Cory:
Unlock Your AI Assistant Now!
Abstract
Background: Current clinical guidelines do not recommend mitral transcatheter edge-to-edge repair (M-TEER) for patients with moderate functional mitral regurgitation (FMR), and the implications of M-TEER in this population are not well documented.
Aims: We aimed to assess M-TEER outcomes in patients with symptomatic moderate FMR compared to those with FMR ≥3+ who were treated with the PASCAL system in the MiCLASP study.
Methods: Patients were stratified by baseline FMR grade (2+ or ≥3+). The echocardiographic core laboratory-assessed mitral regurgitation (MR) reduction, clinical events committee-adjudicated major adverse events (MAE) rate and functional and quality-of-life outcomes were evaluated up to 1 year after M-TEER.
Results: Of the 544 (FMR=322; degenerative MR=163; mixed/other=59) enrolled patients, 101 had baseline FMR 2+ and 197 FMR ≥3+. Both groups achieved significant MR reduction at discharge, which was sustained up to 1 year, with 89.8% of patients achieving MR ≤1+ in the FMR 2+ group and 77.8% in the FMR ≥3+ group (all p<0.001 vs baseline). At 1 year, significant improvements (all p<0.001 vs baseline) in functional capacity (New York Heart Association Class I/II: 67.1% FMR 2+; 70.1% FMR ≥3+) and quality of life (change in the Kansas City Cardiomyopathy Questionnaire overall score: +13.9 points FMR 2+; +13.9 points FMR ≥3+) were achieved in both groups, with high survival (90.0% FMR 2+; 84.2% FMR ≥3+; p=0.176) and low MAE rates (13.9% FMR 2+; 18.3% FMR ≥3+; p=0.413).
Conclusions: In the MiCLASP study, patients with moderate FMR experienced significant MR reduction at 1 year, resulting in clinical and symptomatic benefits comparable to those with ≥moderate-severe FMR, suggesting that select patients with symptomatic moderate FMR can benefit from M-TEER.
Functional mitral regurgitation (FMR) is a complex, multifaceted disease associated with high mortality and significantly reduced quality of life12. Heart failure (HF) with reduced left ventricular ejection fraction (HFrEF) is a frequent finding in these patients and contributes to high morbidity and mortality even when mitral regurgitation (MR) is mild234. The Cardiovascular Outcomes Assessment of the MitraClip Percutaneous Therapy for Heart Failure Patients With Functional Mitral Regurgitation (COAPT) study demonstrated that mitral transcatheter edge-to edge repair (M-TEER) is an effective treatment for FMR patients with HF and MR ≥3+, resulting in significant reductions in the rate of HF hospitalisations and mortality at 2 years compared with medical therapy alone. Based on these findings, M-TEER is deemed to be the treatment of choice for patients with moderate-severe or severe FMR who remain symptomatic despite guideline-directed medical therapy (GDMT)56. Benefits of M-TEER in this population have also been confirmed in the Edwards PASCAL TrAnScatheter Mitral Valve RePair System (CLASP) and Transcatheter Repair of Mitral Regurgitation with Edwards PASCAL Transcatheter Valve Repair System (MiCLASP) studies78910.
In contrast, the Multicentre Study of Percutaneous Mitral Valve MitraClip Device in Patients With Severe Secondary Mitral Regurgitation (MITRA-FR) study failed to demonstrate any benefits of M-TEER in FMR patients. Compared to COAPT, patients in MITRA-FR had substantially more left ventricular (LV) damage at baseline, as evidenced by a larger LV end-diastolic volume (LVEDV) index, suggesting a more advanced stage of LV disease11. These findings imply that FMR patients with excessive LV dilation and myocardial damage may not benefit from M-TEER, with experts speculating that intervening earlier in the disease may provide these patients meaningful clinical benefits and prevent progressively adverse LV remodelling.
However, current clinical guidelines do not recommend M-TEER for patients with moderate FMR, which may reflect an earlier phenotype in the disease spectrum. The MiCLASP study enrolled patients with symptomatic moderate FMR and provided a unique opportunity to evaluate the implications of M-TEER in this population.
Methods
Study design and patient selection
The ongoing, prospective, multicentre, single-arm, European MiCLASP Post Market Clinical Follow-Up (PMCF) study was initiated with the PASCAL transcatheter valve repair system (Edwards Lifesciences) with an eligibility criterion of symptomatic patients with MR ≥2+ commensurate with device labelling. After enrolment of 500 patients, the study was extended to incorporate the newer PASCAL Precision system (also Edwards Lifesciences; both henceforth referred to as the “PASCAL system”) with a revised eligibility criterion of MR ≥3+ in accordance with updated device labelling.
Patients were enrolled in the MiCLASP study based on site assessment of MR using transthoracic echocardiography (TTE) or transoesophageal echocardiography (TOE), clinical presentation, and after being deemed suitable for M-TEER by the local multidisciplinary Heart Team, consisting of a heart failure specialist, interventional cardiologist, mitral valve cardiac surgeon, and an imaging specialist. The Heart Team assessment accounted for persistent symptoms despite receiving optimised medical therapy per local clinical practice. All echocardiograms were subsequently evaluated by the echocardiographic core laboratory (ECL) to reduce variability and bias (Supplementary Table 1). Key exclusion criteria included mitral valve area <4.0 cm2, left ventricular end-diastolic diameter >8.0 cm, and severe aortic, tricuspid and/or pulmonic valve stenosis and/or regurgitation. Detailed inclusion and exclusion criteria for the MiCLASP study have been previously reported10. An independent clinical events committee (CEC) adjudicated all major adverse events (MAE) except for device embolisations, which were adjudicated by the ECL (Supplementary Table 1). Study assessments were conducted at baseline, discharge, 30 days, 6 months, and 1 year.
All patients provided written informed consent. The study was approved by the local ethics committees and health authorities of participating countries and complies with Good Clinical Practice standards in conformance with the Declaration of Helsinki, the International Conference on Harmonisation, and ISO 14155:2011. The MiCLASP study is registered on ClinicalTrials.gov (NCT04430075) and sponsored by Edwards Lifesciences.
Study endpoints
The primary safety endpoint was the 30-day composite MAE rate, comprising cardiovascular mortality, stroke, myocardial infarction (MI), mitral valve reintervention, major access site and vascular complications, major cardiac structural complications, device embolisation, renal complications requiring unplanned dialysis or renal replacement therapy, and severe bleeding (major, extensive, life-threatening, or fatal bleeding, as defined by the Mitral Valve Academic Research Consortium)12. The primary effectiveness endpoint was ECL-assessed MR severity at discharge compared to baseline. Additional secondary effectiveness endpoints assessed at 30 days, 6 months, and 1 year included Kansas City Cardiomyopathy Questionnaire (KCCQ), New York Heart Association (NYHA) Functional Class, and changes in echocardiographic parameters including LVEDV, LV end-systolic volume (LVESV), pulmonary artery systolic pressure, left atrial volume, and transmitral mean gradient. Definitions and outcomes for device, procedural, and clinical success from the MiCLASP study have been previously reported10.
An independent ECL (Cardiovascular Core Lab at Morristown Medical Center, Morristown, NJ, USA) assessed all baseline and follow-up TTE images according to pre-established protocols. MR severity was evaluated by two-dimensional Doppler echocardiography according to modified American Society of Echocardiography (ASE) guidelines and was graded on a scale of 0 to 4+7121314 (Supplementary Table 2). Other key echocardiographic assessments included mitral valve area and gradients, effective regurgitant orifice area (EROA), left atrial volume, left ventricular dimensions, volumes, and ejection fraction, and MR aetiology.
Statistical analysis
Patients with FMR were stratified by ECL-adjudicated baseline MR grade into two groups: moderate FMR (2+) and ≥moderate-severe FMR (≥3+). Continuous variables are presented as median (interquartile range) or mean±standard deviation with the paired Student’s t-test used for comparison between baseline and specific timepoints and the Kruskal-Wallis test used for between-group comparisons of baseline characteristics. An analysis of covariance model with baseline values as covariates was used to compare the mean changes between groups. Categorical variables are reported as percentages, with the Wilcoxon signed-rank test used to compare categorical variables between 2 timepoints and Fisher’s exact test used for between-group comparisons. Statistical significance was set at p<0.05 (2-sided). Deltas were calculated using paired analyses. Time-to-event variables were analysed using Kaplan-Meier survival analysis, and standard error was calculated using the exponential Greenwood formula with log-rank p-values calculated for intergroup comparisons15. A Poisson regression model was used to evaluate pre- and post-procedure HF hospitalisation rates, with days of post-procedure follow-up as an offset; statistical significance was obtained using the Wald chi-square test from the model. All analyses were performed on the intention-to-treat (ITT) population. SAS software, version 9.4 (SAS Institute), was used for all statistical analyses.
Results
Baseline characteristics
The MiCLASP study enrolled 544 patients (FMR=322; degenerative MR=163; mixed/other=59) at 30 sites in 9 European countries (Supplementary Table 3), from whom 1-year outcomes have been previously reported10. Of the 322 FMR patients, 101 had baseline FMR 2+ and 197 had baseline FMR ≥3+, as adjudicated by the ECL (Figure 1). Baseline characteristics are described in Table 1. Notably, there were no significant differences at baseline between the FMR 2+ and FMR ≥3+ groups with respect to NYHA Class III/IV (73.3% FMR 2+; 80.1% FMR ≥3+; p=0.188), KCCQ overall score (51.2±22.3 FMR 2+; 52.2±21.5 FMR ≥3+; p=0.715) and EuroQol 5-dimension health questionnaire (EQ-5D-5L) score (53.7±19.3 FMR 2+; 56.4±17.5 FMR ≥3+; p=0.259). As expected, the LVEDV index, LVESV index, vena contracta width, and EROA were significantly higher in the FMR ≥3+ group (p<0.001 for all). Cardiomyopathy, hypertension, atrial fibrillation, and renal insufficiency or failure were the most common comorbidities present in both groups (p>0.05 for all).
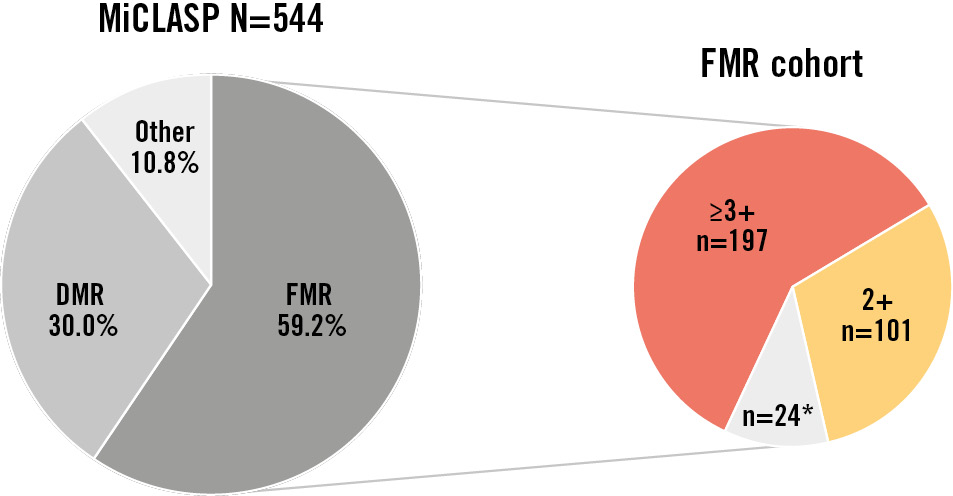
Figure 1. MR aetiology and severity as assessed by the core laboratory. Cardiovascular core lab at Morristown Medical Center, Morristown, NJ, USA. “Other” includes mixed aetiology and missing or non-evaluable baseline echocardiograms. *Includes FMR 1+ (n=20) and non-evaluable baseline echocardiograms (n=4). DMR: degenerative MR; FMR: functional MR; MR: mitral regurgitation
Table 1. Baseline characteristics.
| Characteristics | FMR 2+ n=101 |
FMR ≥3+ n=197 |
p-value |
|---|---|---|---|
|
Age, years |
76.7±8.8 (101) |
75.2±10.80 (197) |
0.321 |
|
Male |
54.5 (55) |
64.5 (127) |
0.104 |
|
Body mass index, kg/m2 |
27.0±4.8 (101) |
26.0±4.5 (195) |
0.064 |
|
NYHA Class III/IV |
73.3 (74) |
80.1 (157) |
0.188 |
|
STS score for mitral valve repair, % |
5.6±4.7 (101) |
5.5±4.6 (197) |
0.834 |
|
EuroSCORE II, % |
7.7±8.2 (100) |
8.6±7.6 (197) |
0.094 |
|
EROA, cm2 |
0.22±0.05 (33) |
0.36±0.12 (108) |
<0.001* |
|
Mitral valve area, cm2 |
6.3±1.3 (53) |
6.6±5.0 (129) |
0.649 |
|
LV ejection fraction, % |
43.8±13.6 (98) |
39.1±13.1 (195) |
0.007 |
|
LVEDV, mL |
156.1±66.3 (87) |
196.0±74.2 (176) |
<0.001* |
|
LVESV, mL |
91.9±57.3 (86) |
125.1±65.5 (176) |
<0.001* |
|
LVEDV index, mL/m² |
82.7±32.0 (87) |
104.3±37.3 (174) |
<0.001* |
|
LVESV index, mL/m² |
48.7±29.2 (86) |
66.4±33.6 (174) |
<0.001* |
|
Vena contracta width, A-P, mm |
4.8±0.9 (83) |
6.6±4.2 (170) |
<0.001* |
|
KCCQ overall score |
51.2±22.3 (98) |
52.2±21.5 (194) |
0.715 |
|
EQ-5D-5L score |
53.7±19.3 (95) |
56.4±17.5 (193) |
0.259 |
|
Comorbidities |
|||
|
Hypertension |
84.2 (85/101) |
85.8 (169/197) |
0.732 |
|
Cardiomyopathy |
39.6 (40/101) |
40.1 (79/197) |
1.000 |
|
Myocardial infarction |
26.7 (27/101) |
24.9 (49/197) |
0.779 |
|
Percutaneous coronary intervention/stent |
40.6 (41/101) |
46.7 (92/197) |
0.328 |
|
TIA or stroke |
14.9 (15/101) |
11.2 (22/197) |
0.360 |
|
Atrial fibrillation |
59.4 (60/101) |
67.5 (133/197) |
0.200 |
|
Pacemaker/ICD |
41.6 (42/101) |
45.2 (89/197) |
0.622 |
|
Dyslipidaemia or hyperlipidaemia |
52.5 (53/101) |
51.8 (102/197) |
1.000 |
|
Heart failure hospitalisations within the last year |
45.5 (46/101) |
57.9 (114/197) |
0.050 |
|
AV block >1st degree or ventricular block |
25.7 (26/101) |
31.0 (61/197) |
0.519 |
|
Diabetes |
28.7 (29/101) |
28.9 (57/197) |
1.000 |
|
Renal insufficiency or failure (≥stage 3) |
41.6 (42/101) |
54.8 (108/197) |
0.821 |
|
Values are presented as % (n), % (n/N) or mean±SD (n). *Indicates p-values of statistical significance. P-values were calculated using the Kruskal-Wallis test (continuous variables) or Fisher’s exact test (categorical variables). A-P: anterior-posterior; AV: atrioventricular; EQ-5D-5L: EuroQol 5-dimension health questionnaire; EROA: effective regurgitant orifice area; EuroSCORE: European System for Cardiac Risk Evaluation; FMR: functional mitral regurgitation; ICD: implantable cardioverter-defibrillator; KCCQ: Kansas City Cardiomyopathy Questionnaire; LV: left ventricular; LVEDV: LV end-diastolic volume; LVESV: LV end-systolic volume; NYHA: New York Heart Association; SD: standard deviation; STS: Society of Thoracic Surgeons; TIA: transient ischaemic attack |
|||
Clinical outcomes
The 30-day CEC-adjudicated composite MAE rate was comparable between groups (4.0% FMR 2+; 4.1% FMR ≥3+; p=1.000) with low rates of cardiovascular mortality (1.0% FMR 2+; 0.5% FMR ≥3+), stroke (0% FMR 2+; 0.5% FMR ≥3+), and mitral valve reintervention (1.0% FMR 2+; 0.5% FMR ≥3+) (Table 2). At 1 year, the composite MAE rate was 13.9% in the FMR 2+ group and 18.3% in the FMR ≥3+ group (p=0.413) (Table 2). The ECL-assessed single-leaflet device attachment (SLDA) rate at 30 days was 1.0% in both the FMR 2+ and FMR ≥3+ groups, respectively, which remained stable up to 1 year (1.0% FMR 2+; 1.0% FMR ≥3+).
The 1-year Kaplan-Meier estimates for survival (90.0% FMR 2+; 84.2% FMR ≥3+; p=0.176), freedom from cardiovascular mortality (92.1% FMR 2+; 87.6% FMR ≥3+; p=0.233), freedom from heart failure hospitalisation (79.4% FMR 2+; 82.9% FMR ≥3+; p=0.499), and freedom from all-cause mortality or heart failure hospitalisation (76.4% FMR 2+; 71.5% FMR ≥3+; p=0.396) were comparable between groups (Figure 2). The CEC-adjudicated annualised HF hospitalisation rate 1 year after M-TEER decreased significantly in both groups (60.6% relative reduction FMR 2+; 64.1% relative reduction FMR ≥3+) compared to 1 year preprocedure (both p<0.001) (Figure 3).
Table 2. CEC-adjudicated events at 30 days and 1 year.
| 30 days | 1 year | |||
|---|---|---|---|---|
| Major adverse events | FMR 2+ n=101 |
FMR ≥3+ n=197 |
FMR 2+ n=101 |
FMR ≥3+ n=197 |
|
Cardiovascular mortality |
1.0 (1/101) |
0.5 (1/197) |
6.9 (7/101) |
10.7 (21/197) |
|
Stroke |
0 (0/101) |
0.5 (1/197) |
2.0 (2/101) |
2.0 (4/197) |
|
Myocardial infarction |
0 (0/101) |
0 (0/197) |
1.0 (1/101) |
0.5 (1/197) |
|
Mitral valve reintervention |
1.0 (1/101) |
0.5 (1/197) |
1.0 (1/101) |
1.5 (3/197) |
|
Major cardiac structural complications1 |
0 (0/101) |
0.5 (1/197) |
0 (0/101) |
0.5 (1/197) |
|
Device embolisation |
0 (0/101) |
0 (0/197) |
0 (0/101) |
0 (0/197) |
|
Renal complications requiring unplanned dialysis or renal replacement therapy |
2.0 (2/101) |
0 (0/197) |
4.0 (4/101) |
3.0 (6/197) |
|
Severe bleeding2 |
1.0 (1/101) |
3.6 (7/197) |
5.0 (5/101) |
7.6 (15/197) |
|
Major access site and vascular complications |
1.0 (1/101) |
0.5 (1/197) |
1.0 (1/101) |
0.5 (1/197) |
|
Composite MAE rate |
4.0 (4/101) |
4.1 (8/197) |
13.9 (14/101) |
18.3 (36/197) |
|
p-value |
1.000 |
0.413 |
||
| Other events | ||||
|
All-cause mortality |
1.0 (1/101) |
0.5 (1/197) |
8.9 (9/101) |
13.7 (27/197) |
|
Heart failure hospitalisation |
2.0 (2/101) |
2.5 (5/197) |
18.8 (19/101) |
14.2 (28/197) |
|
SLDA (core laboratory) |
1.0 (1/101) |
1.0 (2/197) |
1.0 (1/101) |
1.0 (2/197) |
|
Values are presented as % (n/N). 1Due to access-related issues. 2Major, extensive, life-threatening, or fatal bleeding defined by the Mitral Valve Academic Research Consortium. CEC: clinical events committee; FMR: functional mitral regurgitation; MAE: major adverse events; SLDA: single-leaflet device attachment |
||||
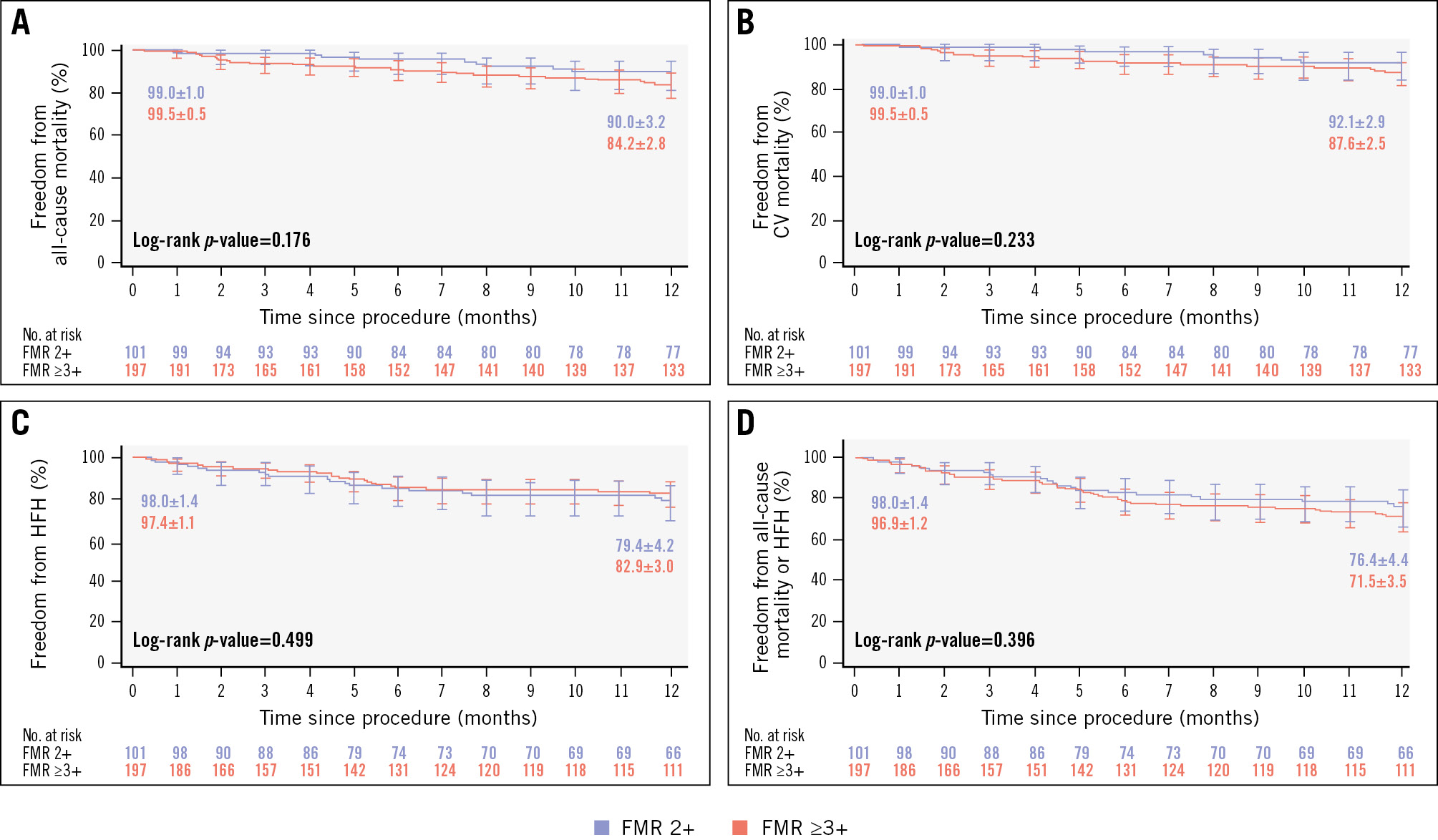
Figure 2. Freedom from CEC-adjudicated mortality and heart failure hospitalisation. Each graph shows Kaplan-Meier estimates±SE, and the error bars represent the 95% CI for 1 year of follow-up: (A) all-cause mortality; (B) cardiovascular (CV) mortality; (C) heart failure hospitalisation (HFH); and (D) all-cause mortality or HFH. CEC: clinical events committee; CI: confidence interval; FMR: functional mitral regurgitation; SE: standard error
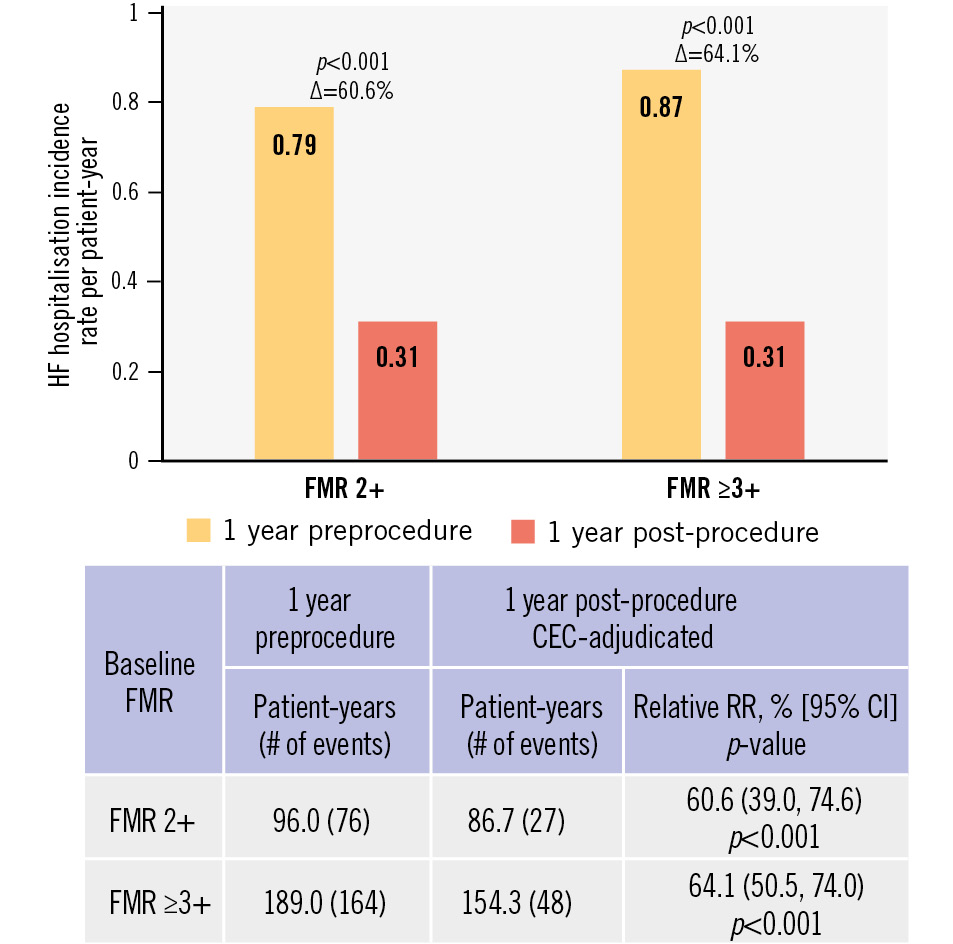
Figure 3. Annualised rate of heart failure hospitalisation. Preprocedure hospitalisation is site-reported; post-procedure hospitalisation is adjudicated by a clinical events committee. CEC: clinical events committee; CI: confidence interval; FMR: functional mitral regurgitation; HF: heart failure; RR: reduction rate
Echocardiographic outcomes
Significant MR reduction from baseline to discharge was observed in both groups, with 91.4% of patients achieving MR ≤1+ in the FMR 2+ group and 74.6% of patients achieving MR ≤1+ in the FMR ≥3+ group (all p<0.001 compared to baseline). This significant MR reduction was sustained up to 1 year with 89.8% of patients achieving MR ≤1+ in the FMR 2+ group (p<0.001 vs baseline) and 77.8% achieving MR ≤1+ in the FMR ≥3+ group (p<0.001 vs baseline). The MR reduction remained durable between discharge and 1 year in both groups (p=0.507 FMR 2+; p=0.730 FMR ≥3+) (Central illustration).
The reduction in MR was accompanied with significant improvements in echocardiographic MR indices (Table 3). In the FMR 2+ group, changes from baseline to 1 year included reductions in LV end-diastolic volume (–17.8 mL; p<0.001), LV end-systolic volume (–9.8 mL; p<0.05), LV end-diastolic diameter (–3.6 mm; p<0.001) and LV end-systolic diameter (–3.7 mm; p<0.001). Similar significant and sustained improvements were also demonstrated at 1 year in the FMR ≥3+ group.
In the FMR 2+ group, the mean transmitral valve gradient increased from 1.6 mmHg at baseline to 3.1 mmHg at discharge and remained stable at 3.1 mmHg up to 1 year (p=0.392 vs discharge), with a similar trend observed in the FMR ≥3+ group (Table 3).
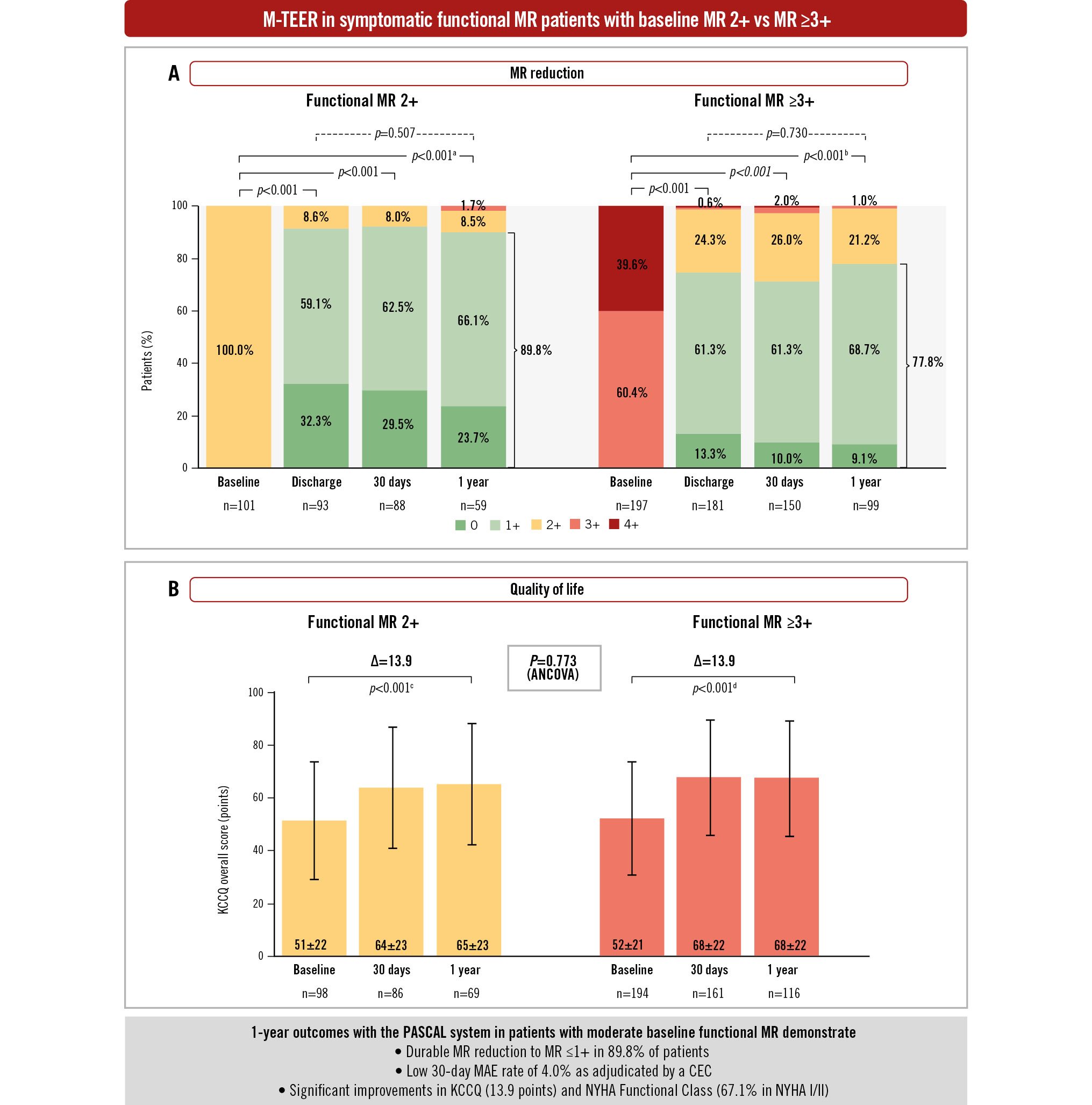
Central illustration. One-year M-TEER outcomes in patients with moderate baseline FMR. Graphs show the unpaired analysis. A) MR severity by transthoracic echocardiography assessed by the echocardiographic core laboratory. P-values were calculated from paired analysis using the Wilcoxon signed-rank test relative to baseline and between discharge and 1 year. B) KCCQ graphs show mean±SD values. Error bars represent 95% CI. Δ and p-values were calculated from the paired analysis using the Student’s t-test. aBaseline vs 1 year (n=59; MR ≤1+=89.8%). bBaseline vs 1 year (n=99; MR ≤1+=77.8%). cBaseline vs 1 year (n=67; mean baseline KCCQ=52.1; mean 1-year KCCQ=66.0). dBaseline vs 1 year (n=114; mean baseline KCCQ=53.7; mean 1-year KCCQ=67.6). The intergroup p-value was calculated using the analysis of covariance (ANCOVA) model. CEC: clinical events committee; CI: confidence interval; FMR: functional mitral regurgitation; KCCQ: Kansas City Cardiomyopathy Questionnaire; MAE: major adverse events; MR: mitral regurgitation; M-TEER: mitral transcatheter edge-to-edge repair; NYHA: New York Heart Association; SD: standard deviation
Table 3. Echocardiographic outcomes by core laboratory up to 1 year.
| FMR 2+ | FMR ≥3+ | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | Baseline | Discharge | Delta (paired n) p-value |
Baseline | 1 year | Delta (paired n) p-value |
Baseline | Discharge | Delta (paired n) p-value |
Baseline | 1 year | Delta> (paired n) p-value |
|
LVEDV, mL |
154.7±65.8 |
149.9±63.1 |
–4.9±19.0 (72) p<0.05 |
155.4±64.2 |
137.6±60.3 |
–17.8±32.7 (50) p<0.001 |
199.1±73.8 |
187.6±70.8 |
–11.6±23.8 (141) p<0.001 |
198.2±74.6 |
162.5±64.7 |
–35.7±40.8 (81) p<0.001 |
|
LVESV, mL |
91.9±55.7 |
89.0±55.3 |
–2.9±11.3 (72) p<0.05 |
88.8±52.2 |
79.0±50.4 |
–9.8±28.1 (49) p<0.05 |
128.1±65.7 |
122.4±63.6 |
–5.7±20.4 (141) p<0.01 |
124.2±66.2 |
102.4±57.6 |
–21.8±36.0 (81) p<0.001 |
|
LVEDD, mm |
59.5±9.3 |
57.2±9.7 |
–2.2±3.1 (75) p<0.001 |
59.5±8.1 |
55.9±7.7 |
–3.6±3.8 (50) p<0.001 |
65.1±9.2 |
63.5±9.3 |
–1.6±2.8 (161) p<0.001 |
65.8±8.6 |
61.7±9.0 |
–4.1±4.4 (92) p<0.001 |
|
LVESD, mm |
47.8±12.3 |
46.2±12.5 |
–1.5±3.7 (70) p<0.01 |
47.6±10.0 |
44.0±10.1 |
–3.7±4.9 (46) p<0.001 |
54.3±12.3 |
53.3±12.6 |
–0.99±4.8 (150) p<0.05 |
54.6±12.2 |
50.4±11.3 |
–4.2±6.5 (89) p<0.001 |
|
LVEF, % |
43.7±13.7 |
44.1±14.2 |
0.44±4.6 (93) p=0.352 |
44.9±11.89 |
46.4±12.2 |
1.4±8.6 (58) p=0.205 |
38.9±13.3 |
38.3±13.4 |
–0.61±4.9 (176) p=0.095 |
39.8±13.5 |
39.6±13.5 |
–0.19±7.1 (99) p=0.788 |
|
Stroke volume, mL |
58.4±16.2 |
62.0±19.9 |
3.6±14.7 (43) p=0.112 |
55.8±14.8 |
60.0±22.2 |
4.2±23.9 (38) p=0.290 |
50.3±17.9 |
54.0±18.1 |
3.8±11.4 (100) p<0.01 |
52.0±16.6 |
56.4±17.6 |
4.5±14.5 (61) p<0.05 |
|
LA volume, mL |
120.4±60.9 |
116.6±52.6 |
–3.8±25.5 (88) p=0.165 |
112.5±42.6 |
107.0±45.8 |
–5.5±22.9 (55) p=0.078 |
138.6±69.9 |
135.0±66.9 |
–3.6±27.6 (171) p=0.087 |
143.4±71.2 |
126.2±67.1 |
–17.2±28.0 (92) p<0.001 |
|
Transmitral mean gradient, mmHg |
1.6±0.8 |
3.1±1.6 |
1.5±1.3 (55) p<0.001 |
1.7±0.8 |
3.1±1.4 |
1.4±1.3 (38) p=0.392a |
2.0±0.8 |
3.3±1.4 |
1.3±1.3 (134) p<0.001 |
1.9±0.7 |
3.2±1.6 |
1.3±1.6 (74) p=0.818a |
|
PASP, mmHg |
40.6±13.9 |
37.7±10.3 |
–2.9±8.8 (58) p<0.05 |
40.5±13.8 |
37.3±9.4 |
–3.2±12.2 (39) p=0.108 |
45.9±11.6 |
39.7±9.6 |
–6.1±10.1 (127) p<0.001 |
47.3±10.7 |
40.5±12.56 |
–6.8±13.3 (71) p<0.001 |
|
Values are mean±SD or mean±SD (n). Paired data presented and used for the calculation of deltas and p-values (using the paired Student’s t-test) compared with baseline. ap-value presented for discharge to 1 year. FMR: functional mitral regurgitation; LA: left atrial; LV: left ventricular; LVEDD: LV end-diastolic diameter; LVEDV: LV end-diastolic volume; LVEF: LV ejection fraction; LVESD: LV end-systolic diameter; LVESV: LV end-systolic volume; PASP: pulmonary artery systolic pressure |
||||||||||||
Functional and quality-of-life outcomes
Significant and sustained improvements in functional and quality-of-life outcomes were observed at 1 year in the FMR 2+ group. Functional capacity improved significantly after M-TEER, with 67.1% of patients in NYHA Class I or II compared to 26.7% at baseline (p<0.001) (Figure 4). The KCCQ overall summary score increased by 13.9±23.0 points (p<0.001 vs baseline) (Central illustration) and the EQ-5D-5L visual analogue scale (VAS) score improved by 7.4±21.8 points (p<0.05 vs baseline) (Supplementary Table 4).
Similar significant improvements were observed at 1 year in the FMR ≥3+ group: 70.1% of patients were in NYHA Class I/II, and there was a 13.9±21.3 point increase in the KCCQ overall summary score and a 6.4±20.7 point increase in the EQ-5D-5L VAS score (all p<0.01 vs baseline) (Central illustration, Figure 4, Supplementary Table 4). At 1 year, differences in functional and quality-of-life outcomes between the 2 groups were not significant (p>0.1 for both).
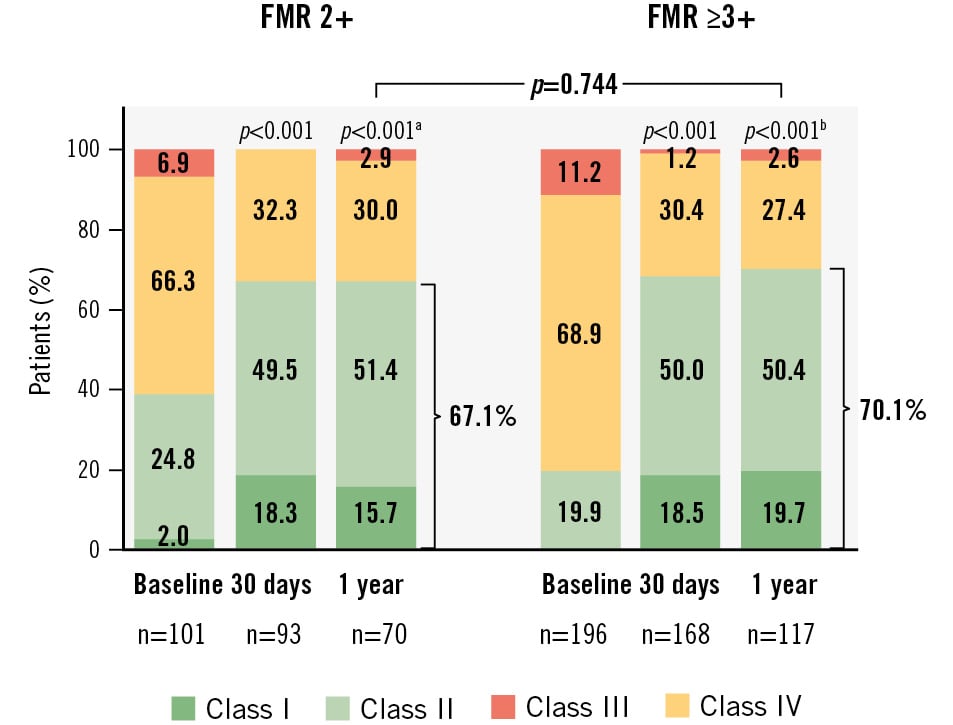
Figure 4. NYHA functional status at 1 year. Graphs show the unpaired analysis. The p-values for intragroup comparison were calculated from paired analysis using the Wilcoxon signed-rank test, and the p-value for intergroup comparison was calculated using Fisher’s exact test. aBaseline vs 1 year (n=70; NYHA Class I/II=67.1%). bBaseline vs 1 year (n=117; NYHA Class I/II=70.1%). FMR: functional mitral regurgitation; NYHA: New York Heart Association

Central illustration. One-year M-TEER outcomes in patients with moderate baseline FMR. Graphs show the unpaired analysis. A) MR severity by transthoracic echocardiography assessed by the echocardiographic core laboratory. P-values were calculated from paired analysis using the Wilcoxon signed-rank test relative to baseline and between discharge and 1 year. B) KCCQ graphs show mean±SD values. Error bars represent 95% CI. Δ and p-values were calculated from the paired analysis using the Student’s t-test. aBaseline vs 1 year (n=59; MR ≤1+=89.8%). bBaseline vs 1 year (n=99; MR ≤1+=77.8%). cBaseline vs 1 year (n=67; mean baseline KCCQ=52.1; mean 1-year KCCQ=66.0). dBaseline vs 1 year (n=114; mean baseline KCCQ=53.7; mean 1-year KCCQ=67.6). The intergroup p-value was calculated using the analysis of covariance (ANCOVA) model. CEC: clinical events committee; CI: confidence interval; FMR: functional mitral regurgitation; KCCQ: Kansas City Cardiomyopathy Questionnaire; MAE: major adverse events; MR: mitral regurgitation; M-TEER: mitral transcatheter edge-to-edge repair; NYHA: New York Heart Association; SD: standard deviation
Discussion
Current clinical guidelines do not recommend M-TEER for patients with symptomatic moderate functional MR. Yet, it is well documented that untreated FMR progresses over time, contributing to unfavourable LV remodelling and secondary cardiac damage24. Offering intervention to patients earlier in the disease spectrum may halt this progression and preserve the health of the LV. We evaluated the clinical and ECL-assessed echocardiographic outcomes of M-TEER with the PASCAL system in MiCLASP patients with moderate FMR (mean EROA 0.22 cm2). Our findings indicate that these patients benefited from M-TEER with a significant and durable MR reduction (89.8% with FMR ≤1+) accompanied by reverse LV remodelling. These echocardiographic findings were associated with symptomatic benefits, demonstrated by significant and sustained improvements in NYHA functional status and quality of life, and a reduction in the annualised HF hospitalisation rate. Importantly, the echocardiographic and clinical benefits achieved by the FMR 2+ group were comparable to the FMR ≥3+ group.
Recently published 1-year outcomes from the MitraClip EXPAND and EXPAND G4 post-market studies in patients with symptomatic baseline moderate FMR (mean EROA 0.20 cm2) demonstrated similar benefits of M-TEER, with significant and sustained improvements in MR grade (96.8% with FMR ≤1+), functional status (79.2% in NYHA Class I/II) and quality of life (+20.5 point improvement in KCCQ summary score)16. Importantly, treatment of moderate FMR with M-TEER resulted in favourable LV reverse remodelling with significant reductions in LVEDV and LVESV, and a 62% reduction in the HF hospitalisation rate. These contemporary findings lend further credence to the thought that earlier treatment of FMR can promote reverse LV remodelling and halt disease progression, along with symptomatic improvements and potential survival benefits17.
Findings from the Randomised Investigation of the MitraClip Device in Heart Failure: 2nd Trial in Patients With Clinically Significant Functional Mitral Regurgitation (Reshape-HF2) study, which was designed to assess the safety and effectiveness of M-TEER in patients with HF and less severe FMR, were recently published181920. Outcomes from this study demonstrated that at 2 years, the rate of first or recurrent hospitalisation for heart failure or cardiovascular death was significantly lower in the device group (M-TEER in conjunction with GDMT) as compared to the control group (medical therapy alone). Importantly, MR was graded as moderate-to-severe in a significant proportion of patients (median EROA 0.23 cm2), providing further evidence that treatment of FMR in patients with an EROA of 0.2-0.3 cm2 can convey significant clinical benefits.
However, some important differences exist between studies. First, all patients enrolled in the moderate FMR cohorts of the MiCLASP and EXPANDed studies were categorised by an ECL to have moderate MR (EROAs of 0.22 cm2 and 0.2 cm2, respectively) at baseline in contrast to only 23% of patients in Reshape-HF2 (EROA <0.2 cm2). Secondly, the Reshape-HF2 study was a randomised clinical study with a GDMT control arm unlike the post-market MiCLASP and EXPANDed studies. Regarding the use of new categories of GDMT with a Class I recommendation for HFrEF like sodium-glucose cotransporter 2 inhibitors (SGLT2i) and angiotensin receptor-neprilysin inhibitors (ARNI; like sacubitril/valsartan), EXPANDed and Reshape-HF2 reported suboptimal use, which would likely be similar to MiCLASP considering the enrolment period. Regardless, each of these studies showed favourable effects of M-TEER in a moderate FMR patient population.
The MiCLASP study results are striking in that functional benefits and freedom from mortality and heart failure hospitalisation were comparable between patients with moderate FMR and those with FMR ≥3+. This observation might appear counterintuitive, given that patients with a greater degree of baseline FMR had larger ventricles both before and after M-TEER, while having comparable comorbidities. However, both groups experienced a marked reduction in MR following M-TEER, thereby alleviating the specific risk of MR progression. This suggests that the presence of MR per se represents a significant risk factor, and treating moderate or greater MR might reduce this risk.
Though the findings from our study are encouraging, several limitations must be taken into consideration. The selection of patients for M-TEER was based on local Heart Team assessment, which incorporated GDMT for heart failure in the decision-making process; however, adherence to GDMT was not routinely collected. The post-market MiCLASP study did not include a control arm, limiting comparison with medical treatment alone. This is important as recent studies have demonstrated the benefits of new classes of medications like SGLT2i and ARNI in reducing MR and improving quality of life in FMR patients with heart failure21. Patient eligibility in the MiCLASP study was based on site evaluation of MR severity rather than prospective review and approval by the ECL. However, after patient enrolment, an ECL assessed all echocardiograms using ASE guidelines to reduce variability and bias in this analysis. Assessment of MR by the treating physician versus the ECL also warrants a discussion. Baseline MR was graded by the treating physician based on both TTE and TOE images using European Union guidelines, whereas the ECL solely relied on TTE for MR assessment using ASE guidelines. Additionally, MR assessment by the ECL was conducted at baseline and at specific timepoints during follow-up, which may not have adequately captured the dynamic range of FMR. MR grading is complex, slightly subjective, operator dependent, and can vary between TTE and TOE assessments22. Hence, the ECL may have underestimated the severity of FMR in some MiCLASP patients. It should be noted that the study limitations discussed above reflect current-day clinical practice and provide a unique opportunity to understand the impact of M-TEER in symptomatic patients with moderate FMR. Regardless, treatment of clinically relevant FMR 2+ with M-TEER resulted in significant clinical benefits at 1 year that were comparable to patients with FMR ≥3+. However, long-term follow-up to evaluate the durability of M-TEER in this population will be important.
Guidelines and consensus documents that guide current clinical practice are based on evidence derived from historical clinical studies523. These studies were restricted to high-risk patients with MR ≥3+ with simple mitral lesions based on the novelty of the therapy, operator inexperience, and older imaging technologies. Since these initial studies, advances including greater operator experience, availability of three-dimensional transoesophageal imaging and introduction of next-generation M-TEER devices have facilitated the treatment of patients currently deemed unsuitable for M-TEER by guidelines and consensus documents. Contemporary learnings from the MiCLASP study and other investigations of moderate MR necessitate re-evaluation of current practice.
While our findings are promising, it is important to highlight that FMR is a heterogeneous condition with diverse phenotypes that may respond differently to GDMT and M-TEER. Hence, well-designed randomised studies under the guidance of a Heart Team with longer durations of follow-up are required to better identify patients with moderate FMR who may benefit from earlier M-TEER and to provide definitive proof of whether early treatment of FMR might prevent progression of the disease.
Limitations
Patient follow-up was challenging because of the COVID-19 pandemic, which resulted in incomplete echocardiographic assessments at some timepoints. The study was not intended or designed to compare or elucidate differences between delivery system iterations.
Conclusions
One-year outcomes from the MiCLASP study suggest that select patients with symptomatic moderate FMR can benefit from M-TEER using the PASCAL system with significant MR reduction and improvements in clinical, functional, and quality-of-life outcomes comparable to those experienced by patients with ≥moderate-severe FMR.
Impact on daily practice
Outcomes from this MiCLASP study subanalysis suggest that select patients with symptomatic moderate baseline functional mitral regurgitation (FMR) can be safely and effectively treated with mitral transcatheter edge-to-edge repair. At 1 year, patients with moderate FMR experienced significant and sustained mitral regurgitation reduction after treatment with the PASCAL system. This was achieved with a low major adverse events rate and accompanied with significant improvements in functional status and quality of life.
Acknowledgements
The authors would like to thank all the patients, sites, and study principal investigators who participated in the MiCLASP study. In addition, they would like to thank Edwards Lifesciences’ TMTT members for their support of this publication: Maithili Shrivastava, PhD; Shiyu (Vanessa) Wang, MS; Allison Weiser, MPH; Suzanne Gilmore, MPIA; and Ted Feldman, MD.
Funding
This work was supported by Edwards Lifesciences.
Conflict of interest statement
P. Lurz has received institutional fees and research grants from Abbott, Edwards Lifesciences, and Recor Medical; honoraria from Edwards Lifesciences, Abbott, Innoventric, Recor Medical, and Boehringer Ingelheim; and has stock options with Innoventric. T. Rassaf has received honoraria, lecture fees, and grant support from Edwards Lifesciences, AstraZeneca, Bayer, Novartis, Berlin Chemie, Daiichi Sankyo, Boehringer Ingelheim, Novo Nordisk, Cardiac Dimensions, and Pfizer, all unrelated to this work; he is cofounder of Bimyo GmbH, a company that develops cardioprotective peptides. P. Luedike received research grants and honoraria for consulting and lectures from Edwards Lifesciences, Novartis, and Pfizer. T. Schmitz receives speaker and proctor honoraria from Edwards Lifesciences and Abbott. T. Geisler received personal fees (lecture honoraria) and institutional research grants from Edwards Lifesciences. V. Rudolph receives research grants from Edwards Lifesciences, Abbott, and Boston Scientific. E. Lubos received grants from Abbott; and personal fees from Abbott, Abiomed, AstraZeneca, Bayer, Edwards Lifesciences, New Valve Technology, and Novartis. I. Eitel receives research grants from Abbott; and speaker honoraria from Abbott, Edwards Lifesciences, and Boston Scientific. R.S. von Bardeleben is a principal investigator for Abbott, DZHK, Edwards Lifesciences, Jenscare, Medtronic, NeoChord, and University of Göttingen IIT; and serves as an advisor, proctor, or speaker for Abbott, Edwards Lifesciences, JenaValve, Jenscare, Medtronic, NeoChord, Philips, and Siemens. N. Brambilla has received speaker and proctor honoraria fees from Boston Scientific, and Edwards Lifesciences; proctor honoraria fees from Abbott; and speaker honoraria fees from Medtronic. S. Berti receives honoraria and is a consultant for Procter & Gamble, Abbott, and Boston Scientific. A. Linke received grants from Novartis, and Edwards Lifesciences; personal fees from Abbott, Abiomed, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Edwards Lifesciences, Medtronic, Novartis, Sanofi Genzyme, and Pfizer; and other fees from Pi-Cardia, Filterlex, and Transverse Medical outside the submitted work. B. Unsöld has received expense-covered procedural training from Edwards Lifesciences and Abbott; and honoraria for lectures and advisory boards from Pfizer, Novartis, AstraZeneca, Alnylam, Bayer, and Sobi. C. Hengstenberg receives institutional research grants from Edwards Lifesciences, Boston Scientific, Abbott, and Medtronic; and is a proctor for Edwards Lifesciences and Boston Scientific. S. Baldus received a research grant from Abbott; and lecture fees from Abbott and Edwards Lifesciences. K. Spargias has received honoraria or consultation fees from Edwards Lifesciences, Abbott, and Medtronic. G. Nickenig has received research funding from the Deutsche Forschungsgemeinschaft, the German Federal Ministry of Education and Research, the EU, Abbott, AGA Medical, AstraZeneca, Bayer, Berlin Chemie, Biosensus, Biotronik, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St. Jude Medical; and has received honoraria for lectures or advisory boards from Abbott, AGA Medical, AstraZeneca, Bayer, Cardiovalve, Berlin Chemie, Biosensus, Biotronik, Bristol-Myers Squibb, Boehringer Ingelheim, Daiichi Sankyo, Edwards Lifesciences, Medtronic, Novartis, Pfizer, Sanofi, and St. Jude Medical. P. Denti is a consultant for InnovHeart, Approxima, Pi-Cardia, and HRV; and receives speaker honoraria from Edwards Lifesciences and Abbott. H. Möllmann receives speaker honoraria/proctor fees from Abbott, Boston Scientific, Edwards Lifesciences, Medtronic, and SMT. W. Rottbauer is a consultant for and receives speaker honoraria from Edwards Lifesciences. F. Praz received travel expenses from Edwards Lifesciences, Abbott, Polares Medical, Medira, and Siemens Healthineers. C. Butter has received lecture fees from Edwards Lifesciences and Abbott; and educational grants from Boston Scientific. N.M. Van Mieghem has received research grants from Abbott, Boston Scientific, Medtronic, Edwards Lifesciences, Daiichi Sankyo, AstraZeneca, and Teleflex; and scientific advisory fees from Anteris, JenaValve, Abbott, Boston Scientific, Medtronic, Amgen, Daiichi Sankyo, Siemens, Pie Medical Imaging, and Teleflex. M.J. Swaans is a proctor/lecturer for Abbott, Boston Scientific, Bioventrix Inc, Cardiac Dimensions, Edwards Lifesciences, GE HealthCare, Medtronic, and Philips Healthcare. A. Witkowski is an advisory board member and has received honoraria from Abbott and Edwards Lifesciences. M.H. Buch participates in the Abbott advisory group; and has received honoraria from Abbott. T. Seidler has received honoraria for lectures, advisory boards, or travel grants from Abbott, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Corvia, Edwards Lifesciences, Medtronic, Myocardia Solutions, Novartis, Pfizer, and Teleflex. L. Marcoff serves as a member of the echocardiography core laboratory for Edwards Lifesciences, and Abbott, for which he receives no direct compensation. K. Koulogiannis is a consultant and advisory board member for Edwards Lifesciences; and a speaker for Abbott. J. Hausleiter is a consultant for, receives speaker honoraria and institutional research support from Edwards Lifesciences. The other authors have no conflicts of interest relevant to the contents of this paper to declare.
Supplementary data
To read the full content of this article, please download the PDF.

