemaker | 3 (3) | 1 (2.5) | 1 (12.5) | 1 (1.9) | 0.284 |
| Prior myocardial infarction | 5 (5) | 1 (2.5) | 0 (0) | 4 (7.7) | 0.393 |
| Prior PCI | 18 (18) | 7 (17.5) | 1 (12.5) | 10 (19.2) | 1.000 |
| Prior CABG | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Diabetes | 9 (9) | 5 (12.5) | 1 (12.5) | 3 (5.8) | 0.491 |
| Prior stroke | 13 (13) | 6 (15.0) | 1 (12.5) | 6 (11.5) | 0.901 |
| Hyperlipidaemia | 21 (21) | 9 (22.5) | 2 (25.0) | 10 (19.2) | 0.809 |
| Hypertension | 64 (64) | 23 (57.5) | 6 (75.0) | 35 (67.3) | 0.534 |
| Peripheral vascular disease | 26 (26) | 9 (22.5) | 2 (25.0) | 15 (28.8) | 0.881 |
| Smoker | 24 (24) | 7 (17.5) | 1 (12.5) | 16 (30.8) | 0.343 |
| Prior LBBB | 3 (3) | 2 (5.0) | 0 (0) | 1 (1.9) | 0.672 |
| Prior RBBB | 3 (3) | 1 (2.5) | 0 (0) | 2 (3.8) | 0.897 |
| STS-PROM score, % | 9.10±5.81 | 9.93±6.07 | 7.37±2.55 | 8.76±6.00 | 0.460 |
| KCCQ score | 58.26±18.64 | 63.37±17.60 | 58.35±12.21 | 53.96±19.67 | 0.166 |
| 6-minute walk distance, m | 309.93±148.23 | 292.14±87.68 | 270.50±66.31 | 325.61±178.73 | 0.760 |
| eGFR, ml/min/1.73 m2 | 94.91±37.30 | 95.44±43.56 | 99.35±31.35 | 93.78±33.14 | 0.921 |
| NT-proBNP, pmol/L | 2,464.13±5,268.72 | 1,372.01±2,636.40 | 4,388.25±7,198.60 | 3,055.43±6,385.37 | 0.191 |
| NYHA Class | |||||
| I | 4 (4) | 0 (0) | 0 (0) | 4 (7.7) | 0.144 |
| II | 32 (32) | 17 (42.5) | 0 (0) | 15 (28.8) | 0.046 |
| III | 53 (53) | 22 (55) | 7 (87.5) | 24 (46.2) | 0.094 |
| IV | 11 (11) | 1 (2.5) | 1 (12.5) | 9 (17.3) | 0.089 |
| Echocardiographic and CT characteristics | |||||
| LVEF, % | 53.02±10.65 | 53.66±10.64 | 43.67±11.92 | 53.96±9.97 | 0.033 |
| LVEDD, mm | 60.95±8.00 | 59.88±9.27 | 65.01±12.05 | 61.17±5.75 | 0.245 |
| LVESD, mm | 43.52±10.45 | 41.70±10.49 | 52.96±13.37 | 43.25±9.11 | 0.033 |
| VCW, cm | 0.74±0.16 | 0.72±0.17 | 0.70±0.08 | 0.77±0.17 | 0.315 |
| EROA, cm2 | 0.35±0.13 | 0.36±0.14 | 0.31±0.05 | 0.35±0.13 | 0.371 |
| MR moderate to severe | 33 (33) | 10 (25) | 3 (37.5) | 20 (38.5) | 0.236 |
| TR moderate to severe | 18 (18) | 9 (22.5) | 1 (12.5) | 8 (15.4) | 0.708 |
| Annulus perimeter, mm | 80.34±5.38 | 80.34±4.95 | 85.21±5.91 | 79.60±5.33 | 0.021 |
| Angle, ° | 54.87±10.78 | 52.23±10.42 | 48.25±12.28 | 57.98±9.96 | 0.007 |
| LVOT perimeter, mm | 83.40±9.07 | 82.41±6.28 | 102.26±10.91 | 81.25±7.21 | 0.000 |
| Ascending aortic diameter, mm | 38.24±4.35 | 34.84±2.40 | 35.40±1.85 | 41.29±3.47 | 0.000 |
| STJ diameter, mm | 34.51±4.63 | 31.42±2.74 | 31.76±1.99 | 37.31±4.32 | 0.000 |
| Left coronary sinus or major axis, mm | 35.62±4.06 | 34.49±3.83 | 32.21±3.20 | 37.01±3.82 | 0.000 |
| Right coronary sinus or minor axis, mm | 34.58±4.24 | 32.85±3.80 | 34.23±4.66 | 35.96±4.07 | 0.002 |
| Non-coronary sinus, mm | 35.63±4.00 | 34.34±4.06 | 33.76±4.13 | 36.90±3.56 | 0.003 |
| Variables are given as numbers (percentage) or mean value±SD. AF: atrial fibrillation; BMI: body mass index; BSA: body surface area; CABG: coronary artery bypass grafting; COPD: chronic obstructive pulmonary disease; CT: computed tomography; eGFR: estimated glomerular filtration rate; EROA: effective regurgitant orifice area; KCCQ: Kansas City Cardiomyopathy Questionnaire; LBBB: left bundle branch block; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; LVOT: left ventricular outflow tract; MR: mitral regurgitation; NT-proBNP: N-terminal pro-brain natriuretic peptide; NYHA: New York Heart Association; PCI: percutaneous coronary intervention; RBBB: right bundle branch block; SD: standard deviation; STJ: sinotubular junction; STS-PROM: Society of Thoracic Surgeons Predicted Risk of Mortality; TR: tricuspid regurgitation; VCW: vena contracta width | |||||
Clinical outcomes
The primary efficacy endpoint, defined as device success, was achieved in 91 cases (91%). Nine patients required a second valve implantation (valve-in-valve) due to suboptimal initial results. One case involved displacement of the first valve into the ascending aorta, while 8 cases were attributed to excessive implantation depth. Additionally, 1 patient required surgical intervention due to heart failure resulting from paravalvular leak. Notably, all 9 device failures occurred within the first two-thirds of the trial cohort, with no device failures observed in the final third, suggesting a significant learning curve effect.
The 30-day safety outcomes were favourable, with no procedure-related mortality, major bleeding events, or renal failure. The stroke rate was 3%, affecting three patients, while 9 patients (9%) required a new permanent pacemaker implantation (Central illustration). New left bundle branch block developed in 8 patients (8%), and 2 patients (2%) were hospitalised due to heart failure. Thirty-day clinical outcomes are summarised in Table 2.
Clinical follow-up demonstrated significant improvements in patient symptoms and cardiac function. Left ventricular remodelling was observed at 1-year follow-up, with a significant reduction in LVESD from 43.52±8.48 mm to 31.13±4.15 mm (p<0.001), accompanied by a corresponding improvement in left ventricular end-diastolic diameter (LVEDD) (Figure 4). At 30 days post-procedure, NYHA Functional Class distribution was as follows: 28 patients in Class I, 65 in Class II, and 7 in Class III, with no patients remaining in Class IV. At 1-year follow-up, 94 of the initial 100 patients completed echocardiographic and functional evaluations, with 35 patients in Class I, 55 in Class II, and 4 in Class III. The remaining 6 patients were lost to follow-up or deceased. Among those followed, 85% demonstrated an improvement of at least 1 NYHA Class from baseline (Figure 4).
Table 2. Thirty-day clinical outcomes.
| Endpoints at 30 days | Patients (n=100) | Type 1 | Type 2 | Type 3 | p-value |
|---|---|---|---|---|---|
| Primary efficacy endpoint | |||||
| Device success | 91 (91) | 37 (93) | 8 (100) | 46 (88) | 0.663 |
| Primary safety endpoints | |||||
| Death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Cardiac death | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Stroke | 3 (3) | 2 (5.0) | 0 (0) | 1 (1.9) | 0.672 |
| Surgery related to device | 1 (1) | 0 (0) | 0 (0) | 1 (1.9) | 1.000 |
| MI | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Renal failure | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Rehospitalisation due to heart failure | 2 (2) | 1 (2.5) | 0 (0) | 1 (1.9) | 1.000 |
| New LBBB | 8 (8) | 3 (7.5) | 1 (12.5) | 4 (7.7) | 0.729 |
| New permanent pacemaker | 9 (9) | 2 (5) | 0 (0.0) | 7 (13.5) | 0.336 |
| Major or life-threatening bleeding | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Major vascular complication | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1.000 |
| Variables are given as numbers (percentage). LBBB: left bundle branch block; MI: myocardial infarction | |||||
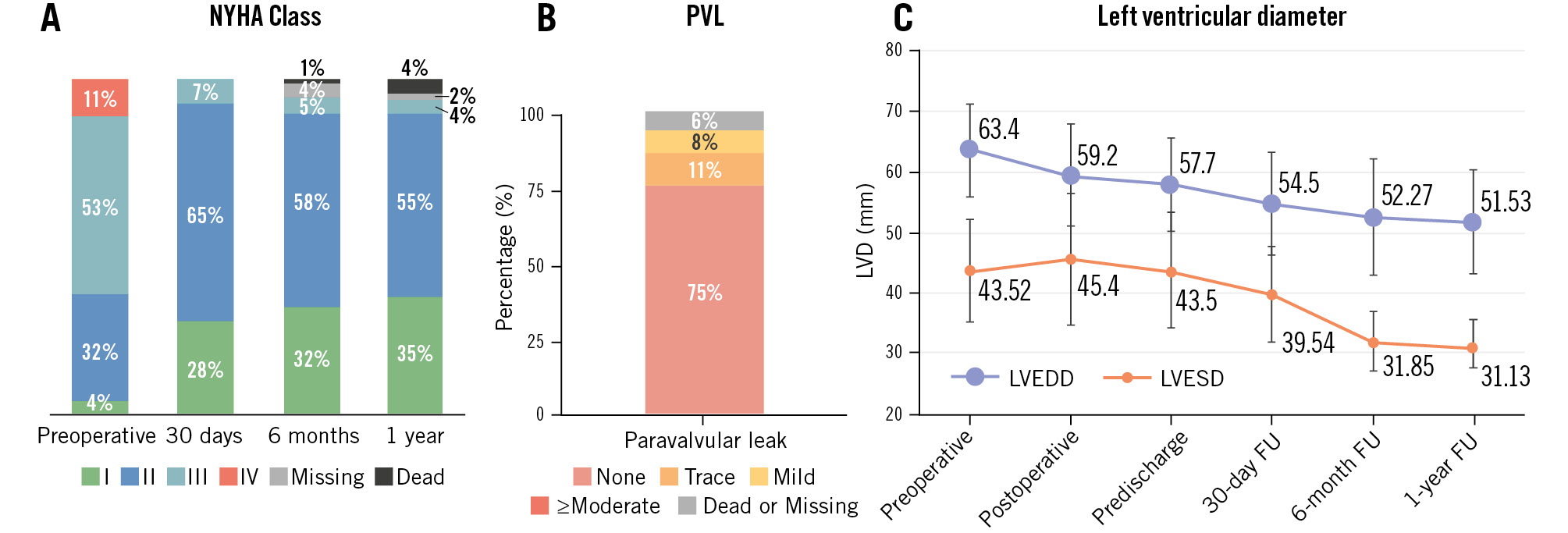
Figure 4. Clinical outcomes. A) Improvement in NYHA Functional Class at 1-year follow-up. B) Absence of moderate or severe PVL at 1-year follow-up. C) Reduction in left ventricular diameters at follow-up. FU: follow-up; LVEDD: left ventricular end-diastolic diameter; LVESD: left ventricular end-systolic diameter; NYHA: New York Heart Association; PVL: paravalvular leak
Postprocedural CT results
Postprocedural CT analysis was performed in 52 patients, with FEA completed in 43 cases. Nine patients were excluded due to valve-in-valve implantation or inadequate image quality. The mean THV implantation depth was 8.30±3.63 mm below the virtual annular plane. The maximum contact force was primarily localised between the annulus and the sinotubular junction (STJ; anatomical annulus) in 36 of 43 patients (83.7%). Contact forces in the anatomical annulus region were significantly higher compared to those observed in the LVOT and ascending aorta regions. These biomechanical findings provide strong support for the AURORA classification system, underscoring the importance of comprehensive anatomical assessment for optimal THV anchoring (Figure 3).
Discussion
TAVI in patients with pure AR has historically posed significant technical challenges, primarily due to inadequate anchoring forces between the THV and native structures. This limitation has resulted in relatively low device success rates and a high incidence of moderate or greater paravalvular leak. The AURORA trial introduces an innovative approach to TAVI in pure AR by moving beyond the traditional single-plane virtual annulus assessment. Rather than relying solely on annular measurements and aggressive oversizing, this study incorporated a multiplanar evaluation of anatomical structures to optimise THV anchoring. This comprehensive anatomical assessment yielded two key outcomes: a high device success rate (91%) and a notably low permanent pacemaker implantation rate (9%). These findings suggest that detailed multiplanar anatomical evaluation may be more effective than conventional oversizing strategies in achieving stable THV anchoring while minimising complications. Additionally, biomechanical analysis confirmed this approach by demonstrating that contact forces were predominantly concentrated within the anatomical zones defined by our classification system.
Postprocedural CT analysis, combined with FEA, provided critical validation of our anatomical classification system. The findings indicated that the maximum contact forces were primarily localised between the virtual annulus and the STJ, specifically within the region encompassing the anatomical annulus and native leaflets. This biomechanical evidence supports the hypothesis that a comprehensive anatomical assessment is essential for optimising THV anchoring.
The AURORA classification system offers several key advantages. First, it enhances patient selection in regions where AR-dedicated valves are unavailable – such as mainland China – by delivering outcomes comparable to those with dedicated devices. Second, it establishes a framework for future valve design, emphasising the importance of multiple anchoring zones along the aortic root.
A particularly noteworthy outcome was the low permanent pacemaker implantation rate of 9%, which is significantly lower than previously reported aortic regurgitation TAVI rates1718 and those reported by studies evaluating dedicated AR valves19. This favourable outcome can be attributed to our strategic focus on anatomical compatibility rather than aggressive oversizing. By prioritising native structural length with a 10% oversizing threshold over maximal oversizing rates, we achieved stable anchoring while minimising the pressure on the conduction system. Furthermore, emphasising anatomical annulus anchoring facilitated controlled THV deployment depths, further reducing conduction system injury risk.
The observed 3% stroke rate in our study is comparable to the 2% rate reported in the recent ALIGN-AR Trial19. Although our protocol, which includes rapid pacing and potential valve recapturing, could theoretically increase stroke risk, differences in patient populations, device characteristics, and the study’s limited sample size preclude a direct statistical comparison.
The cylindrical frame design of the VitaFlow Valve, distinct from more tapered self-expanding valves, may have contributed to our favourable outcomes by optimising contact with the anatomical annulus tissue, thereby enhancing anchoring forces. While dedicated AR devices primarily achieve stabilisation through specialised anchoring mechanisms in the aortic sinuses, the AURORA classification may still provide valuable anatomical insights. Although originally developed for conventional THVs, its systematic evaluation of multiple anatomical zones could aid in optimising sizing strategies even for dedicated AR devices, particularly in complex anatomies where relying solely on virtual annulus measurements may be inadequate.
Limitations
Despite these promising results, several limitations must be acknowledged. The study’s single-arm, non-blinded, and non-randomised design introduces potential bias. Furthermore, the AURORA classification system has only been tested with the VitaFlow Valve system (MicroPort), and its applicability to other TAVI devices requires further validation. Future randomised controlled trials comparing this approach with AR-dedicated valves will be necessary to establish more robust evidence. Additionally, longer-term follow-up is required to assess the durability of these promising early outcomes.
Conclusions
In conclusion, the AURORA trial demonstrates that comprehensive anatomical assessment and strategic device positioning enable favourable outcomes in pure AR cases using conventional THVs. This approach not only provides an immediate solution for regions lacking AR-dedicated valves but also offers valuable insights for future device development. The low pacemaker implantation rate suggests that optimal anatomical matching may be superior to aggressive oversizing strategies, reinforcing the importance of detailed anatomical evaluation in TAVI procedures.
Impact on daily practice
The AURORA classification system enhances anatomical assessment for transcatheter aortic valve implantation (TAVI) in pure aortic regurgitation (AR) patients by moving beyond the traditional virtual annulus-based evaluation. By incorporating multiplanar measurements of the entire aortic root, it enables better patient selection and improved procedural outcomes. The low permanent pacemaker rate suggests that precise anatomical matching may be more effective than aggressive oversizing. This approach is especially useful where AR-dedicated valves are unavailable, allowing effective use of conventional TAVI devices. The dual-anchoring theory and anatomical classification offer a structured framework for interventionalists, potentially reducing device failure and improving long-term outcomes.
Funding
The trial was investigator initiated and received funding from the Capital’s Funds for Health Improvement and Research (2022-2-2065). MicroPort had no role in the study design, data collection and analysis, or manuscript preparation.
Conflict of interest statement
The authors have no conflicts of interest to declare.

