Abstract
Intravascular and intracardiac masses are usually represented by thrombi, tumours, and vegetations. They can affect both the right and left chambers of the heart and the venous and arterial circulation. Traditionally, their treatment is surgical or, in some circumstances, based on systemic anticoagulation/fibrinolysis. However, the complexity and frailty of patients who sometimes present with these conditions have pushed surgeons to find alternative minimally invasive effective treatments. While small masses can be removed with multiple devices, large masses are a more challenging problem. Vacuum-assisted aspiration systems such as the AngioVac System were developed to treat intravenous and right-sided heart thrombi. The application of the AngioVac System was widened to right-sided endocarditis and, later, to left-sided thrombi and vegetations. This review summarises the clinical results of different uses of the vacuum-assisted aspiration system to treat intravenous and intracardiac masses.
Intracardiac masses, either located in the left or right heart chambers, have various origins but are mainly represented by thrombi, primary or secondary tumours, and endocarditis vegetations12. Patients presenting with these conditions might be frail and have an unstable haemodynamic status. In such conditions, surgery may be affected by high perioperative mortality and morbidity12.
Percutaneous vacuum-assisted aspiration of large intravascular/intracardiac masses has become a less invasive option for this subgroup of patients who cannot take advantage of conventional surgery3. While different solutions have been developed to deal with small masses, the only available system able to manage large masses is the AngioVac System (AngioDynamics). It was developed and approved to address right-sided heart disease45678; however, off-label utilisations have been reported to remove left-sided masses through transseptal/transapical access in high-risk patients9101112. It enables a vacuum-assisted aspiration of large heart and vascular masses, providing a circulatory support system which supports the patient’s haemodynamics and returns large blood volumes to the patient.
The use of vacuum-assisted systems for intracardiac and intravascular masses is not yet included in current international guidelines, as the safety and efficacy data have been derived only from small retrospective studies, and large retrospective and clinical randomised trials are lacking.
We here summarise the clinical experience, indications, and controversies of the percutaneous aspiration system to treat right- and left-sided large masses.
The AngioVac System and procedure
The AngioVac System comprises a large-bore cannula, an extracorporeal circulation circuit including a filter connected to a centrifugal pump, and a reinfusion cannula to return blood to the patient. The original standard setup is a venovenous extracorporeal circuit with the aspiration cannula inserted in the internal jugular or femoral vein and the reinfusion cannula placed in the femoral vein (Figure 1). The 22 Fr aspiration cannula has a 20 or 180 degree angulated tip (Figure 2). A closed circuit is built, and a standard centrifugal pump generates vacuum aspiration. The suctioned material is trapped in the circuit filter placed ahead of the pump’s inlet.
The procedure is performed in a standard operating room under general anaesthesia. Real-time transoesophageal echocardiographic (TOE) guidance is mandatory and can be integrated with fluoroscopy. The aspiration cannula’s insertion site depends on the mass location and can be surgical or percutaneous. As per hospital protocol, systemic heparinisation is necessary with a target activated clotting time (ACT). The reinfusion cannula insertion site depends on the aspiration site.
The Central illustration summarises the possible circuit setup for aspiration of masses located in the right and left sections.
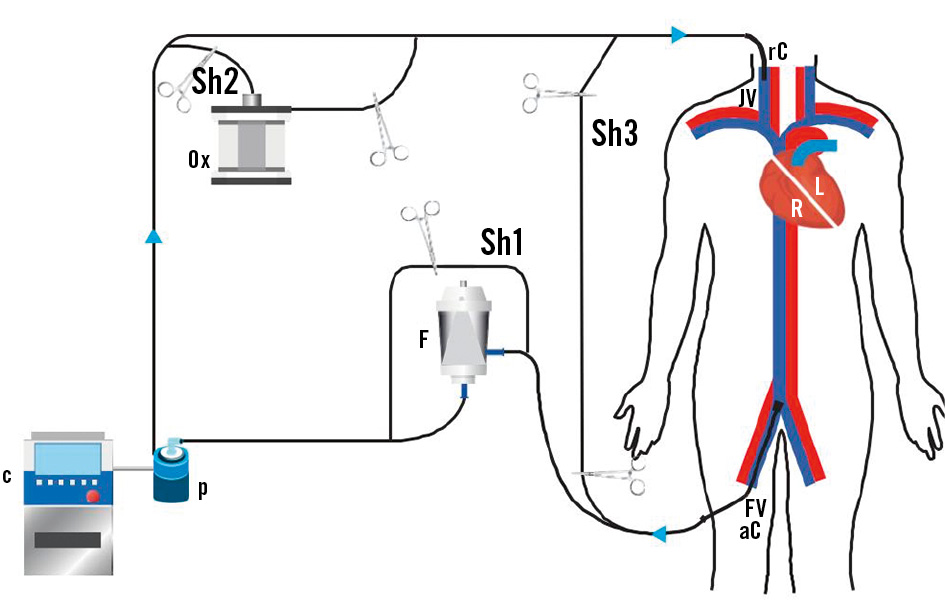
Figure 1. Standard cannulation setup. The aspiration cannula (aC) is inserted into the venous system through the femoral vein (FV). Thrombi and/or vegetations are aspirated with the venous blood and trapped in the filter (F). Filtered blood is then circulated by a console-controlled (C) centrifugal pump (P) and returned to the patient through the reinfusion venous cannula (rC) in the jugular vein (JV). The represented setup includes three shunting lines: the first one (Sh1) to bypass the filter, the second one (Sh2) to include an oxygenator (Ox) and the third one (Sh3) to exclude the whole circuit from the patient. R: right side; L: left side
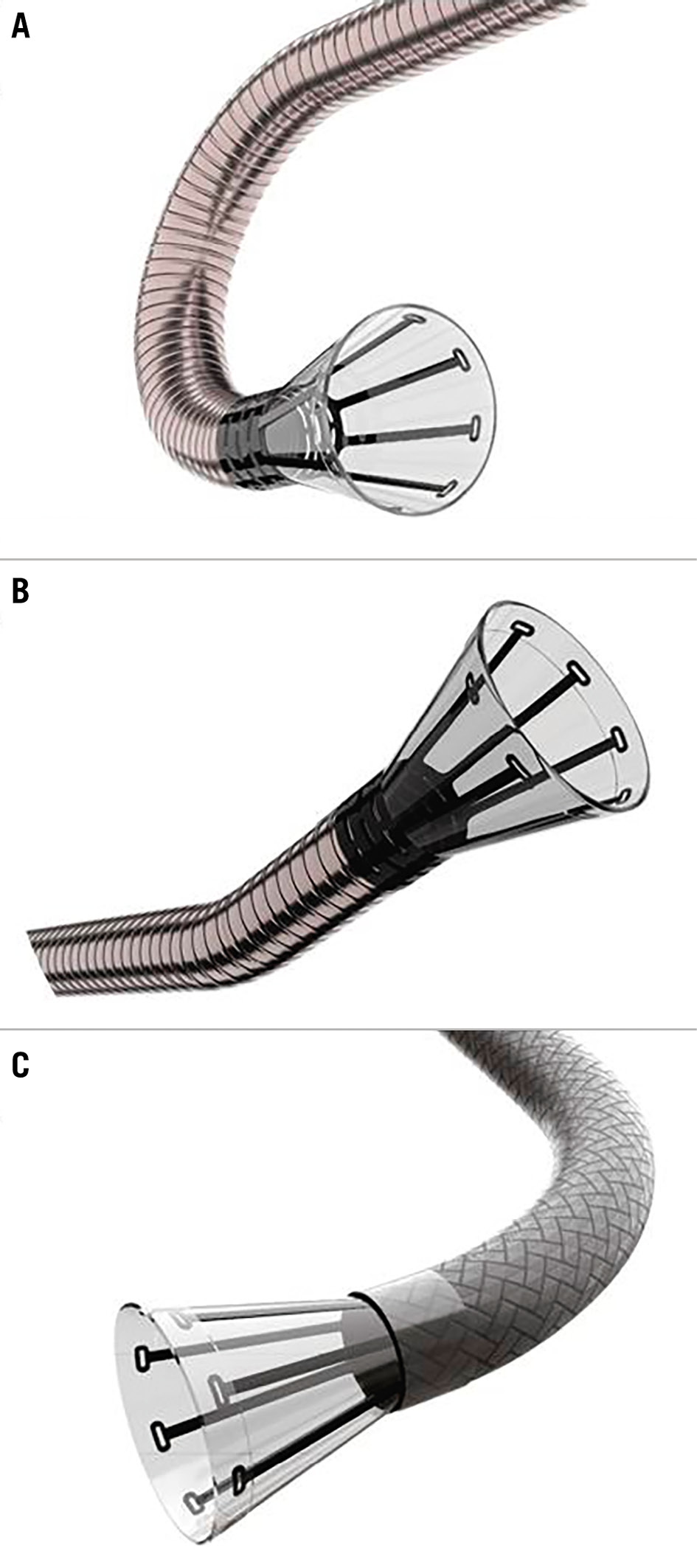
Figure 2. Available AngioVac inflow cannulae. A) 22 Fr size with a 180° angle tip, (B) 22 Fr size with a 20° angle tip, and (C) 18 Fr size with an 85° angle tip.
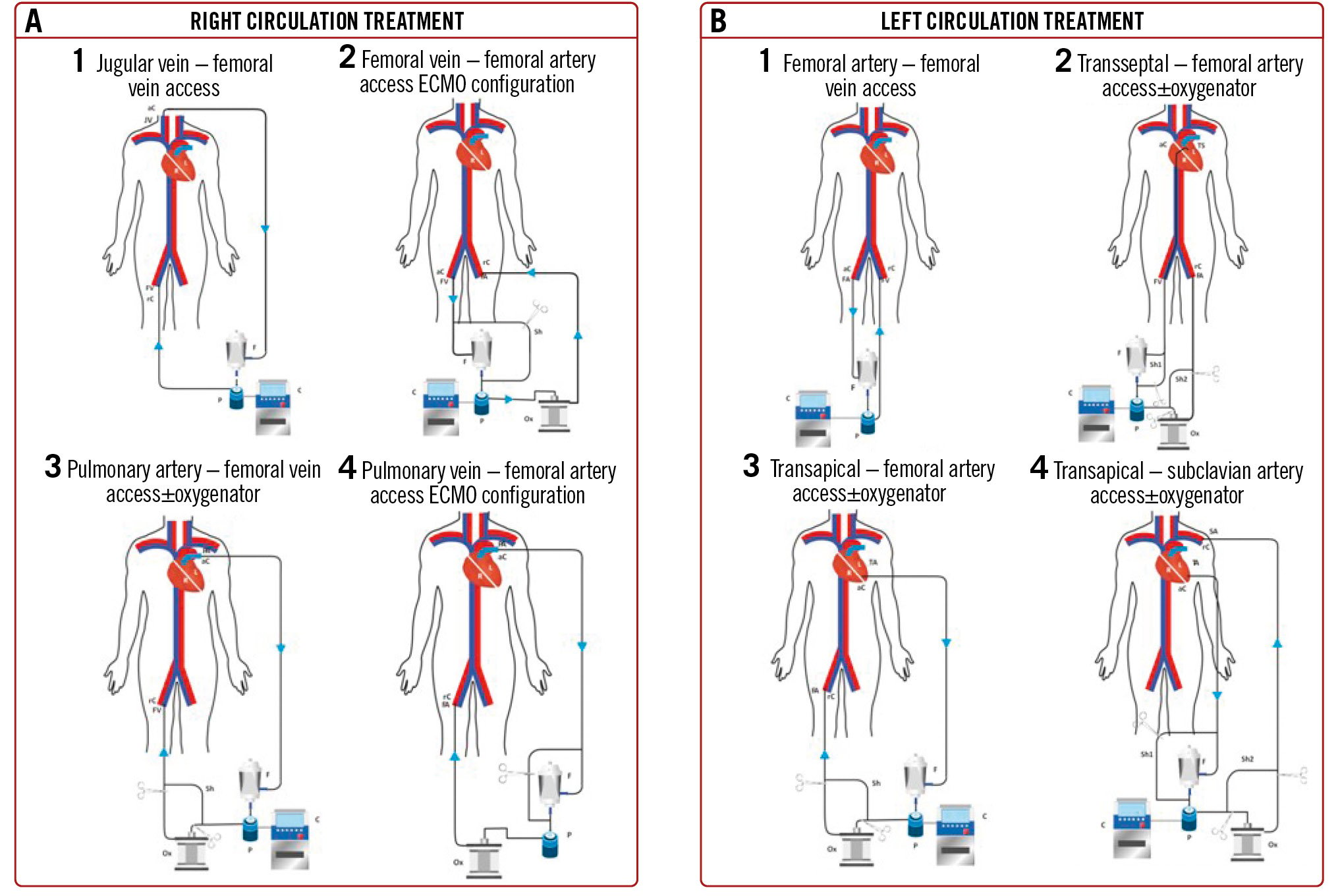
Central illustration. Circuit setups. A) Right-sided masses. A1) Jugular vein – femoral vein access: superior caval, tricuspid, atrial and jugular vein mass suction. This is preferable to treat haemodynamically stable patients, since the reinfusion cannula is positioned in the femoral vein, and therefore, there is no chance to mechanically assist the patient. A2) Femoral vein – femoral artery access ECMO configuration: right-sided masses suction and circulatory assistance. A shunt line allows for filter bypassing, in case of need for assistance >6 h. A3) Pulmonary artery – femoral vein access±oxygenator: for pulmonary embolism. By switching the reinfusion cannula into the femoral artery, the system can be shifted to a temporary ECMO. A4) Pulmonary artery – femoral artery access ECMO configuration: this configuration enables the operator to treat pulmonary embolism, ensuring mechanical support to the circulation. The oxygenator and the shunt line provide the chance to bypass the filter in case of need for >6 h of use, transforming the circuit into an ECMO. B) Left-sided masses. B1) Femoral artery – femoral vein access: this configuration has been described for abdominal aorta mass suction procedures. B2) Transseptal – femoral artery access±oxygenator: this setup has been described to approach left atrial/mitral valve/LV masses. The optional use of an oxygenator increases the filtering and assists blood oxygenation in case the patient needs prolonged mechanical circulatory support; in this case, a shunt line allows for filter bypassing and prolonged left mechanical assistance. B3) Transapical – femoral artery access±oxygenator: mitral and aortic valve masses. This setup includes two shunt lines: one to bypass the filter in case of need for prolonged left mechanical assistance and one to allow the potential use of an oxygenator. B4) Transapical – subclavian artery access±oxygenator: the reinfusion cannula can be positioned in the subclavian artery when femoral arteries are not available. This configuration allows for prolonged left mechanical assistance by means of a shunt line bypassing the filter. ECMO: extracorporeal membrane oxygenation; LV: left ventricular
Current clinical application
We systematically searched PubMed’s MEDLINE, Google Scholar and Ovid for single- and multicentre studies and registries assessing the use of the AngioVac System to treat intravascular (venous and arterial) and intracardiac (right- and left-sided) masses. We found 149 relevant articles that mentioned the use of the AngioVac System. Preoperative, procedural, and postoperative results were examined, and relevant data were summarised in the present review. The most important articles are reported in Table 1.
Table 1. Main articles relative to the use of AngioVac with reported mortality and success rates.
| Authors | No. of cases | Mass type | Success | Death | Study type |
|---|---|---|---|---|---|
| Donaldson CW et al3 | 14 | IVC thrombus/RHT (11)PE (5) | 73% | 13% (30 d) | Retrospective single-centre |
| Hameed I et al4 | 182 | VC/RHT (101)RH endocarditis (81) | 71.6% (VC/RHT 80.5%, PE 30.4%)74.5% | 23.3% (VC/RHT 14.8%, PE 32.3%)14.6% | Meta-analysis |
| Jabaar AA et al5 | 13 | VC | 83% | NA | Systematic review |
| Resnick SA et al7 | 7 | IVC/RHT (6)PE (1) | 86% | 0 | Retrospective single-centre |
| Moriarty JM et al8 | 16 | IVC (10)RHT (6) | 100%67% | 87.5% | Retrospective single-centre |
| Qintar M et al11 | 10 | LH endocarditis | 80% | 0 | Retrospective single-centre |
| Al Badri A et al17 | 7 | IVC/RHT (5)Leads vegetation (2) | 86% | 0 | Retrospective single-centre |
| Al-Hakim R et al22 | 5 | PE | 40% | 80% (30 d) | Retrospective single-centre |
| D’Ayala M et al23 | 12 | IVC (4)RHT (5)PE (3) | 100%60%33% (partial) | NA | Retrospective single-centre |
| Mhanna M et al28 | 301 | RH endocarditis | 79.1% | 89.7% | Meta-analysis |
| George B et al29 | 33 | TV endocarditis | 90.0% | 61% | Retrospective single-centre |
| Patel N et al30 | 3 | Leads vegetation | 100% | 0 | Retrospective single-centre |
| Schaerf RHM et al31 | 20 | Leads vegetation | 100% | 0 | Retrospective single-centre |
| Starck C et al32 | 78 | RH endocarditis | 91.7% | 0 | Retrospective single-centre |
| Tarzia V et al33 | 13 | Leads vegetation | 92.3% | 0 | Retrospective single-centre |
| d: days; IVC: inferior vena cava; LH: left heart; PE: pulmonary embolism; RH: right heart; RHT: right heart thrombosis; TV: tricuspid valve; VC: vena cava | |||||
Intracardiac right heart and intravenous masses
THROMBI AND TUMOURS
The presence of right heart thrombi or tumours (RHT) is often associated with extensive iliocaval thrombosis and/or pulmonary embolism (PE)113 and carries poor outcomes13. Patients with RHT usually benefit from surgery more than from anticoagulation or thrombolytic therapy alone14. The AngioVac System was initially proposed for high-risk patients234515. This procedure was safe in the described cases161718 (Figure 3, Moving image 1). The success rate may vary depending on the centres’ experience and an acute versus chronic origin of the thrombi, as confirmed by a recent meta-analysis showing complete removal of RHT in between 68% and 85% of cases depending on the thrombosis acuteness16. Similarly, Resnick7 and Al Badri17 reported partial or completely unsuccessful thrombi aspiration only in the case of chronic conditions.
In iliocaval thrombosis, success rates were reported up to 85%, with a 14.8% rate of procedure-related mortality41617. Donaldson et al3 reported a 73% success rate and hospital survival of 87% in a small population presenting extensive thrombosis. In contrast to other experiences41617, they reported a high incidence of postoperative transfusion (55%) and access site complications (4%), probably related to patient frailty.
The AngioVac System has also been used to locally debulk intravenous neoplastic infiltration of the inferior vena cava in patients with kidney tumours, restoring venous drainage from the inferior part of the body1920, followed by nephrectomy (Figure 4, Moving image 2).
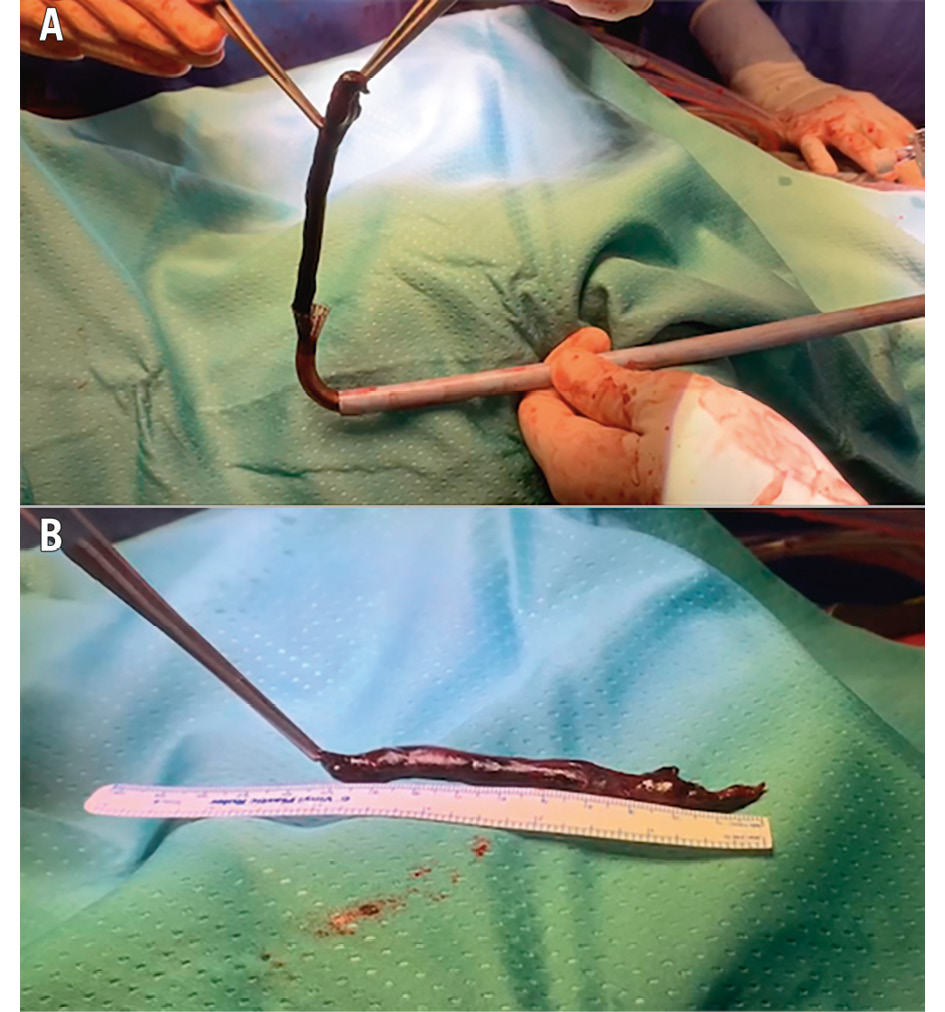
Figure 3. Superior vena cava-right atrium thrombus. Patient with port-a-cath thrombosis. A) The aspirated thrombus is extracted from the funnel-shaped tip of the AngioVac cannula. B) The entire thrombus is shown, measuring over 10 cm.
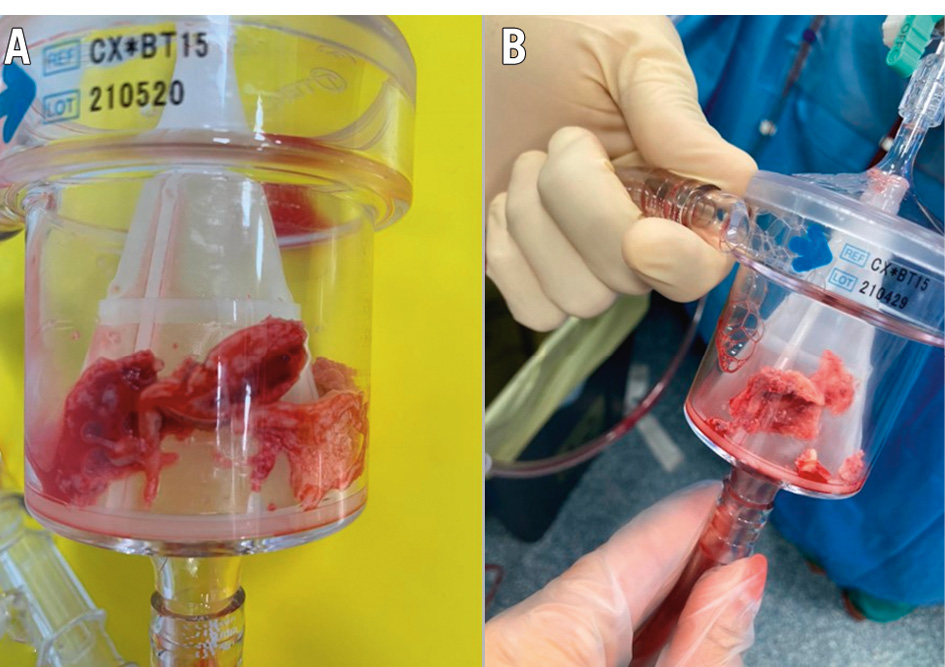
Figure 4. Inferior vena cava thrombus/tumour infiltration. The patient was affected by kidney carcinoma and inferior vena cava neoplastic infiltration and thrombosis. A) Filter containing the aspirated material; (B) fragments of the intravenous mass.
PULMONARY EMBOLISM
PE has an incidence of 0.95 per 1,000 subjects per year and a mortality rate of 1% for submassive PEs to more than 65% for massive PEs21. Treatment mainly relies on systemic thrombolysis and may require extracorporeal membrane oxygenator (ECMO) support in case of severe haemodynamic instability, as indicated by international guidelines21. Surgical embolectomy is indicated in case of massive PEs involving proximal pulmonary artery branches; however, in frail patients, the risk might be prohibitive21.
The AngioVac System has been previously used to treat PE as an alternative to standard medical or surgical management11. PE treatment with percutaneous aspiration is limited to high-risk and haemodynamically unstable patients, and outcomes are poor when compared to RHT and iliocaval aspiration. Indeed, Al-Hakim et al had a 40% success rate in their 5 patients with 80% perioperative mortality22. Another single-centre case series, including 12 patients with massive PE, had a 33% success rate with high in-hospital mortality and intraoperative complications, including right ventricular (RV) perforation and tricuspid valve (TV) apparatus tearing23. Renew et al recently described a successful case of a massive saddle-shaped PE treated using the AngioVac with an ECMO circuit to provide circulatory support and oxygenation24. In all described cases, the procedure was performed under TOE and fluoroscopy guidance.
ENDOCARDITIS
Most commonly, vegetation in the right heart involves the TV (native or prosthetic) and intravenous rhythm device leads25. Despite advancements in diagnosis and treatment, hospital mortality is still significant26. Surgery is indicated in the presence of severe TV regurgitation and vegetations larger than 2 cm, persistent sepsis despite ad hoc therapy, and recurrent PE27.
Percutaneous aspiration has emerged as an alternative to surgery in prohibitive surgical risk patients46 (Figure 5). Experience is still limited to small single-centre case series. However, a meta-analysis28 showed up to 75% clinical success (combination of residual vegetation size <50%, in-hospital survival, absence of persistent bacteraemia, and satisfactory valve function not requiring further interventions) and 15% perioperative mortality. In a single-centre experience, George et al had a 61% reduction of the vegetation size in 33 patients with 90% in-hospital survival29.
The AngioVac System has also been used to remove vegetation of intravenous rhythm device leads. Patel et al and Schaerf et al had a technical success rate of >80%, no infection recurrence, and >90% in-hospital survival3031. Starck’s group32 treated 35 patients (83 leads) with systemic infections, obtaining complete vegetation debulking with no intraoperative death in 88.6% of the patients, while Tarzia et al33 reported 92.3% procedural success with a 7.7% intraoperative complication rate. In all series, vegetation debulking was followed by lead extraction.
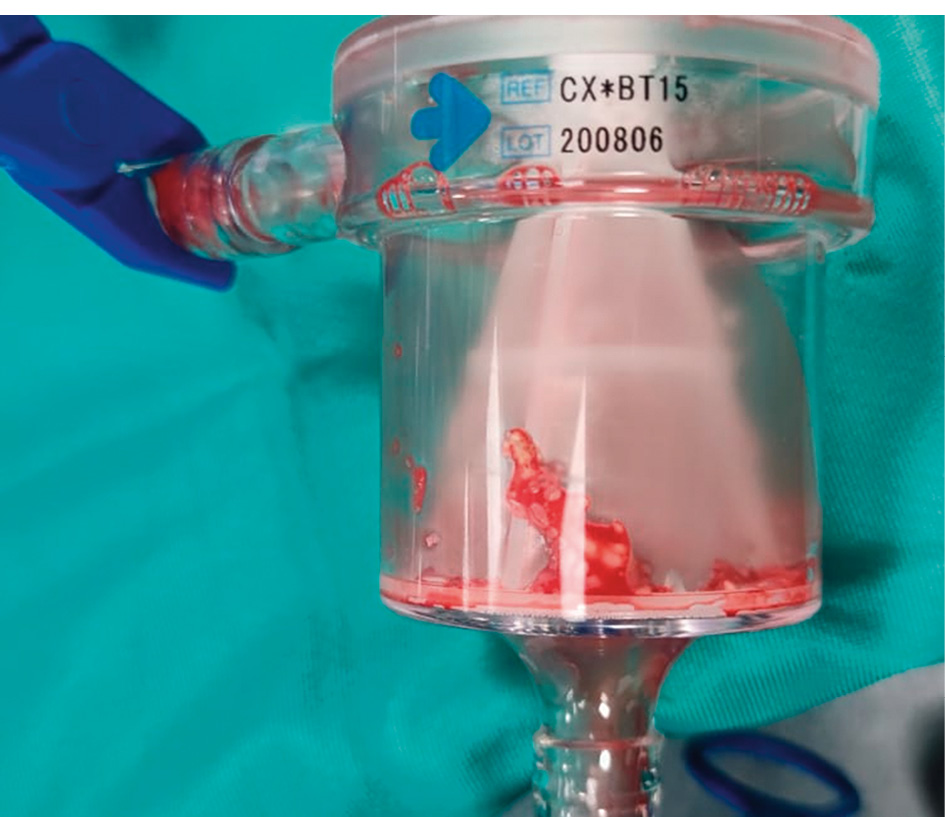
Figure 5. Tricuspid endocarditis. The filter containing the suctioned vegetation.
Intracardiac left heart and intra-aortic masses
ENDOCARDITIS
The prevalence of mitral and aortic native and prosthesis endocarditis has increased over the last decade. Surgery is indicated in the presence of acute heart failure, local tissue destruction, large vegetations, and persistent bacteraemia despite optimal prolonged antibiotic therapy27. About 20% of patients with infective endocarditis are not referred for surgery because of their high surgical risk34. The percutaneous aspiration of left-sided vegetation has been proposed9101112 (Figure 6). Its clinical application is still limited, and no multicentre data are available. Gerosa et al were the first to describe a case of transapical (TA) aspiration of mitral prosthesis vegetation with patient survival9. Other cases have been described, mainly with a transseptal left atrium (LA) approach101112. Differently from Gerosa’s group, who used left heart extracorporeal assistance (left ventricle [LV] to common femoral artery) with the interposition of an oxygenator, other groups preferred a venovenous cannulation with aspiration in the LA through the interatrial septum and reinfusion in the contralateral femoral vein without an oxygenator. In most cases, a percutaneous cerebral protection device was used to prevent potential vegetation embolisation to the supraoptic vessels. Xu et al recently described a case of mitral prosthesis endocarditis that was successfully treated with staged transseptal vegetation aspiration and mitral valve-in-valve implantation using a transcatheter valve in a patient who had been deemed unsuitable for conventional redo surgery because of high surgical risk35.
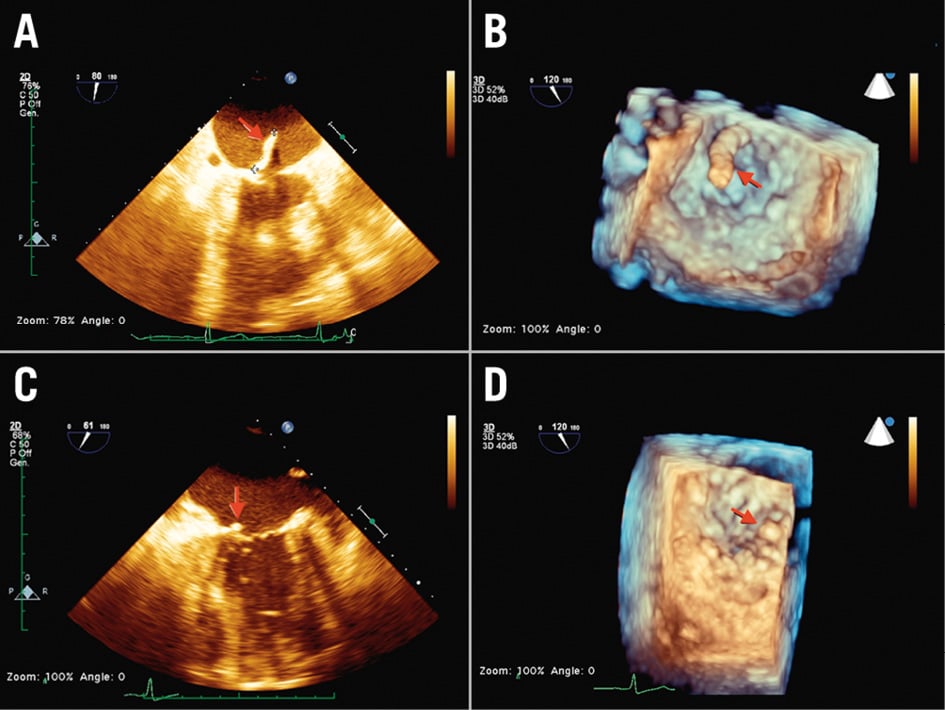
Figure 6. Native mitral valve endocarditis. Transapical aspiration access and femoral artery reinfusion. A,B) Preoperative 2D/3D TOE: vegetation attached to the posterior mitral leaflet and floating in the left atrium during systole (red arrow). C,D) Postoperative 2D/3D TOE: the vegetation is almost completely removed (red arrow). 2D: two-dimensional; 3D: three-dimensional; TOE: transoesophageal echocardiography
AORTIC MASSES
Thoracic aortic mural thrombus is relatively uncommon. Its management is not standardised and depends on its location and extent, patient comorbidities, and the physician’s preferences. The literature describes the application of percutaneous aspiration combined with a cerebral embolic protection device to remove bulky thrombi in the ascending and descending aorta in high-risk patients. Tsilimparis et al36 aspirated a large ascending aorta thrombus from the left subclavian artery extending close to the supra-aortic vessels without complications. Frisoli37 and Kang38 described ascending and descending thoracic aorta thrombus removal from the common femoral artery and reinfusion through the contralateral femoral vein. Rolon et al39 described a case of extensive descending aorta thrombus with infrarenal occlusion, inferior limb, and small bowel ischaemia. The patient underwent infrarenal surgical thrombectomy, aortobisiliac bypass and AngioVac aspiration of the supraceliac part of the thrombus. In this case, the suction cannula was advanced in the aorta using one of the branches of the vascular graft, and blood was reinfused in the contralateral femoral vein. The patient survived without major complications. Transcaval aortic access was described by Qintar et al, who successfully managed a case of distal arch thrombosis in a 55-year-old woman with multiple cerebral and upper limb embolisations40.
Discussion
The AngioVac System was first developed to treat large intravenous masses1516, but its use has expanded to the aspiration of intracardiac masses1718, including valves91011122829, lead vegetations3132, and PE2123. While published results suggest that the AngioVac System is a safe and effective minimally invasive option to treat large intravenous thrombi and kidney-related tumours15161718, data supporting its use in right-sided intracardiac masses and PE are insufficient. Still, in the case of chronic iliocaval thrombosis, results might be suboptimal for acute and subacute thrombosis16. The use of the AngioVac System for PE has shown high perioperative mortality2122, and successful cases have been just anecdotal23. Its application in this subset is mainly limited by the size and stiffness of the aspiration cannula and the risk of damaging the TV subvalvular apparatus or perforating the RV, as reported in the literature2122. Based on our experience, a possible solution to avoid TV crossing and RV navigation is introducing the AngioVac cannula into the pulmonary trunk through a left minithoracotomy in the second intercostal space or mini-upper sternotomy. Pulmonary artery (PA) cannulation can be performed either directly with a DrySeal sheath (W. L. Gore & Associates) or with the interposition of an 8-10 mm vascular graft to prevent arterial tearing during the manipulation of the AngioVac cannula. However, even with this setup, the complete removal of distal emboli is unlikely (Figure 7).
Therefore, the use of AngioVac for PE treatment should be reserved only for prohibitive-risk haemodynamically unstable patients to debulk massive embolisation in the main PA and its branches and then proceed either with a percutaneous smaller catheter or surgical embolectomy in a second stage when the patient is more stable or with intravenous anticoagulation with or without ECMO support depending on the patient’s clinical conditions. Moreover, when this option is chosen, we prefer an ECMO-like setup with the interposition of an oxygenator (Central illustration). This would indeed provide circulatory support and blood oxygenation during the procedure and might be switched to midterm paracorporeal circulatory support (ECMO or right ventricular assist device) until complete recovery of RV function and improvement of pulmonary circulation are achieved.
As shown in the Central illustration, we usually prefer adding an oxygenator to the circuit to improve filter capacity and to provide stable oxygenation during aspiration. This is of paramount importance in case the aspiration cannula is on the right side or in the pulmonary system and the infusion cannula is inserted in the arterial system. Furthermore, when treating intravascular tumours that tend to fragment into numerous very small particles, the presence of an oxygenator provides extra filtering capacity, preventing the reinfusion of part of the debris. In case of an increased risk of bleeding and therefore necessity of lower ACT values, the oxygenator can be excluded thanks to a shunt line. This setup gives extreme versatility to the circuit, providing the opportunity to mechanically assist the patient even when the procedure has been completed.
In case of right-sided endocarditis, available data suggest that the use of the AngioVac System should be reserved for frail patients with high surgical risk and no TV regurgitation2728. In this case, the rationale is to reduce the size of the vegetation, possibly lowering the microbial burden with a positive impact on the effect of antibiotic therapy28. Surgery remains mandatory in the presence of local infection extension or when the valve or prosthesis malfunctions, causing acute heart failure and haemodynamic instability. Moreover, suction on the TV carries an increased risk of chordal tearing that can lead to acute TV regurgitation. Initial treatment of lead endocarditis with AngioVac is possible to reduce the microbial burden and the risk of PE; however, in all cases, it must be followed by conventional lead extraction3132.
Treatment of left-sided endocarditis9101112 and aortic thrombi3536373839 with the AngioVac System is relatively new, and data are scant. Therefore, its use should be limited to inoperable patients when medical therapy is ineffective. As described in the literature, in all cases, a cerebral embolism protection device should be used in consideration of the high risk of distal embolism during wire/sheath insertion and mass manipulation4041.
Treatment of mitral endocarditis can be achieved either with a transapical or transseptal approach9101112 with comparable outcomes. While avoiding an open-chest procedure and puncture of the left ventricular apex, the transseptal approach entails the creation of an iatrogenic septal atrial defect that, in some selected cases, might need to be closed with a closure device. Placement of material inside the heart in infected patients should be carefully considered and if possible avoided. Apical insertion of the aspiration cannula has the advantage of allowing for contemporary treatment of aortic and mitral valves in case of multivalvular endocarditis (Figure 8). However, the best access site should be chosen based on each patient’s characteristics and the location of the vegetations (atrial or ventricular aspect of the valve; involvement of one or two valves). Direct access to the mitral valve might also be obtained with insertion of the aspiration cannula via the right upper pulmonary vein with a mini-thoracotomy approach. Combined transseptal AngioVac and valve-in-valve implantation for mitral prosthesis endocarditis has been described34. Even if attractive, this option should be considered very carefully, because the experience is currently limited to a single case, and complete removal of the infected material is not achievable.
Transcatheter vacuum-assisted aspiration has become a new part of the therapeutic strategy for intracardiac and intravascular masses in specific high-risk clinical scenarios; it can work either in isolation or as a step in a combination of different procedures. It offers reduced invasiveness and, so far, good clinical results with respect to the type of treated patients. However, in consideration of its novelty, preprocedural multidisciplinary Heart Team evaluation is mandatory. Depending on the disease, the planning should include access site definition, circuit setup, further therapeutic steps (either medical or interventional/surgical) and optimal imaging guidance.
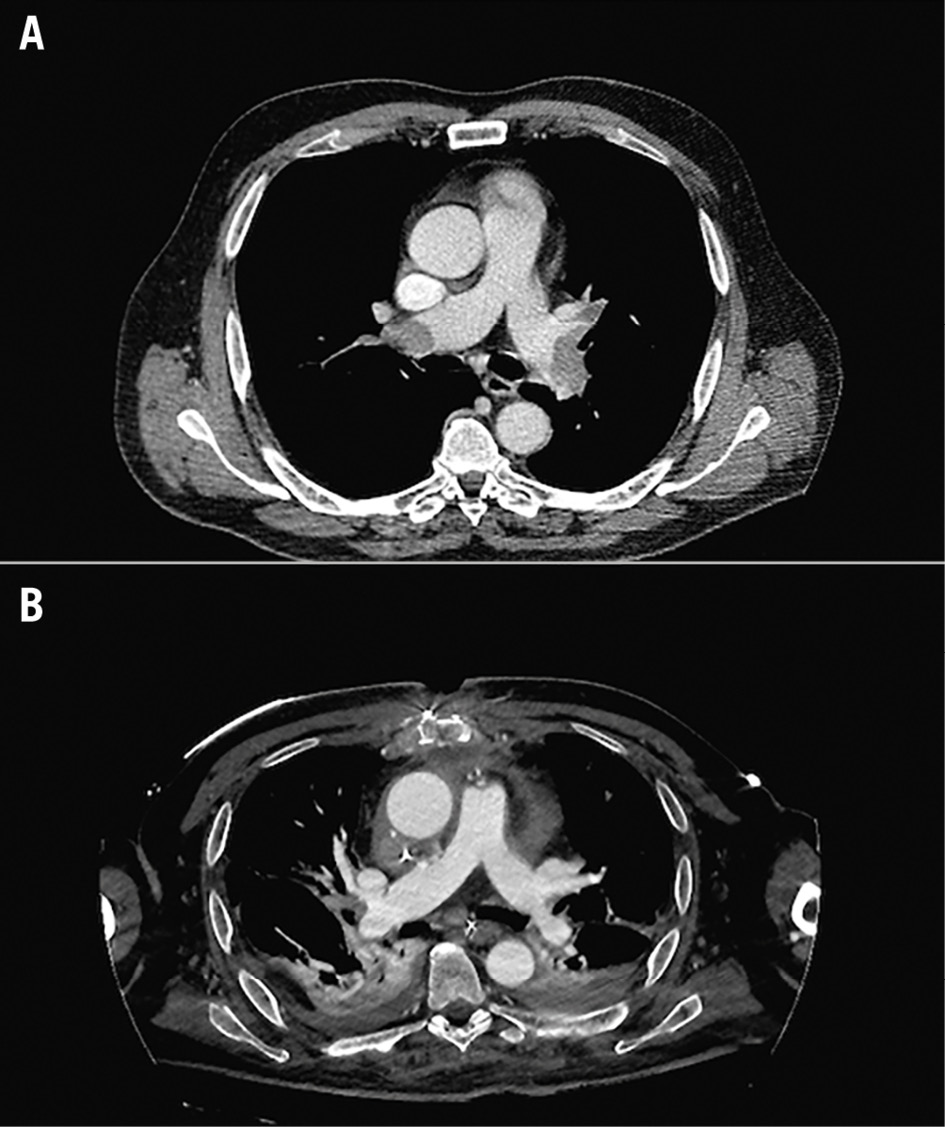
Figure 7. Massive pulmonary embolism. A) Preprocedural angio-CT: the principal pulmonary branches show almost complete occlusion. B) Postprocedural angio-CT: almost complete reperfusion is appreciable on both sides. CT: computed tomography

Central illustration. Circuit setups. A) Right-sided masses. A1) Jugular vein – femoral vein access: superior caval, tricuspid, atrial and jugular vein mass suction. This is preferable to treat haemodynamically stable patients, since the reinfusion cannula is positioned in the femoral vein, and therefore, there is no chance to mechanically assist the patient. A2) Femoral vein – femoral artery access ECMO configuration: right-sided masses suction and circulatory assistance. A shunt line allows for filter bypassing, in case of need for assistance >6 h. A3) Pulmonary artery – femoral vein access±oxygenator: for pulmonary embolism. By switching the reinfusion cannula into the femoral artery, the system can be shifted to a temporary ECMO. A4) Pulmonary artery – femoral artery access ECMO configuration: this configuration enables the operator to treat pulmonary embolism, ensuring mechanical support to the circulation. The oxygenator and the shunt line provide the chance to bypass the filter in case of need for >6 h of use, transforming the circuit into an ECMO. B) Left-sided masses. B1) Femoral artery – femoral vein access: this configuration has been described for abdominal aorta mass suction procedures. B2) Transseptal – femoral artery access±oxygenator: this setup has been described to approach left atrial/mitral valve/LV masses. The optional use of an oxygenator increases the filtering and assists blood oxygenation in case the patient needs prolonged mechanical circulatory support; in this case, a shunt line allows for filter bypassing and prolonged left mechanical assistance. B3) Transapical – femoral artery access±oxygenator: mitral and aortic valve masses. This setup includes two shunt lines: one to bypass the filter in case of need for prolonged left mechanical assistance and one to allow the potential use of an oxygenator. B4) Transapical – subclavian artery access±oxygenator: the reinfusion cannula can be positioned in the subclavian artery when femoral arteries are not available. This configuration allows for prolonged left mechanical assistance by means of a shunt line bypassing the filter. ECMO: extracorporeal membrane oxygenation; LV: left ventricular
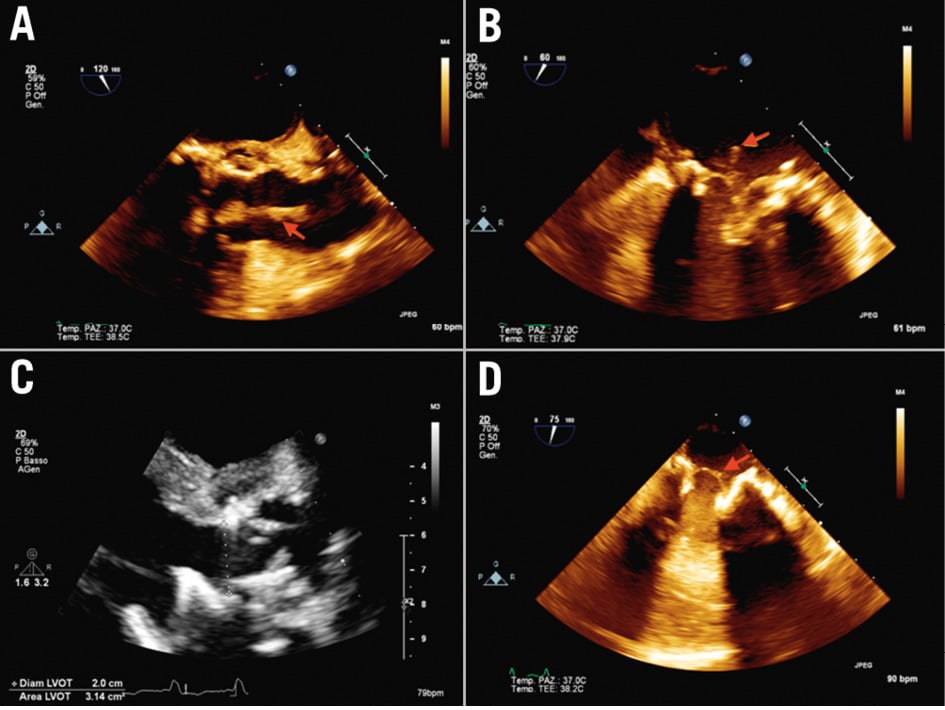
Figure 8. Mitral and aortic prosthesis endocarditis. Transapical aspiration access and femoral artery reinfusion. A) 2D preoperative transoesophageal echocardiography (TOE) showing the presence of a mass (red arrow) attached to the aortic prosthesis and floating in the aortic root, (B) 2D preoperative TOE showing a mass (red arrow) attached to the mitral prosthesis on the atrial side, (C) postoperative 2D TOE showing no residual mass on the aortic prosthesis, (D) postoperative 2D TOE showing a residual minimal stump (red arrow) on the mitral prosthesis. 2D: two-dimensional
Conclusions
Transcatheter vacuum-assisted aspiration has become an alternative to conventional surgery in different clinical scenarios to treat high-risk patients. However, based on the current experience, it should be indicated only in iliocaval and right heart thrombosis. Application to right- and left-sided endocarditis, PE and aortic thrombi is still not supported by sufficiently reliable results. It should therefore be limited to carefully selected inoperable/high-risk patients after multidisciplinary Heart Team evaluation.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplementary data
To read the full content of this article, please download the PDF.
Moving image 1. 10 cm long thrombus removed from the right atrium/superior vena cava. The patient presented with distal pulmonary embolism and a mass floating in the right atrium impinging the tricuspid valve.
Moving image 2. Inferior vena cava thrombus/tumour infiltration specimens inside the AngioVac filter.

